Thymoquinone (TQ) Inhibits Inflammation and Migration of THP-1 Macrophages: Mechanistic Insights into the Prevention of Atherosclerosis Using In-Vitro and In-Silico Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture and Maintenance of THP-1 Cells
2.2. Differentiation of THP-1 Monocytes into Macrophages
2.3. Lactate Dehydrogenase (LDH) Assay
2.4. Quantitative Real-Time PCR
2.5. Monocyte Migration Assay
2.6. Cholesterol Efflux Assay
2.7. SwissTargetPrediction for TQ
2.8. Web Gestalt Analysis of TQ Putative Protein Targets
2.9. The Open Targets Platforms Analysis
2.10. Ingenuity Pathway Analysis
2.11. Statistical Analysis
3. Results
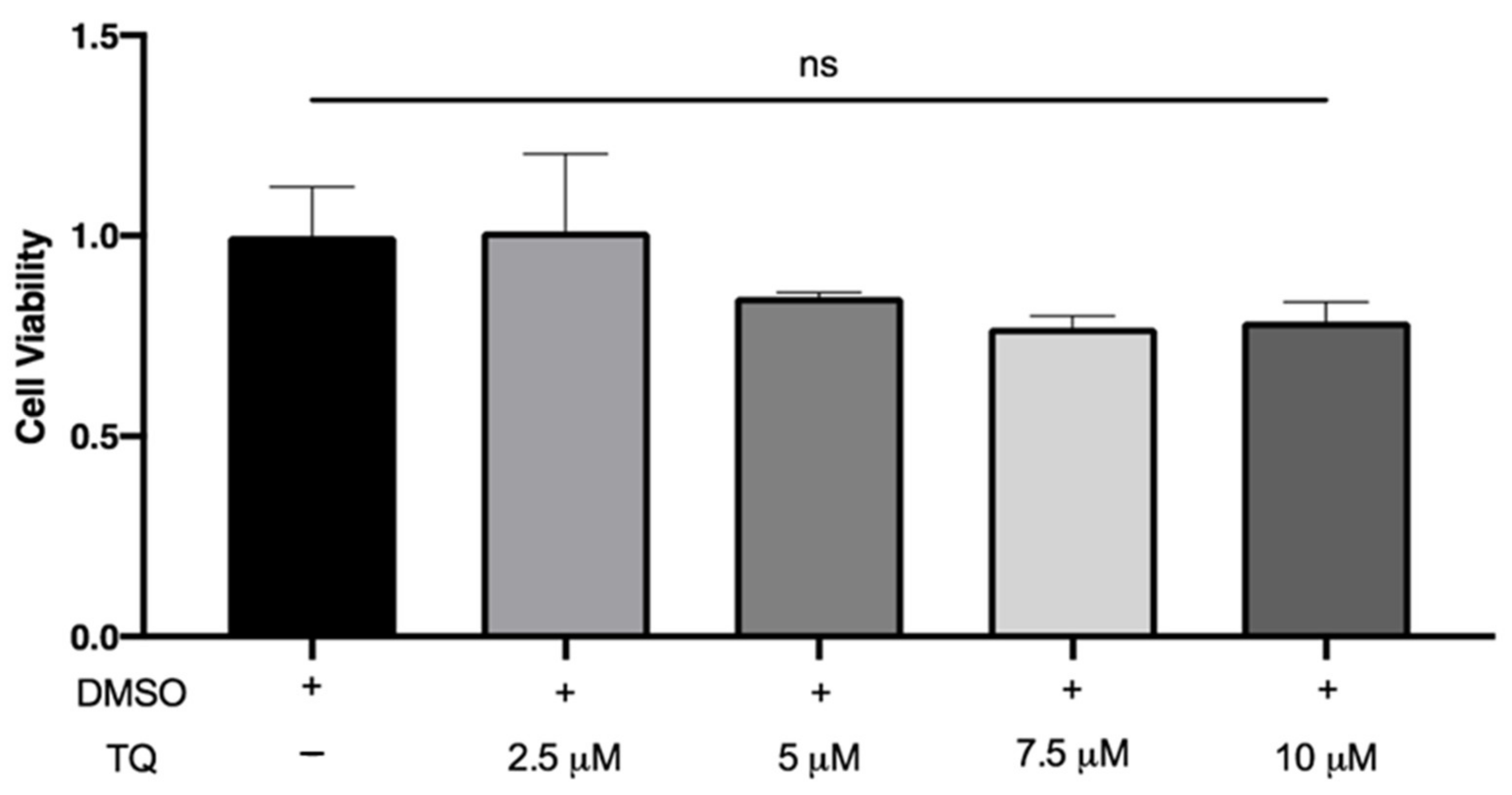
3.1. TQ Did Not Affect the Viability of THP-1 Macrophages
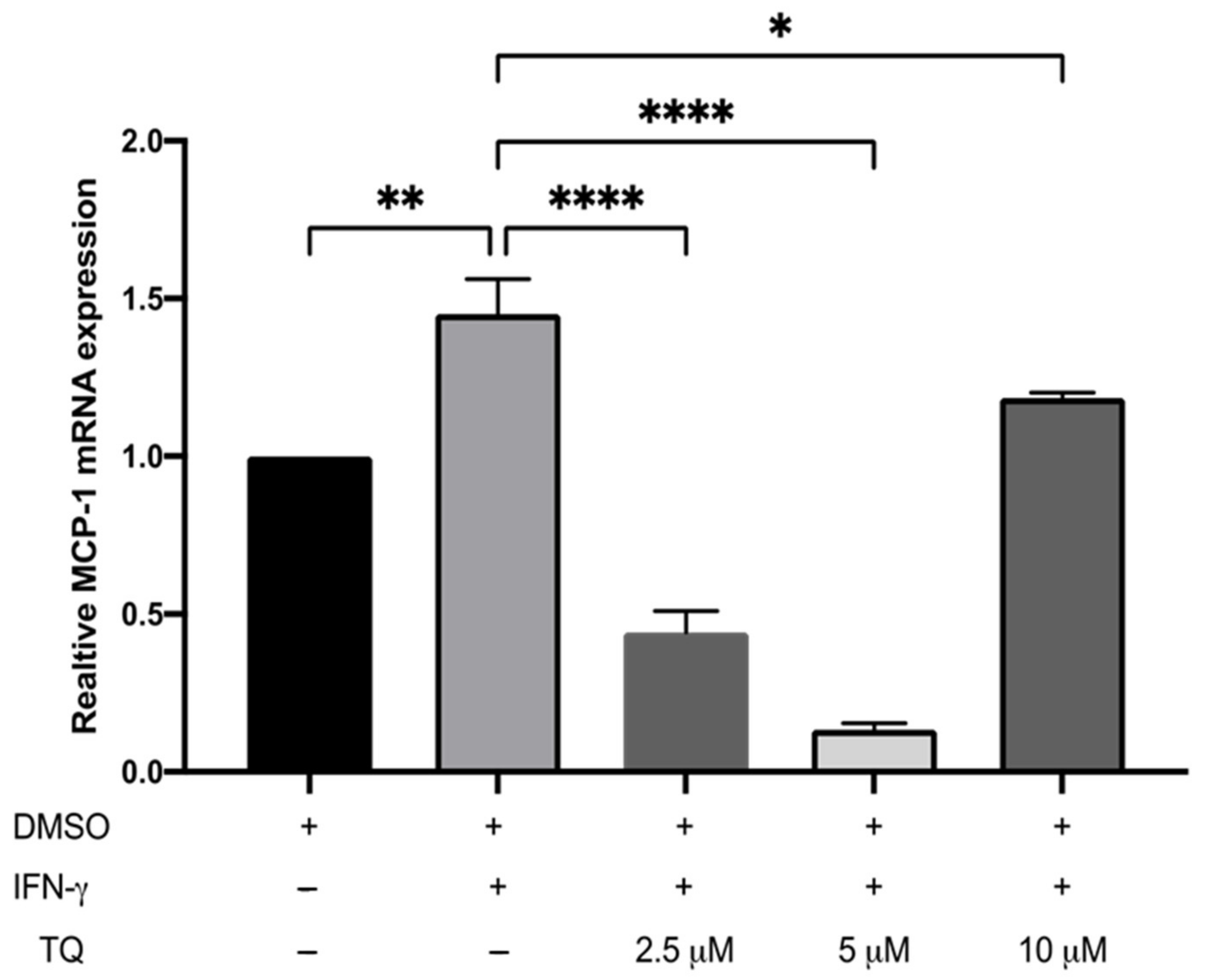
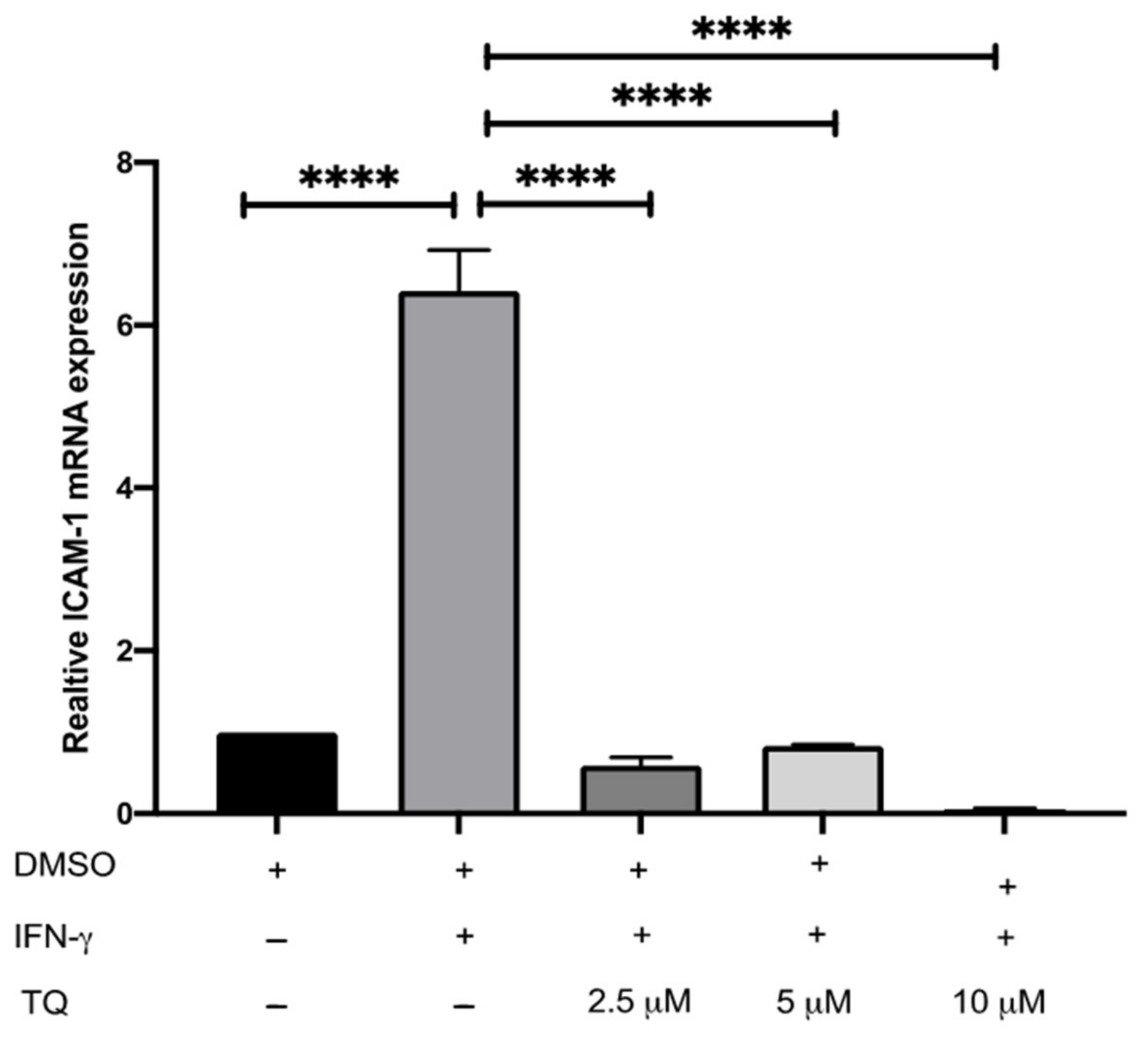
3.2. TQ Inhibits IFN-γ-Induced ICAM-1 and MCP-1 Gene Expression in Human THP-1 Macrophages
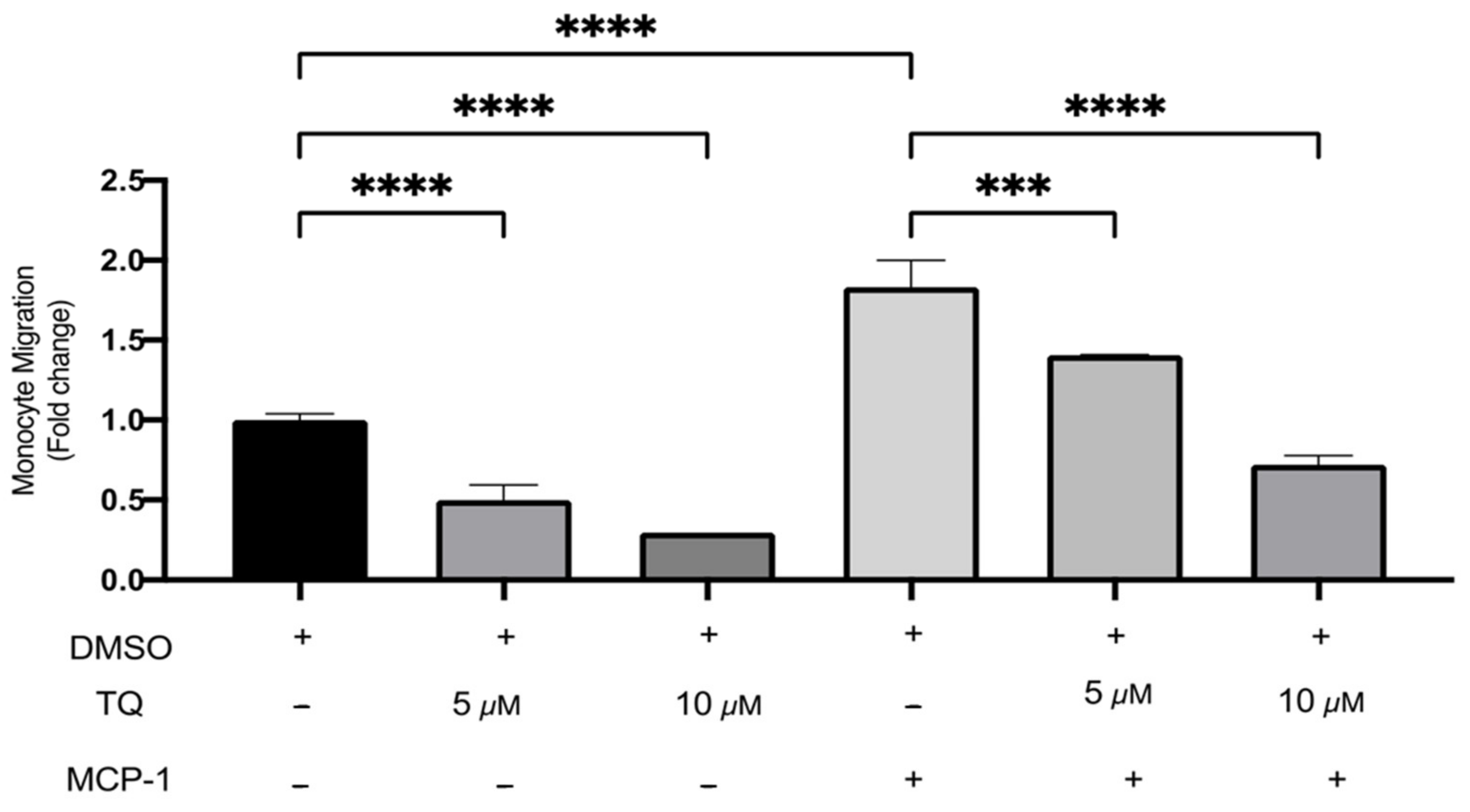
3.3. TQ Inhibits Human Monocyte Migration towards MCP-1
3.4. TQ Has no Effect on Cholesterol Efflux from Human THP-1 Macrophages
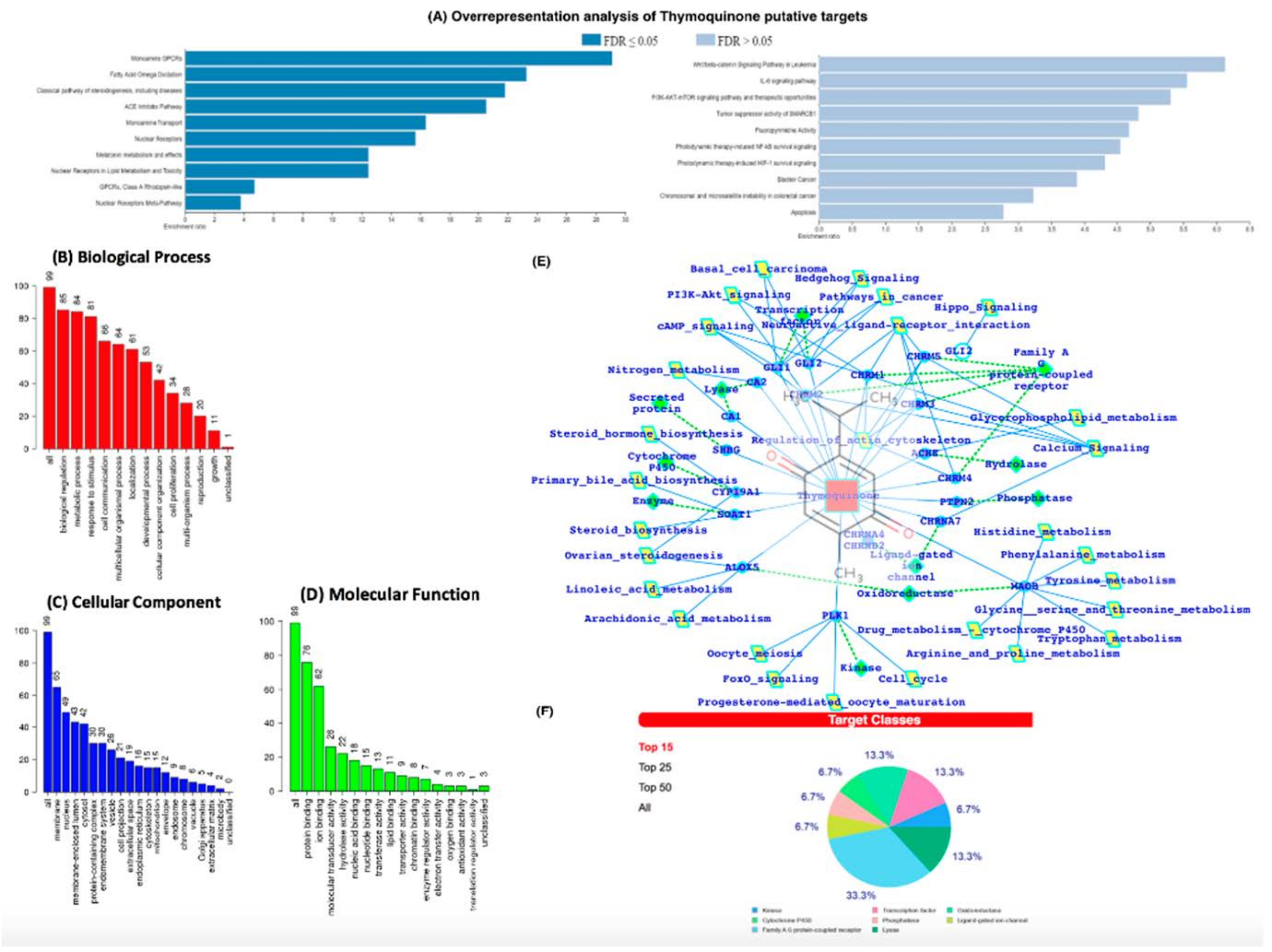
3.5. In-Silico Analysis Reveals TQ Molecular Targets Implicated in Atherosclerosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lusis, A.J. Genetics of atherosclerosis. Trends Genet. 2012, 28, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors for Coronary Artery Disease. 2021 Jun 5. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.L.; Ramji, D.P. The influence of dysfunctional signaling and lipid homeostasis in mediating the inflammatory responses during atherosclerosis. Biochim. Biophys. Acta. 2015, 1852, 1498–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramji, D.P.; Davies, T.S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Li, A.C.; Glass, C.K. The macrophage foam cell as a target for therapeutic intervention. Nat. Med. 2002, 8, 1235–1242. [Google Scholar] [CrossRef]
- Cejkova, S.; Králová-Lesná, I.; Poledne, R. Monocyte adhesion to the endothelium is an initial stage of atherosclerosis development. Cor Et Vasa 2016, 58, e419–e425. [Google Scholar] [CrossRef]
- McLaren, J.E.; Michael, D.R.; Ashlin, T.G.; Ramji, D.P. Cytokines, macrophage lipid metabolism and foam cells: Implications for cardiovascular disease therapy. Prog. Lipid Res. 2011, 50, 331–347. [Google Scholar] [CrossRef]
- Glaser, J.; Holzgrabe, U. Focus on PAINS: False friends in the quest for selective anti-protozoal lead structures from Nature? Med. Chem. Comm. 2016, 7, 214–223. [Google Scholar] [CrossRef]
- Nader, M.A.; el-Agamy, D.S.; Suddek, G.M. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch. Pharm. Res. 2010, 33, 637–643. [Google Scholar] [CrossRef]
- Asgary, S.; Ghannadi, A.; Dashti, G.; Helalat, A.; Sahebkar, A.; Najafi, S. Nigella sativa L. improves lipid profile and prevents atherosclerosis: Evidence from an experimental study on hypercholesterolemic rabbits. J. Funct. Foods 2013, 5, 228–234. [Google Scholar] [CrossRef]
- Ragheb, A.; Attia, A.; Elbarbr, F.; Prasad, K.; Shoker, A.; Medicine, A. Attenuated combined action of cyclosporine A and hyperlipidemia on atherogenesis in rabbits by thymoquinone. Evid.-Based Complementary Altern. Med. 2011, 2011, 620319. [Google Scholar] [CrossRef]
- Idris-Khodja, N.; Schini-Kerth, V. Thymoquinone improves aging-related endothelial dysfunction in the rat mesenteric artery. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 749–758. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Kim, S. Getting the most out of PubChem for virtual screening. Expert Opin. Drug Discov. 2016, 11, 843–855. [Google Scholar] [CrossRef] [Green Version]
- Bahlas, S.; Damiati, L.A.; Al-Hazmi, A.S.; Pushparaj, P.N. Decoding the Role of Sphingosine-1-Phosphate in Asthma and Other Respiratory System Diseases Using Next Generation Knowledge Discovery Platforms Coupled with Luminex Multiple Analyte Profiling Technology. Front. Cell Dev. Biol. 2020, 19, 444. [Google Scholar] [CrossRef]
- Gfeller, D.; Michielin, O.; Zoete, V. Shaping the interaction landscape of bioactive molecules. Bioinformatics 2013, 29, 3073–3079. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. Swiss TargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Zhang, G.; Zhou, Y.; Lin, C.; Chen, S.; Lin, Y.; Mai, S.; Huang, Z. Reverse Screening Methods to Search for the Protein Targets of Chemopreventive Compounds. Front. Chem. 2018, 6, 138. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. Application of the SwissDrugDesign Online Resources in Virtual Screening. Int. J. Mol. Sci. 2019, 20, 4612. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koscielny, G.; An, P.; Carvalho-Silva, D.; Cham, J.A.; Fumis, L.; Gasparyan, R.; Hasan, S.; Karamanis, N.; Maguire, M.; Papa, E.; et al. Open Targets: A platform for therapeutic target identification and validation. Nucleic Acids Res. 2017, 45, D985–D994. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Silva, D.; Pierleoni, A.; Pignatelli, M.; Ong, C.; Fumis, L.; Karamanis, N.; Carmona, M.; Faulconbridge, A.; Hercules, A.; McAuley, E.; et al. Open Targets Platform: New developments and updates two years on. Nucleic Acids Res. 2019, 47, D1056–D1065. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, D.; Hercules, A.; Carmona, M.; Suveges, D.; Gonzalez-Uriarte, A.; Malangone, C.; Miranda, A.; Fumis, L.; Carvalho-Silva, D.; Spitzer, M.; et al. Open Targets Platform: Supporting systematic drug-target identification and prioritisation. Nucleic Acids Res. 2021, 49, D1302–D1310. [Google Scholar] [CrossRef]
- Khaladkar, M.; Koscielny, G.; Hasan, S.; Agarwal, P.; Dunham, I.; Rajpal, D.; Sanseau, P. Uncovering novel repositioning opportunities using the Open Targets platform. Drug Discov. Today 2017, 22, 1800–1807. [Google Scholar] [CrossRef]
- Jafri, M.A.; Kalamegam, G.; Abbas, M.; Al-Kaff, M.; Ahmed, F.; Bakhashab, S.; Rasool, M.; Naseer, M.I.; Sinnadurai, V.; Pushparaj, P.N. Deciphering the Association of Cytokines, Chemokines, and Growth Factors in Chondrogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Using an Ex Vivo Osteochondral Culture System. Front. Cell Dev. Biol. 2020, 7, 380. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Eldakhakhny, B.M.; Sadoun Al, H.; Choudhry, H.; Mobashir, M. In-Silico Study of Immune System Associated Genes in Case of Type-2 Diabetes with Insulin Action and Resistance, and/or Obesity. Front. Endocrinol. 2021, 12, 281. [Google Scholar] [CrossRef]
- Bajrai, L.H.; Sohrab, S.S.; Mobashir, M.; Kamal, M.A.; Rizvi, M.A.; Azhar, E.I. Understanding the role of potential pathways and its components including hypoxia and immune system in case of oral cancer. Sci. Rep. 2021, 11, 19576. [Google Scholar] [CrossRef]
- Bajrai, L.; Sohrab, S.S.; Alandijany, T.A.; Mobashir, M.; Parveen, S.; Kamal, M.A. Gene expression profiling of early acute febrile stage of dengue infection and its comparative analysis with Streptococcus pneumoniae infection. Front. Cell. Infect. Microbiol. 2021, 11, 707905. [Google Scholar] [CrossRef]
- Campbell, C.Y.; Rivera, J.J.; Blumenthal, R.S. Residual risk in statin-treated patients: Future therapeutic options. Curr. Cardiol. Rep. 2007, 9, 499–505. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Sampson, U.K.; Fazio, S.; Linton, M.F. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: The evidence, etiology, and therapeutic challenges. Curr. Atheroscler. Rep. 2012, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Noels, H.J. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Fardoun, M.; Al-Shehabi, T.; El-Yazbi, A.; Issa, K.; Zouein, F.; Maaliki, D.; Iratni, R.; Eid, A.H. Ziziphus nummularia Inhibits Inflammation-Induced Atherogenic Phenotype of Human Aortic Smooth Muscle Cells. Oxid. Med. Cell. Longev. 2017, 2017, 4134093. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Chen, X.; Ma, J.; Hassan, W.; Wu, H.; Ling, J.; Shang, J. β-Elemene attenuates atherosclerosis in apolipoprotein E-deficient mice via restoring no levels and alleviating oxidative stress. Biomed. Pharm. 2017, 95, 1789–1798. [Google Scholar] [CrossRef]
- Park, S.H.; Koo, H.J.; Sung, Y.Y.; Kim, H.K. The protective effect of Prunella vulgaris ethanol extract against vascular inflammation in TNF-α-stimulated human aortic smooth muscle cells. BMB Rep. 2013, 46, 352–357. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Feng, H.; Guo, S.; Han, Y.; Chen, X. Danshenol A inhibits TNF-α-induced expression of intercellular adhesion molecule-1 (ICAM-1) mediated by NOX4 in endothelial cells. Sci. Rep. 2017, 7, 12953. [Google Scholar] [CrossRef] [Green Version]
- Jameel, S.; Huwait, E.; Al-Massabi, R.; Saddeek, S.; Gauthaman, K.; Prola, A. Punicalagin Regulates Key Processes Associated with Atherosclerosis in THP-1 Cellular Model. Pharmaceuticals 2020, 13, 372. [Google Scholar] [CrossRef]
- Chehl, N.; Chipitsyna, G.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB 2009, 11, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Umar, S.; Hedaya, O.; Singh, A.K.; Ahmed, J.T. Thymoquinone inhibits TNF-α-induced inflammation and cell adhesion in rheumatoid arthritis synovial fibroblasts by ASK1 regulation. Pharmacology 2015, 287, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazwani, M.; Zhang, Y.; Gao, X.; Fan, J.; Li, J.; Li, S. Anti-fibrotic effect of thymoquinone on hepatic stellate cells. Phytomedicine 2014, 21, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Kolli-Bouhafs, K.; Boukhari, A.; Abusnina, A.; Velot, E.; Gies, J.P.; Lugnier, C.; Rondé, P. Thymoquinone reduces migration and invasion of human glioblastoma cells associated with FAK, MMP-2 and MMP-9 down-regulation. Investig. New Drugs 2012, 30, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Li, X.; Chen, H.; Han, Y.; Fan, Y.J. Thymoquinone inhibits angiotensin II-induced proliferation and migration of vascular smooth muscle cells through the AMPK/PPARγ/PGC-1α pathway. Biology 2016, 35, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kuang, X.R.; Lv, P.T.; Yan, X.X. Thymoquinone inhibits proliferation and invasion of human nonsmall-cell lung cancer cells via ERK pathway. Tumor Biol. 2015, 36, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Beg, Z.H. Hypolipidemic and antioxidant activities of thymoquinone and limonene in atherogenic suspension fed rats. Food Chem. 2013, 138, 1116–1124. [Google Scholar] [CrossRef]
- Bamosa, A.; Ali, B.A.; Al-Hawsawi, Z.A. The effect of thymoquinone on blood lipids in rats. Indian J. Physiol. Pharmacol. 2002, 46, 195–201. [Google Scholar]

| Genes | Primer Sequence |
|---|---|
| MCP-1 | F: 5′-CGCTCAGCCAGATGC-AATCAATG-3′ R: 5′-ATGGTCTTGAAGATCA-CAGCTT-CTTTGG-3′ |
| ICAM-1 | F: 5′-GACCAGAGGTTGAAC-CCCAC-3′ R: 5′-GCGCCGGAAAGCTG-TAGAT-3′ |
| GAPDH | F: 5′-CTTTTGCGTCGCCAG-CCGAG-3′ R: 5′-GCCCAATACGACCAAA TCCGTTGACT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huwait, E.; Al-Gharawi, N.; Al-Ghamdi, M.A.; Gari, M.; Prola, A.; Natesan Pushparaj, P.; Kalamegam, G. Thymoquinone (TQ) Inhibits Inflammation and Migration of THP-1 Macrophages: Mechanistic Insights into the Prevention of Atherosclerosis Using In-Vitro and In-Silico Analysis. Curr. Issues Mol. Biol. 2022, 44, 1740-1753. https://doi.org/10.3390/cimb44040120
Huwait E, Al-Gharawi N, Al-Ghamdi MA, Gari M, Prola A, Natesan Pushparaj P, Kalamegam G. Thymoquinone (TQ) Inhibits Inflammation and Migration of THP-1 Macrophages: Mechanistic Insights into the Prevention of Atherosclerosis Using In-Vitro and In-Silico Analysis. Current Issues in Molecular Biology. 2022; 44(4):1740-1753. https://doi.org/10.3390/cimb44040120
Chicago/Turabian StyleHuwait, Etimad, Nouf Al-Gharawi, Maryam A. Al-Ghamdi, Mamdooh Gari, Alexandre Prola, Peter Natesan Pushparaj, and Gauthaman Kalamegam. 2022. "Thymoquinone (TQ) Inhibits Inflammation and Migration of THP-1 Macrophages: Mechanistic Insights into the Prevention of Atherosclerosis Using In-Vitro and In-Silico Analysis" Current Issues in Molecular Biology 44, no. 4: 1740-1753. https://doi.org/10.3390/cimb44040120
APA StyleHuwait, E., Al-Gharawi, N., Al-Ghamdi, M. A., Gari, M., Prola, A., Natesan Pushparaj, P., & Kalamegam, G. (2022). Thymoquinone (TQ) Inhibits Inflammation and Migration of THP-1 Macrophages: Mechanistic Insights into the Prevention of Atherosclerosis Using In-Vitro and In-Silico Analysis. Current Issues in Molecular Biology, 44(4), 1740-1753. https://doi.org/10.3390/cimb44040120








