The Increase in Circulating Levels of Pro-Inflammatory Chemokines, Cytokines, and Complement C5 in Canines with Impaired Kidney Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Complete Blood Count (CBC)
2.3. Histopathology
2.4. Blood Collection and RNA Extraction
2.5. Pathway-Focused Gene Expression Analysis
2.6. Statistical Analysis
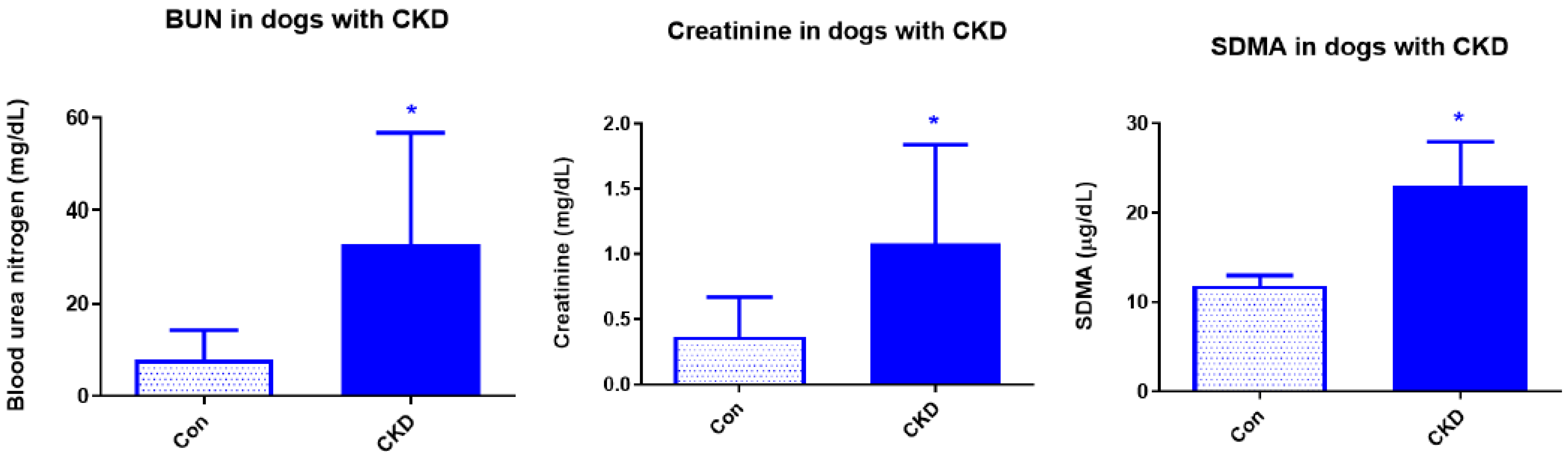
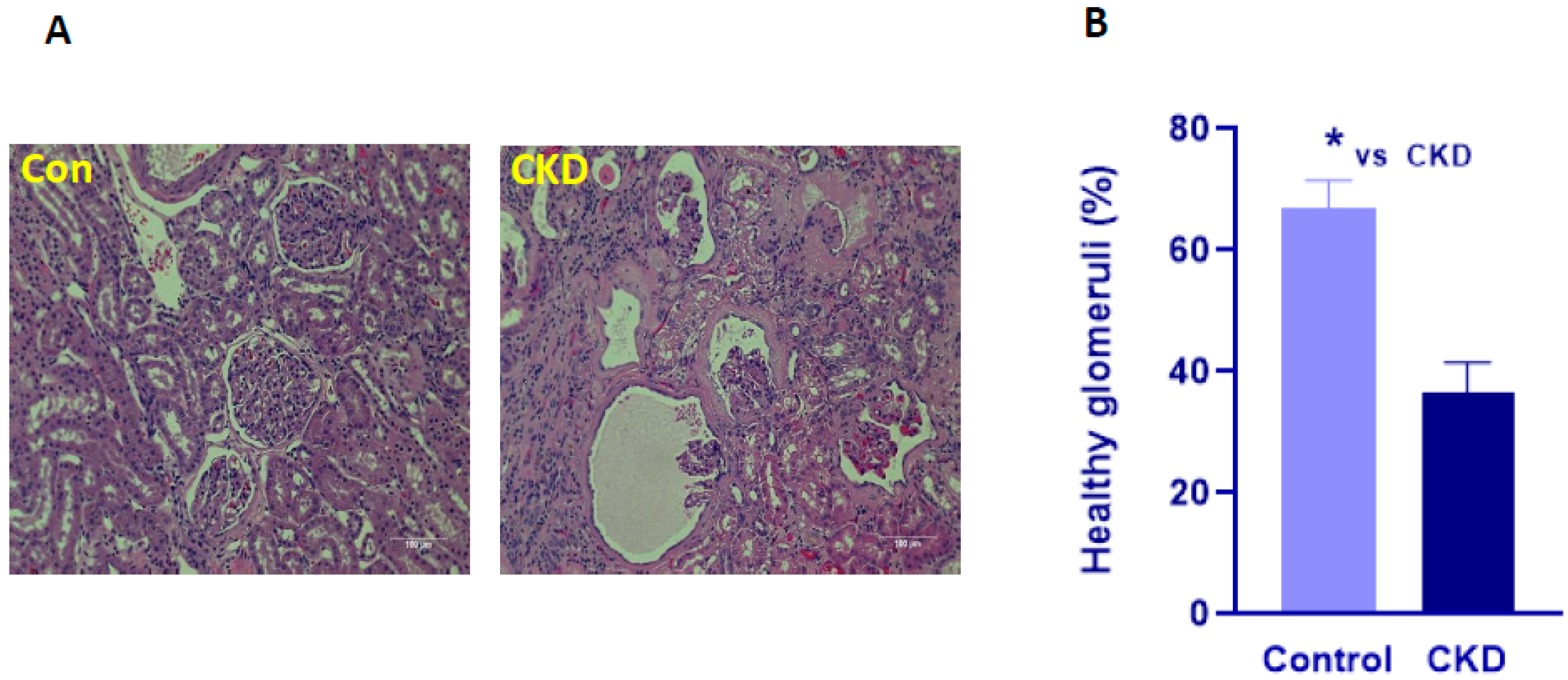
3. Results
3.1. Complete Blood Count
3.2. Histopathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Polzin, D.J. Chronic kidney disease in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Polzin, D.J. Evidence-based step-wise approach to managing chronic kidney disease in dogs and cats. J. Vet. Emerg. Crit. Care 2013, 23, 205–215. [Google Scholar] [CrossRef]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Almes, K.; Jewell, D.E. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J. Vet. Intern. Med. 2016, 30, 794–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babyak, J.M.; Weiner, D.E.; Noubary, F.; Sharp, C.R. Prevalence of Elevated Serum Creatinine Concentration in Dogs Presenting to a Veterinary Academic Medical Center (2010–2014). J. Vet. Intern. Med. 2017, 31, 1757–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galle, J. Oxidative stress in chronic renal failure. Nephrol. Dial. Transplant. 2001, 16, 2135–2137. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R. Proinflammatory effects of oxidative stress in chronic kidney disease: Role of additional angiotensin II blockade. Am. J. Physiol. Renal. Physiol. 2003, 284, F863–F869. [Google Scholar] [CrossRef] [Green Version]
- Himmelfarb, J. Relevance of oxidative pathways in the pathophysiology of chronic kidney disease. Cardiol. Clin. 2005, 23, 319–330. [Google Scholar] [CrossRef]
- Diaz-Buxo, J.A.; Woods, H.F. Protecting the endothelium: A new focus for management of chronic kidney disease. Hemodial. Int. 2006, 10, 42–48. [Google Scholar] [CrossRef]
- Halfen, D.P.; Caragelasco, D.S.; Nogueira, J.P.S.; Jeremias, J.T.; Pedrinelli, V.; Oba, P.M.; Ruberti, B.; Pontieri, C.F.F.; Kogika, M.M.; Brunetto, M.A. Evaluation of Electrolyte Concentration and Pro-Inflammatory and Oxidative Status in Dogs with Advanced Chronic Kidney Disease under Dietary Treatment. Toxins 2019, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Perini-Perera, S.; Del-Ángel-Caraza, J.; Pérez-Sánchez, A.P.; Quijano-Hernández, I.A.; Recillas-Morales, S. Evaluation of Chronic Kidney Disease Progression in Dogs With Therapeutic Management of Risk Factors. Front. Vet. Sci. 2021, 8, 621084. [Google Scholar] [CrossRef]
- Floege, J.; Burns, M.W.; Alpers, C.E.; Yoshimura, A.; Pritzl, P.; Gordon, K.; Seifert, R.A.; Bowen-Pope, D.F.; Couser, W.G.; Johnson, R.J. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 1992, 41, 297–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, G.; Nakano, K.; Sawada, M.; Uno, K.; Shibayama, Y.; Ienaga, K.; Kondo, M. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991, 40, 1007–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noels, H.; Lehrke, M.; Vanholder, R.; Jankowski, J. Lipoproteins and fatty acids in chronic kidney disease: Molecular and metabolic alterations. Nat. Rev. Nephrol. 2021, 17, 528–542. [Google Scholar] [CrossRef]
- Speer, T.; Ridker, P.M.; von Eckardstein, A.; Schunk, S.J.; Fliser, D. Lipoproteins in chronic kidney disease: From bench to bedside. Eur. Heart J. 2021, 42, 2170–2185. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Huys, G.R.B.; Joossens, M.; Van Biesen, W.; Glorieux, G.; Vaneechoutte, M. Isolation and Quantification of Uremic Toxin Precursor-Generating Gut Bacteria in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2020, 21, 1986. [Google Scholar] [CrossRef] [Green Version]
- Stenvinkel, P.; Ketteler, M.; Johnson, R.J.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimbürger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia-the good, the bad, and the ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [CrossRef] [Green Version]
- Keepers, T.R.; Gross, L.K.; Obrig, T.G. Monocyte chemoattractant protein 1, macrophage inflammatory protein 1 alpha, and RANTES recruit macrophages to the kidney in a mouse model of hemolytic-uremic syndrome. Infect. Immun. 2007, 75, 1229–1236. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.S.; Pulskens, W.P.; Sadler, J.J.; Butter, L.M.; Teske, G.J.; Ulland, T.K.; Eisenbarth, S.C.; Florquin, S.; Flavell, R.A.; Leemans, J.C.; et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA 2009, 106, 20388–20393. [Google Scholar] [CrossRef] [Green Version]
- Vilaysane, A.; Chun, J.; Seamone, M.E.; Wang, W.; Chin, R.; Hirota, S.; Li, Y.; Clark, S.A.; Tschopp, J.; Trpkov, K.; et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 2010, 21, 1732–1744. [Google Scholar] [CrossRef] [Green Version]
- Segerer, S.; Nelson, P.J.; Schlondorff, D. Chemokines, chemokine receptors, and renal disease: From basic science to pathophysiologic and therapeutic studies. J. Am. Soc. Nephrol. 2000, 11, 152–176. [Google Scholar] [CrossRef]
- Vielhauer, V.; Eis, V.; Schlondorff, D.; Anders, H.J. Identifying chemokines as therapeutic targets in renal disease: Lessons from antagonist studies and knockout mice. Kidney Blood Press. Res. 2004, 27, 226–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrero, J.J.; Yilmaz, M.I.; Lindholm, B.; Stenvinkel, P. Cytokine dysregulation in chronic kidney disease: How can we treat it? Blood Purif. 2008, 26, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.Y.; Ka, S.M.; Chang, J.M.; Chen, H.C.; Shui, H.A.; Li, C.Y.; Hua, K.F.; Chang, W.L.; Huang, J.J.; Yang, S.S.; et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Biol. Med. 2011, 51, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Faustino, V.D.; Arias, S.C.; Ávila, V.F.; Foresto-Neto, O.; Zambom, F.F.; Machado, F.G.; Machado dos Reis, L.; Malheiros, D.M.; Volpini, R.A.; Camara, N.O.; et al. Simultaneous activation of innate and adaptive immunity participates in the development of renal injury in a model of heavy proteinuria. Biosci. Rep. 2018, 38, BSR20180762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianciolo, R.; Brown, C.; Mohr, C.; Nabity, M.; Van der Lugt, J.; McLeland, S.; Aresu, L.; Benali, S.; Spangler, B.; Amerman, H.; et al. Atlas of Renal Lesions in Proteinuric Dogs, V1. The Ohio State University Web Site. 2018. Available online: https://ohiostate.pressbooks.pub/vetrenalpathatlas/ (accessed on 5 February 2020).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Melendez, L.D.; Jewell, D.E. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J. Vet. Intern. Med. 2015, 29, 808–814. [Google Scholar] [CrossRef]
- Anders, H.J.; Vielhauer, V.; Schlöndorff, D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 2003, 63, 401–415. [Google Scholar] [CrossRef] [Green Version]
- Chung, A.C.; Lan, H.Y. Chemokines in renal injury. J. Am. Soc. Nephrol. 2011, 22, 802–809. [Google Scholar] [CrossRef] [Green Version]
- Nomiyama, H.; Hieshima, K.; Nakayama, T.; Sakaguchi, T.; Fujisawa, R.; Tanase, S.; Nishiura, H.; Matsuno, K.; Takamori, H.; Tabira, Y.; et al. Human CC chemokine liver-expressed chemokine/CCL16 is a functional ligand for CCR1, CCR2 and CCR5, and constitutively expressed by hepatocytes. Int. Immunol. 2001, 13, 1021–1029. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, T.; Kato, Y.; Hieshima, K.; Nagakubo, D.; Kunori, Y.; Fujisawa, T.; Yoshie, O. Liver-expressed chemokine/CC chemokine ligand 16 attracts eosinophils by interacting with histamine H4 receptor. J. Immunol. 2004, 173, 2078–2083. [Google Scholar] [CrossRef]
- Tsou, C.L.; Gladue, R.P.; Carroll, L.A.; Paradis, T.; Boyd, J.G.; Nelson, R.T.; Neote, K.; Charo, I.F. Identification of C-C chemokine receptor 1 (CCR1) as the monocyte hemofiltrate C-C chemokine (HCC)-1 receptor. J. Exp. Med. 1998, 188, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Hofstra, C.L.; Desai, P.J.; Thurmond, R.L.; Fung-Leung, W.P. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J. Pharmacol. Exp. Ther. 2003, 305, 1212–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Wu, N.; Xiang, Y.; Wu, L.; Li, C.; Yuan, Z.; Jia, X.; Zhang, Z.; Zhong, L.; Li, Y. Prognostic value of chemokines in patients with newly diagnosed atrial fibrillation. Int. J. Cardiol. 2020, 320, 83–89. [Google Scholar] [CrossRef]
- Hayatbakhsh, M.M.; Gowhari Shabgah, A.; Pishgouyi, S.; Tavakol Afshari, J.; Zeidabadi, H.; Mohammadi, M. The Serum Levels of CCL2 and CCL16 Expression in Patients with Irritable Bowel Syndrome. Rep. Biochem. Mol. Biol. 2019, 8, 9–14. [Google Scholar]
- Sayyed, S.G.; Ryu, M.; Kulkarni, O.P.; Schmid, H.; Lichtnekert, J.; Grüner, S.; Green, L.; Mattei, P.; Hartmann, G.; Anders, H.J. An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int. 2011, 80, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninichuk, V.; Anders, H.J. Chemokine receptor CCR1: A new target for progressive kidney disease. Am. J. Nephrol. 2005, 25, 365–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, J.Y.; Lu, D.; Lin, Z.Y.; Cen, B.N.; Wei, X.Y.; Xie, H.Y.; Zheng, S.S.; Xu, X. CC motif chemokine ligand 16 inhibits the progression of liver cirrhosis via inactivating hepatic stellate cells. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 440–448. [Google Scholar] [CrossRef]
- Brüünsgaard, H.; Pedersen, B.K. Age-related inflammatory cytokines and disease. Immunol. Allergy Clin. N. Am. 2003, 23, 15–39. [Google Scholar] [CrossRef]
- Persson, T.; Monsef, N.; Andersson, P.; Bjartell, A.; Malm, J.; Calafat, J.; Egesten, A. Expression of the neutrophil-activating CXC chemokine ENA-78/CXCL5 by human eosinophils. Clin. Exp. Allergy 2003, 33, 531–537. [Google Scholar] [CrossRef]
- Mestas, J.; Burdick, M.D.; Reckamp, K.; Pantuck, A.; Figlin, R.A.; Strieter, R.M. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J. Immunol. 2005, 175, 5351–5357. [Google Scholar] [CrossRef] [Green Version]
- Higurashi, M.; Ohya, Y.; Joh, K.; Muraguchi, M.; Nishimura, M.; Terawaki, H.; Yagui, K.; Hashimoto, N.; Saito, Y.; Yamada, K. Increased urinary levels of CXCL5, CXCL8 and CXCL9 in patients with Type 2 diabetic nephropathy. J. Diabetes Complicat. 2009, 23, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Krupickova, L.; Fialova, M.; Novotny, M.; Svachova, V.; Mezerova, K.; Cecrdlova, E.; Viklicky, O.; Striz, I. Chemokine Profiles Are Affected in Serum of Patients with Acute Rejection of Kidney Allograft. Mediat. Inflamm. 2021, 2021, 5513690. [Google Scholar] [CrossRef]
- de Oliveira, J.G.; Xavier, P.D.; Sampaio, S.M.; Tavares, I.S.; Mendes, A.A. The synthesis by fine-needle aspiration biopsy cultures of IL-7, IL-16 and IL-18 is significantly associated with acute rejection in kidney transplants. Nephron 2002, 92, 622–628. [Google Scholar] [CrossRef]
- Wang, S.; Diao, H.; Guan, Q.; Cruikshank, W.W.; Delovitch, T.L.; Jevnikar, A.M.; Du, C. Decreased renal ischemia-reperfusion injury by IL-16 inactivation. Kidney Int. 2008, 73, 318–326. [Google Scholar] [CrossRef] [Green Version]
- An, X.; Liao, G.; Chen, Y.; Luo, A.; Liu, J.; Yuan, Y.; Li, L.; Yang, L.; Wang, H.; Liu, F.; et al. Intervention for early diabetic nephropathy by mesenchymal stem cells in a preclinical nonhuman primate model. Stem Cell Res. Ther. 2019, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Chen, M. Complement Activation in Progression of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 423–441. [Google Scholar] [PubMed]
- Willows, J.; Brown, M.; Sheerin, N.S. The role of complement in kidney disease. Clin. Med. 2020, 20, 156–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Wu, K.Y.; Ma, N.; Wei, L.L.; Garstka, M.; Zhou, W.; Li, K. The C5a/C5aR2 axis promotes renal inflammation and tissue damage. JCI Insight 2020, 5, e134081. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.L.; Gullipalli, D.; Ueda, Y.; Sato, S.; Zhou, L.; Miwa, T.; Tung, K.S.; Song, W.C. C5 inhibition prevents renal failure in a mouse model of lethal C3 glomerulopathy. Kidney Int. 2017, 91, 1386–1397. [Google Scholar] [CrossRef]
- Portilla, D.; Xavier, S. Role of intracellular complement activation in kidney fibrosis. Br. J. Pharmacol. 2021, 178, 2880–2891. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Nandakumar, K.S.; Tedesco, F. Inhibiting the C5-C5a receptor axis. Mol. Immunol. 2011, 48, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Cofiell, R.; Kukreja, A.; Bedard, K.; Yan, Y.; Mickle, A.P.; Ogawa, M.; Bedrosian, C.L.; Faas, S.J. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood 2015, 125, 3253–3262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nentwig, A.; Schweighauser, A.; Maissen-Villiger, C.; Bruckmaier, R.M.; Zurbriggen, A.; van Dorland, H.A.; Francey, T. Assessment of the expression of biomarkers of uremic inflammation in dogs with renal disease. Am. J. Vet. Res. 2016, 77, 218–224. [Google Scholar] [CrossRef]
- White, J.R.; Imburgia, C.; Dul, E.; Appelbaum, E.; O’Donnell, K.; O’Shannessy, D.J.; Brawner, M.; Fornwald, J.; Adamou, J.; Elshourbagy, N.A.; et al. Cloning and functional characterization of a novel human CC chemokine that binds to the CCR3 receptor and activates human eosinophils. J. Leukoc. Biol. 1997, 62, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Geng, M.; Ding, J.; Lv, K.; Du, H.; Ding, J.; Pei, W.; Hu, X.; Gu, J.; et al. CCL24 Protects Renal Function by Controlling Inflammation in Podocytes. Dis. Markers. 2021, 2021, 8837825. [Google Scholar] [CrossRef]
- Hsieh, P.F.; Liu, S.F.; Lee, T.C.; Huang, J.S.; Yin, L.T.; Chang, W.T.; Chuang, L.Y.; Guh, J.Y.; Hung, M.Y.; Yang, Y.L. The role of IL-7 in renal proximal tubule epithelial cells fibrosis. Mol. Immunol. 2012, 50, 74–82. [Google Scholar] [CrossRef]
- Romanova, Y.; Laikov, A.; Markelova, M.; Khadiullina, R.; Makseev, A.; Hasanova, M.; Rizvanov, A.; Khaiboullina, S.; Salafutdinov, I. Proteomic analysis of human serum from patients with chronic kidney disease. Biomolecules 2020, 10, 257. [Google Scholar] [CrossRef] [Green Version]
- Mai, H.L.; Nguyen, T.V.H.; Branchereau, J.; Poirier, N.; Renaudin, K.; Mary, C.; Belarif, L.; Minault, D.; Hervouet, J.; Le Bas-Berdardet, S.; et al. Interleukin-7 receptor blockade by an anti-CD127 monoclonal antibody in nonhuman primate kidney transplantation. Am. J. Transplant. 2020, 20, 101–111. [Google Scholar] [CrossRef]
- Meghraoui-Kheddar, A.; Barthelemy, S.; Boissonnas, A.; Combadière, C. Revising CX3CR1 Expression on Murine Classical and Non-classical Monocytes. Front. Immunol. 2020, 11, 1117. [Google Scholar] [CrossRef]
- Hamdan, D.; Robinson, L.A. Role of the CX3CL1-CX3CR1 axis in renal disease. Am. J. Physiol. Renal Physiol. 2021, 321, F121–F134. [Google Scholar] [CrossRef]
- Sheridan, G.K.; Wdowicz, A.; Pickering, M.; Watters, O.; Halley, P.; O’Sullivan, N.C.; Mooney, C.; O’Connell, D.J.; O’Connor, J.J.; Murphy, K.J. CX3CL1 is up-regulated in the rat hippocampus during memory-associated synaptic plasticity. Front. Cell. Neurosci. 2014, 12, 233. [Google Scholar] [CrossRef]
- Engel, D.R.; Krause, T.A.; Snelgrove, S.L.; Thiebes, S.; Hickey, M.J.; Boor, P.; Kitching, A.R.; Kurts, C. CX3CR1 reduces kidney fibrosis by inhibiting local proliferation of profibrotic macrophages. J. Immunol. 2015, 194, 1628–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, G.J.; Hewitson, T.D. Animal models of chronic kidney disease: Useful but not perfect. Nephrol. Dial. Transplant. 2013, 28, 2432–24388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouchelon, J.L.; Atkins, C.E.; Bussadori, C.; Oyama, M.A.; Vaden, S.L.; Bonagura, J.D.; Chetboul, V.; Cowgill, L.D.; Elliot, J.; Francey, T.; et al. Cardiovascular-renal axis disorders in the domestic dog and cat: A veterinary consensus statement. J. Small Anim. Pract. 2015, 56, 537–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]

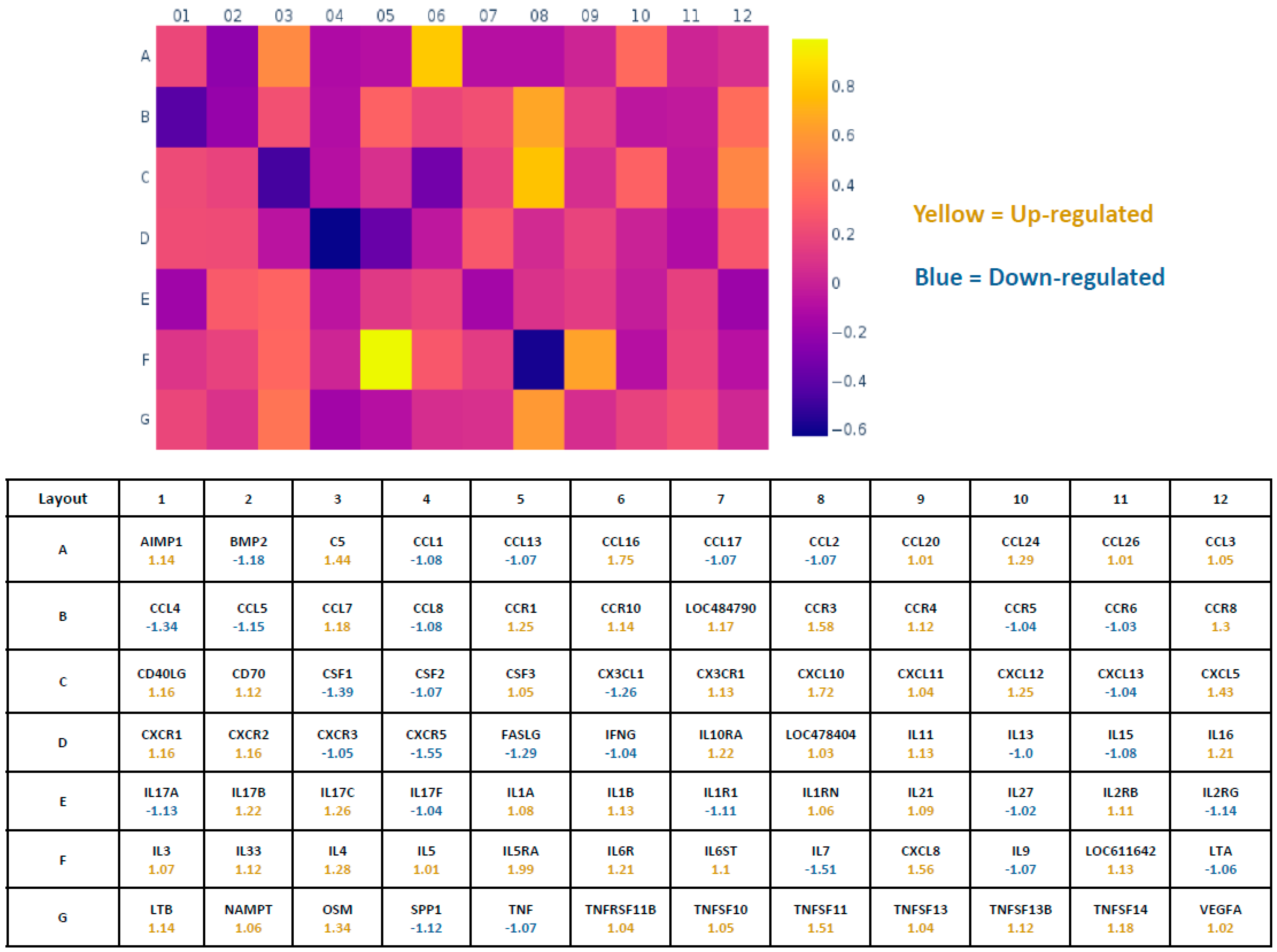
| Cytokine 1 | Calculated Amount at 10 Years of Age 2 | Slope (Increase in Amount Per Year) | Standard Error | p-Value |
|---|---|---|---|---|
| CCL4 | 0.0236 | 0.0631 | 0.0221 | 0.01 |
| CCL5 | 0.1241 | 0.1092 | 0.0426 | 0.02 |
| CD70 | 0.0258 | 0.0074 | 0.0033 | 0.04 |
| IL17B | 0.0024 | 0.0005 | 0.0002 | 0.04 |
| IL2RB | 0.3335 | 0.1164 | 0.0442 | 0.02 |
| FASLG | 0.0271 | 0.0255 | 0.0081 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavener, S.K.; Jewell, D.E.; Panickar, K.S. The Increase in Circulating Levels of Pro-Inflammatory Chemokines, Cytokines, and Complement C5 in Canines with Impaired Kidney Function. Curr. Issues Mol. Biol. 2022, 44, 1664-1676. https://doi.org/10.3390/cimb44040114
Tavener SK, Jewell DE, Panickar KS. The Increase in Circulating Levels of Pro-Inflammatory Chemokines, Cytokines, and Complement C5 in Canines with Impaired Kidney Function. Current Issues in Molecular Biology. 2022; 44(4):1664-1676. https://doi.org/10.3390/cimb44040114
Chicago/Turabian StyleTavener, Selena K., Dennis E. Jewell, and Kiran S. Panickar. 2022. "The Increase in Circulating Levels of Pro-Inflammatory Chemokines, Cytokines, and Complement C5 in Canines with Impaired Kidney Function" Current Issues in Molecular Biology 44, no. 4: 1664-1676. https://doi.org/10.3390/cimb44040114
APA StyleTavener, S. K., Jewell, D. E., & Panickar, K. S. (2022). The Increase in Circulating Levels of Pro-Inflammatory Chemokines, Cytokines, and Complement C5 in Canines with Impaired Kidney Function. Current Issues in Molecular Biology, 44(4), 1664-1676. https://doi.org/10.3390/cimb44040114






