Laminin 511-E8 Fragment Offers Superior Adhesion Properties for Gastric Cancer Cells Compared with Full-Length Laminin 511
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
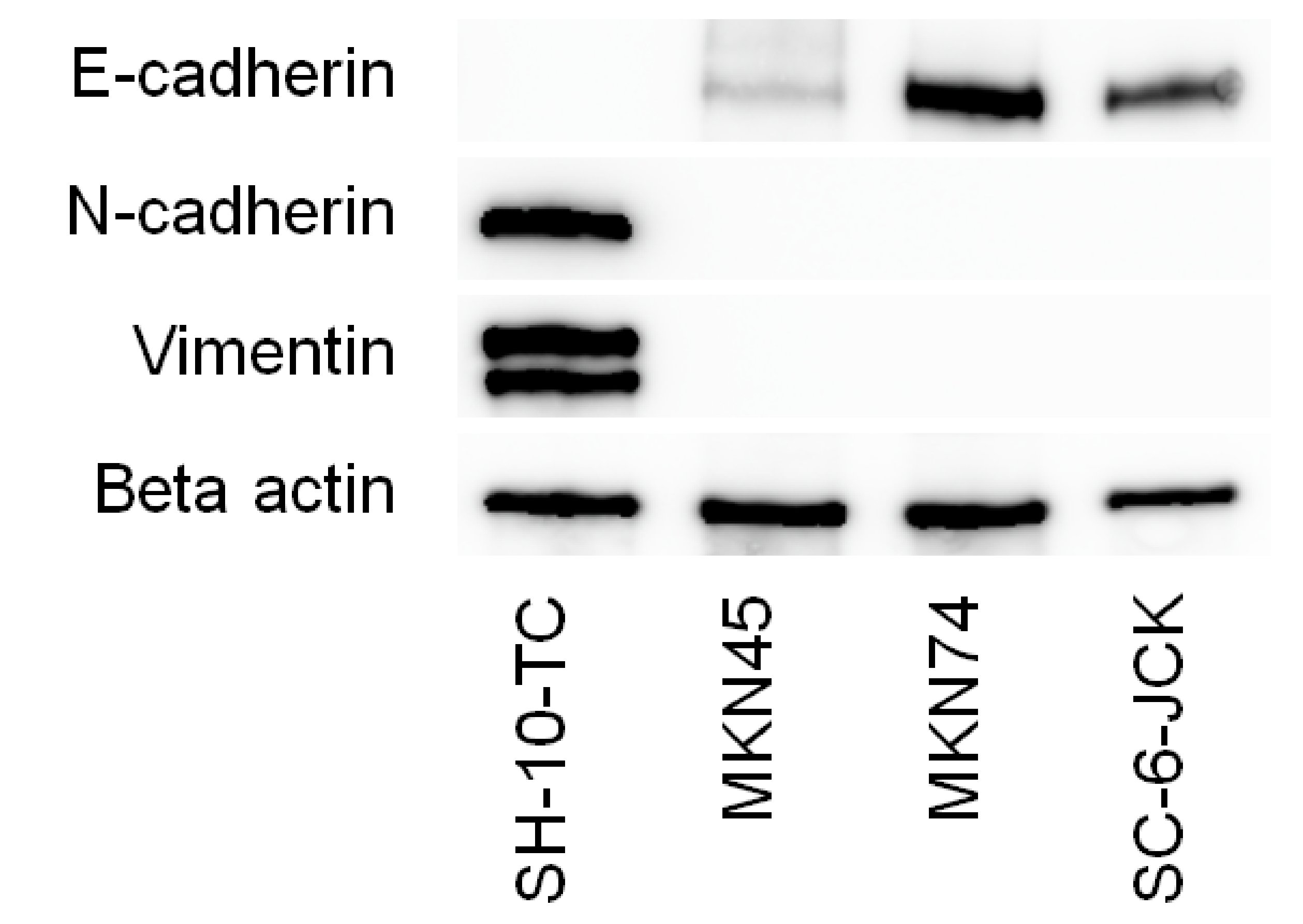
2.2. Western Blot Analysis
2.3. Cellular Proliferation Assays
2.4. Immunostaining
2.5. Calculation of Adhesive Cells
2.6. Quantitative mRNA Expression Analysis
2.7. Blocking Study
2.8. Statistical Analysis
3. Results
3.1. Mesenchymal/Epithelial Phenotypes of the Gastric Cancer Cell Lines Tested
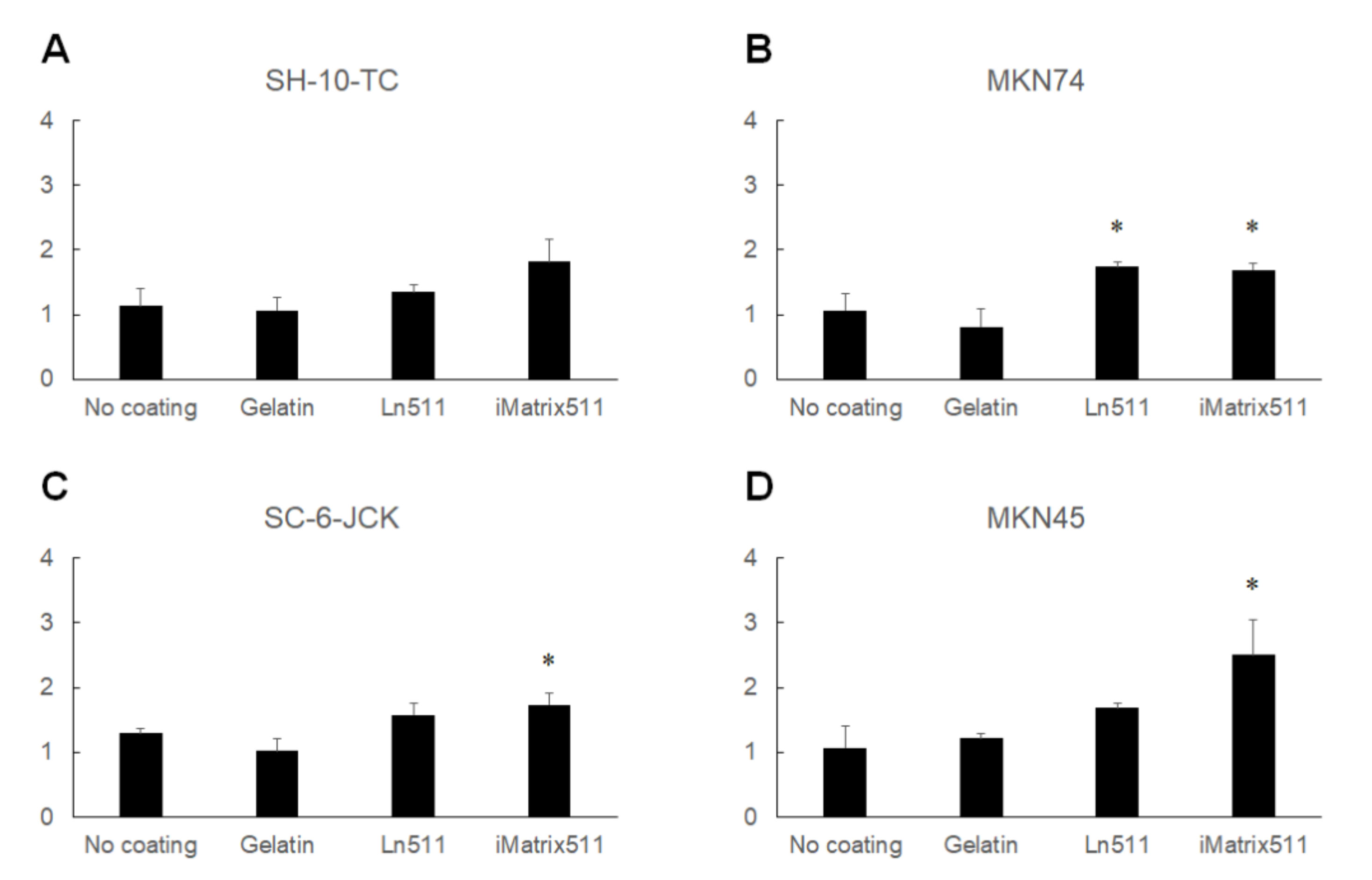
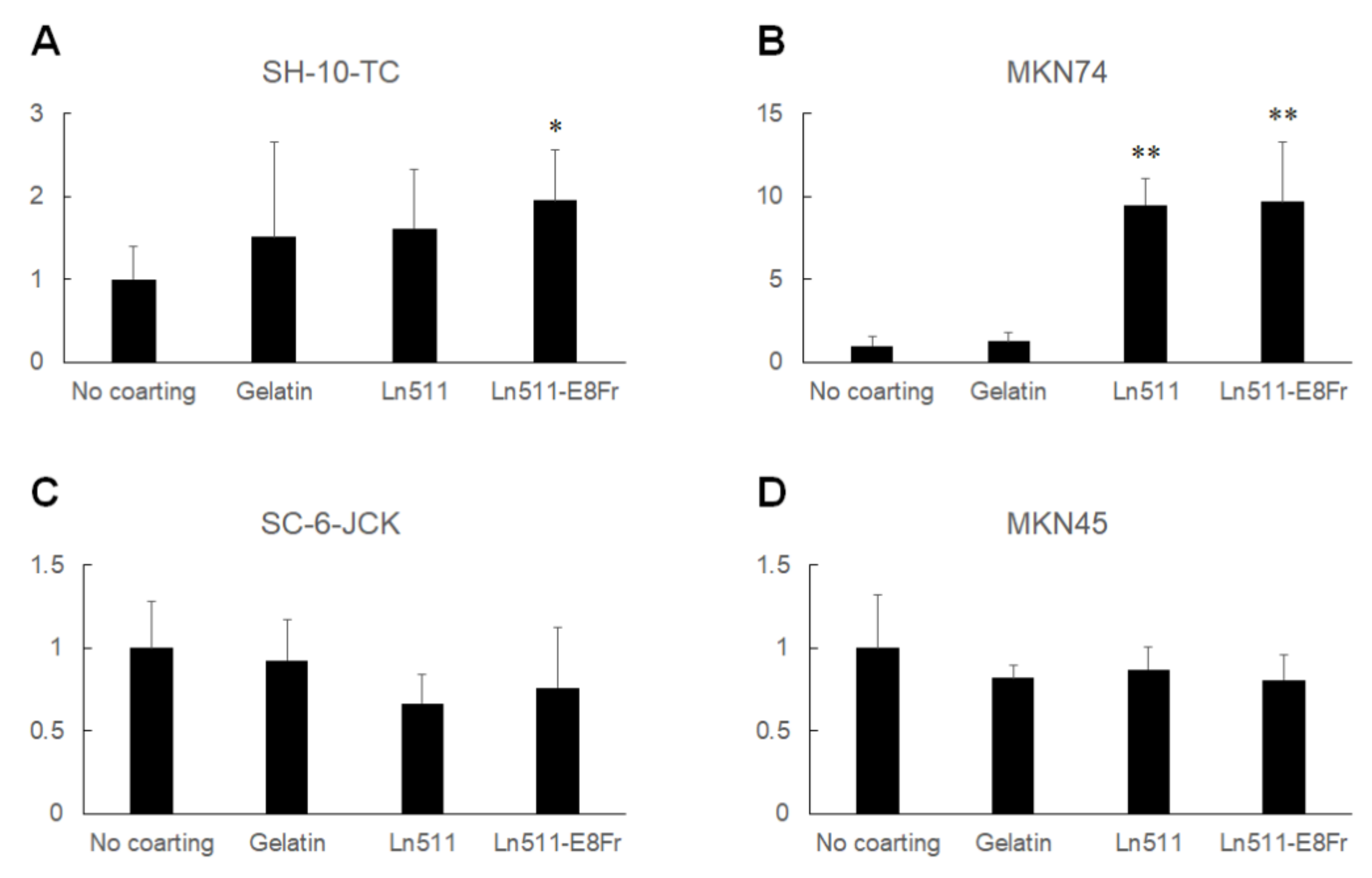
3.2. Ln511-E8 Fragment Promotes Gastric Cancer Cell Proliferation
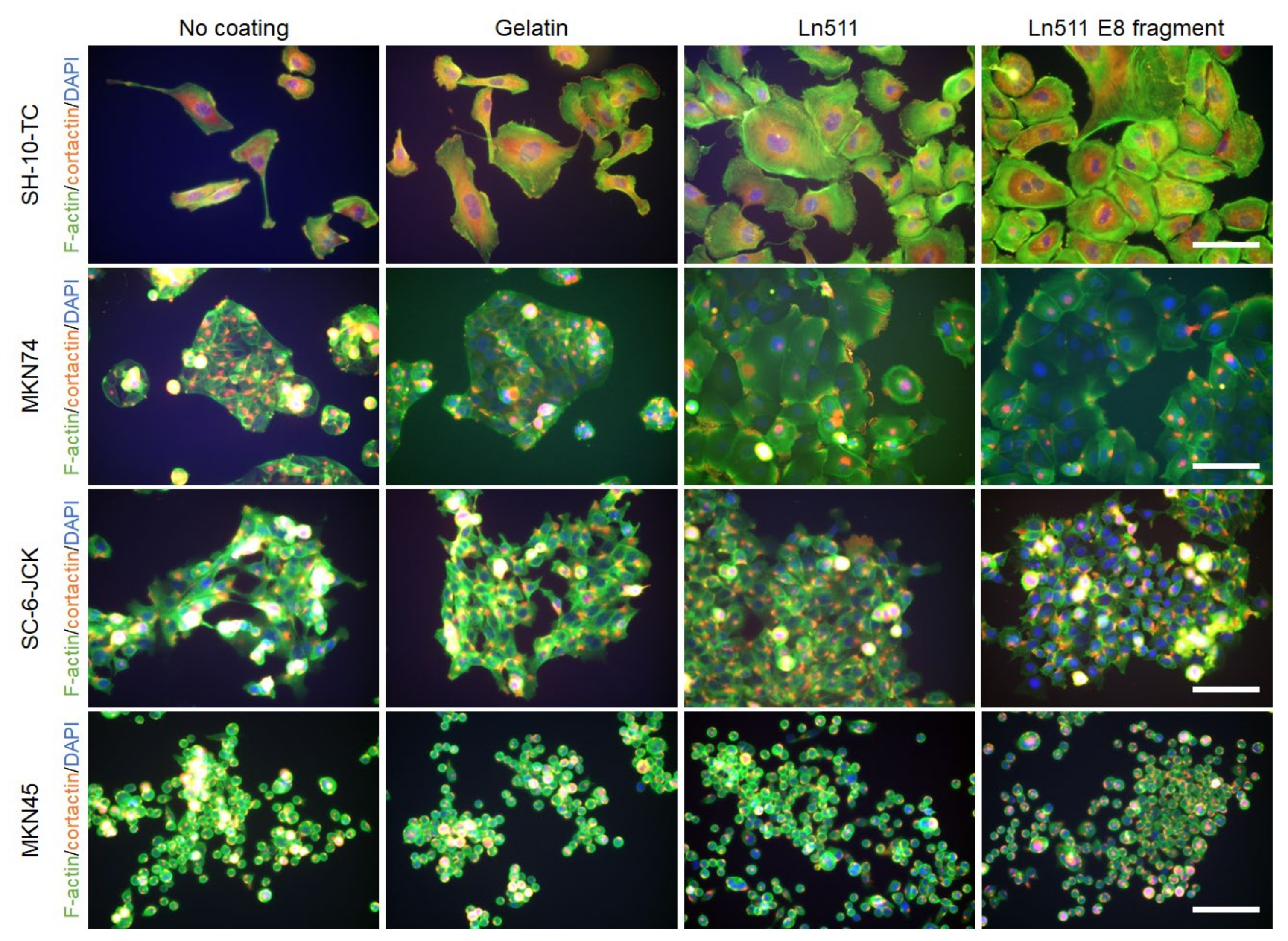
3.3. Ln511 and Ln511-E8 Fragments Modulate the Morphology of Some Gastric Cancer Cells
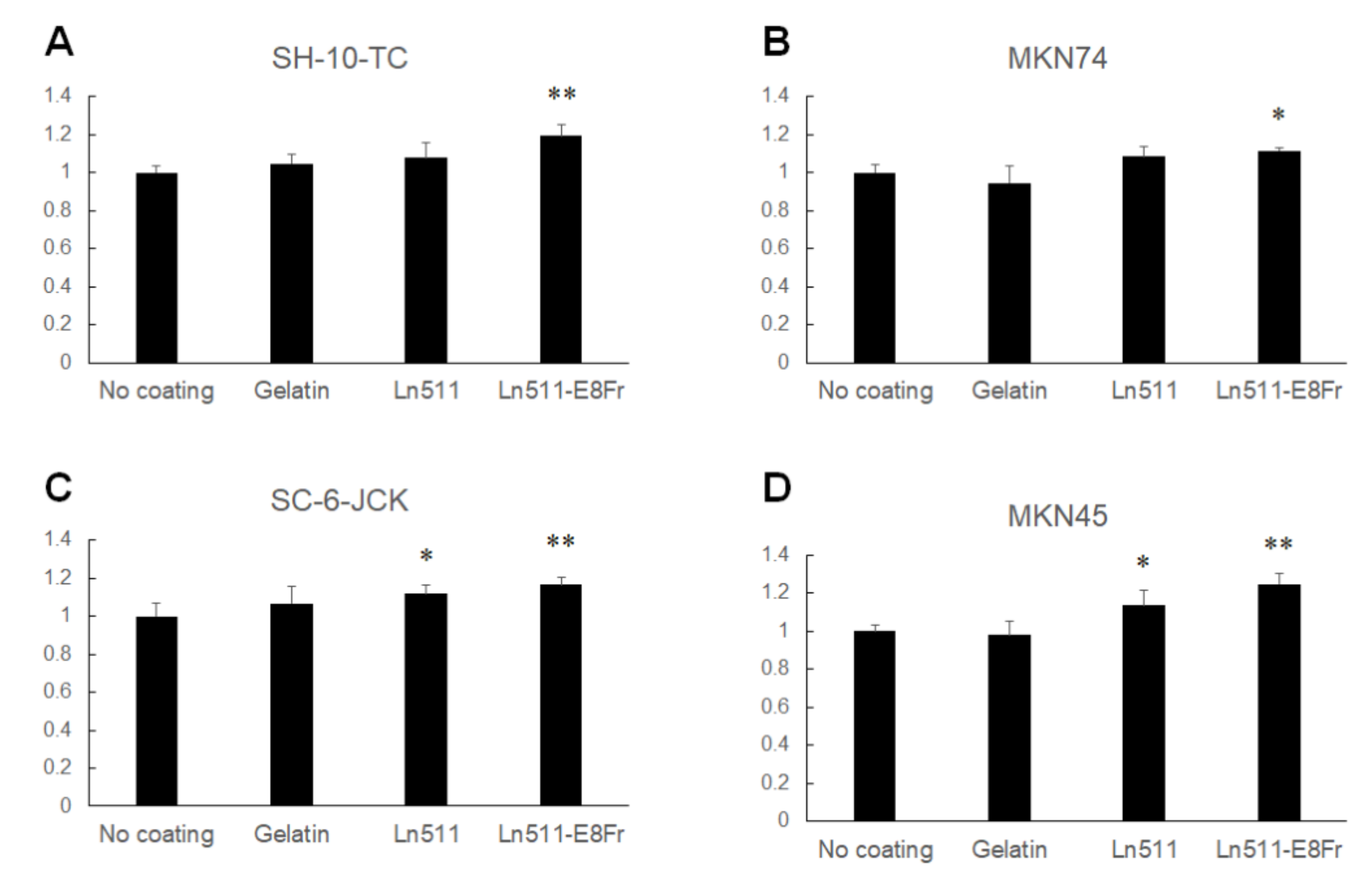
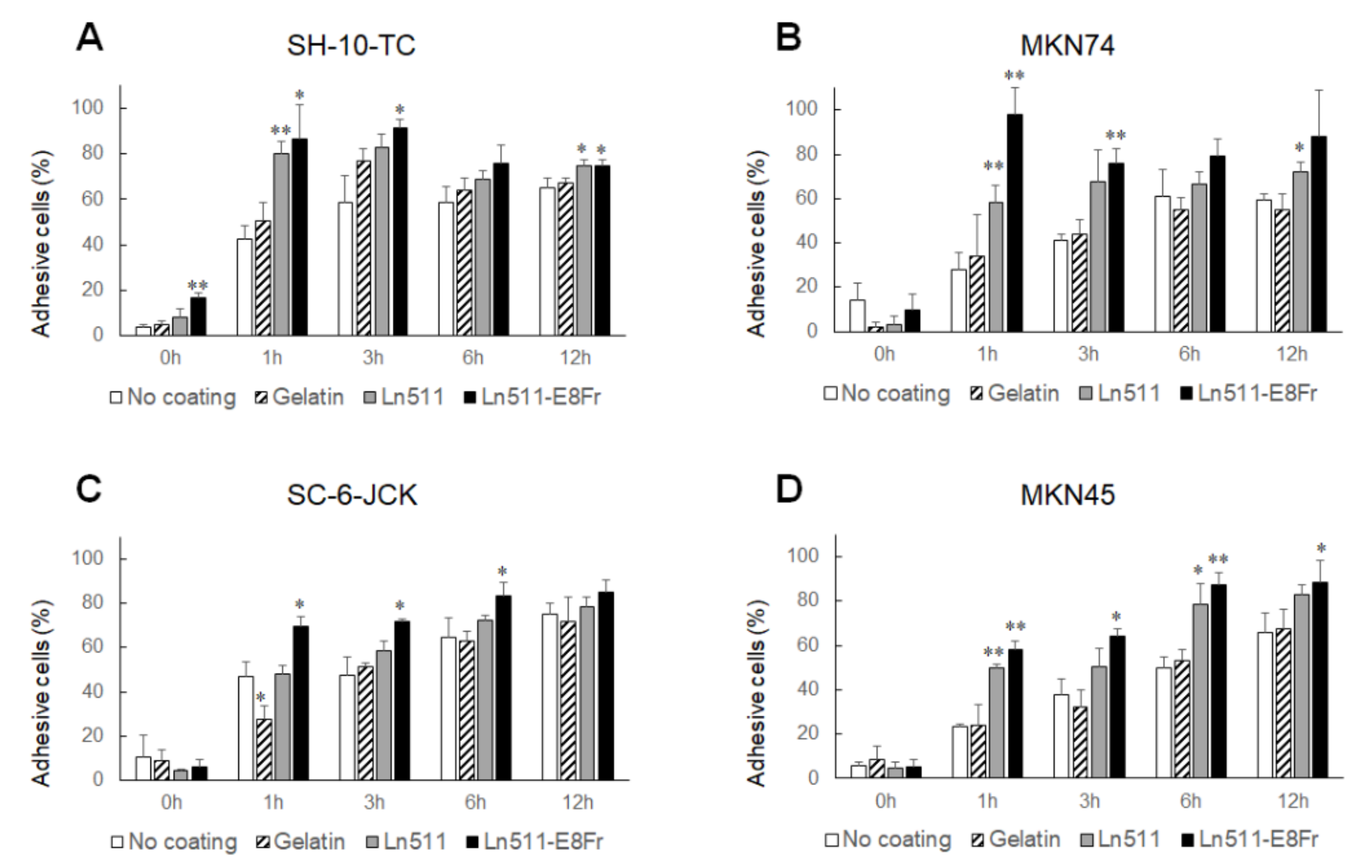
3.4. Ln511-E8 Fragment Augments the Adhesion of Gastric Cancer Cells
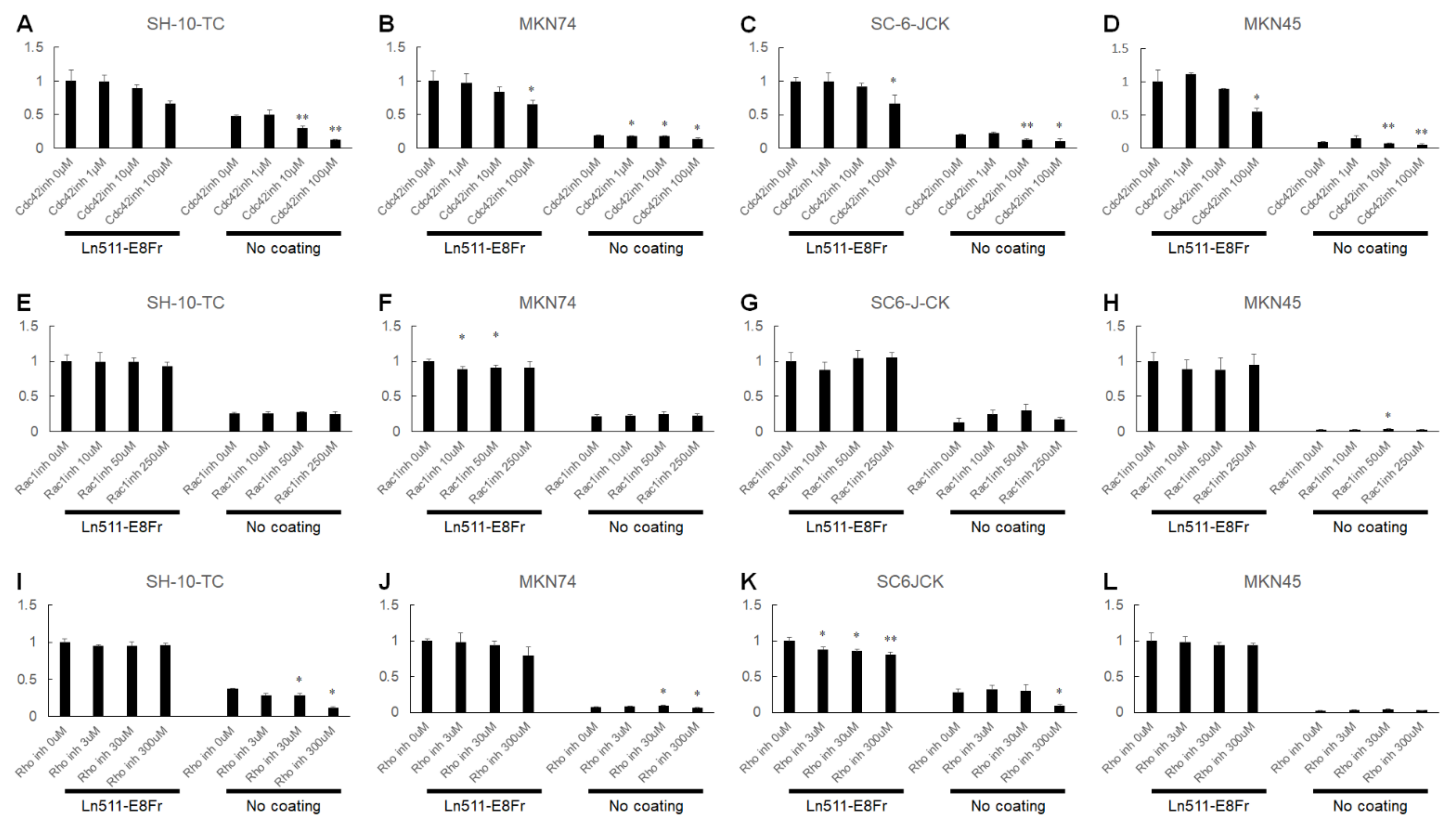
3.5. Possible Involvement of Cdc42 in the Ln511-E8 Fragment-Induced Enhanced Adhesion of Gastric Cancer Cells
4. Discussion
- i.
- The Ln511-E8 fragment promotes the proliferation of SH-10-TC, MKN74, SC-6-JCK, and MKN45 cells;
- ii.
- The cytoplasmic areas of gastric cancer cells cultured on the Ln511-E8 fragment were significantly larger than those without the coating material in SH-10-TC and MKN74 cells;
- iii.
- SH-10-TC, MKN74, SC-6-JCK, and MKN45 cells promptly adhered to Ln511-E8 fragment-coated plates.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bond, K.H.; Chiba, T.; Wynne, K.P.H.; Vary, C.P.H.; Sims-Lucas, S.; Coburn, J.M.; Oxburgh, L. The Extracellular Matrix Environment of Clear Cell Renal Cell Carcinoma Determines Cancer Associated Fibroblast Growth. Cancers 2021, 13, 5873. [Google Scholar] [CrossRef] [PubMed]
- Camargo, S.; Gofrit, O.N.; Assis, A.; Mitrani, E. Paracrine Signaling from a Three-Dimensional Model of Bladder Carcinoma and from Normal Bladder Switch the Phenotype of Stromal Fibroblasts. Cancers 2021, 13, 2972. [Google Scholar] [CrossRef] [PubMed]
- Meireles Da Costa, N.; Mendes, F.A.; Pontes, B.; Nasciutti, L.E.; Ribeiro Pinto, L.F.; Palumbo Júnior, A. Potential Therapeutic Significance of Laminin in Head and Neck Squamous Carcinomas. Cancers 2021, 13, 1890. [Google Scholar] [CrossRef] [PubMed]
- Ndoye, A.; Miskin, R.P.; Di Persio, C.M. Integrin α3β1 Represses Reelin Expression in Breast Cancer Cells to Promote Invasion. Cancers 2021, 13, 344. [Google Scholar] [CrossRef]
- Iwamuro, M.; Shiraha, H.; Oyama, A.; Uchida, D.; Horiguchi, S.; Okada, H. Laminin-411 and -511 Modulate the Proliferation, Adhesion, and Morphology of Gastric Cancer Cells. Cell Biochem. Biophys. 2021, 79, 407–418. [Google Scholar] [CrossRef]
- Takagi, R.; Tanuma-Takahashi, A.; Akiyama, S.; Kaneko, W.; Miura, C.; Yamato, M.; Shimizu, T.; Umezawa, A. Laminin-511-derived recombinant fragment and Rho kinase inhibitor Y-27632 facilitate serial cultivation of keratinocytes differentiated from human embryonic stem cells. Regen. Ther. 2021, 18, 242–252. [Google Scholar] [CrossRef]
- Yuzuriha, A.; Nakamura, S.; Sugimoto, N.; Kihara, S.; Nakagawa, M.; Yamamoto, T.; Sekiguchi, K.; Eto, K. Extracellular laminin regulates hematopoietic potential of pluripotent stem cells through integrin β1-ILK-β-catenin-JUN axis. Stem Cell Res. 2021, 53, 102287. [Google Scholar] [CrossRef]
- Sugawara, Y.; Hamada, K.; Yamada, Y.; Kumai, J.; Kanagawa, M.; Kobayashi, K.; Toda, T.; Negishi, Y.; Katagiri, F.; Hozumi, K.; et al. Characterization of dystroglycan binding in adhesion of human induced pluripotent stem cells to laminin-511 E8 fragment. Sci. Rep. 2019, 9, 13037. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kawase, E. Efficient and scalable culture of single dissociated human pluripotent stem cells using recombinant E8 fragments of human laminin isoforms. Curr. Protoc. Stem Cell Biol. 2015, 32, 1C.18.1–1C.18.8. [Google Scholar] [CrossRef]
- Miyazaki, T.; Futaki, S.; Suemori, H.; Taniguchi, Y.; Yamada, M.; Kawasaki, M.; Hayashi, M.; Kumagai, H.; Nakatsuji, N.; Sekiguchi, K.; et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 2012, 3, 1236. [Google Scholar] [CrossRef] [Green Version]
- Mydel, P.; Shipley, J.M.; Adair-Kirk, T.L.; Kelley, D.G.; Broekelmann, T.J.; Mecham, R.P.; Senior, R.M. Neutrophil elastase cleaves laminin-332 (laminin-5) generating peptides that are chemotactic for neutrophils. J. Biol. Chem. 2008, 283, 9513–9522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurchenco, P.D.; Cheng, Y.S. Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J. Biol. Chem. 1993, 268, 17286–17299. [Google Scholar] [CrossRef]
- Ido, H.; Harada, K.; Futaki, S.; Hayashi, Y.; Nishiuchi, R.; Natsuka, Y.; Li, S.; Wada, Y.; Combs, A.C.; Ervasti, J.M.; et al. Molecular dissection of the alpha-dystroglycan- and integrin-binding sites within the globular domain of human laminin-10. J. Biol. Chem. 2004, 279, 10946–10954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutzmann, R.; Huber, J.; Schmetz, K.A.; Oberbäumer, I.; Hartl, L. Structural study of long arm fragments of laminin. Evidence for repetitive C-terminal sequences in the A-chain, not present in the B-chains. Eur. J. Biochem. 1988, 177, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Vuoristo, S.; Toivonen, S.; Weltner, J.; Mikkola, M.; Ustinov, J.; Trokovic, R.; Palgi, J.; Lund, R.; Tuuri, T.; Otonkoski, T. A novel feeder-free culture system for human pluripotent stem cell culture and induced pluripotent stem cell derivation. PLoS ONE 2013, 8, e76205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiering, D.; Hodgson, L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adhes. Migr. 2011, 5, 170–180. [Google Scholar] [CrossRef] [Green Version]
- Bonfim-Melo, A.; Ferreira, É.R.; Mortara, R.A. Rac1/WAVE2 and Cdc42/N-WASP Participation in Actin-Dependent Host Cell Invasion by Extracellular Amastigotes of Trypanosoma cruzi. Front. Microbiol. 2018, 9, 360. [Google Scholar] [CrossRef]
- Edwards, D.C.; Sanders, L.C.; Bokoch, G.M.; Gill, G.N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1999, 1, 253–259. [Google Scholar] [CrossRef]
- Arjonen, A.; Kaukonen, R.; Ivaska, J. Filopodia and adhesion in cancer cell motility. Cell Adhes. Migr. 2011, 5, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.K.; Cho, S.J.; Yoon, C.; Lee, J.H.; Park, D.J.; Yoon, S.S. Increased RhoA Activity Predicts Worse Overall Survival in Patients Undergoing Surgical Resection for Lauren Diffuse-Type Gastric Adenocarcinoma. Ann. Surg. Oncol. 2016, 23, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- Shibata, S.; Hayashi, R.; Okubo, T.; Kudo, Y.; Katayama, T.; Ishikawa, Y.; Toga, J.; Yagi, E.; Honma, Y.; Quantock, A.J.; et al. Selective Laminin-Directed Differentiation of Human Induced Pluripotent Stem Cells into Distinct Ocular Lineages. Cell Rep. 2018, 25, 1668–1679.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamuro, M.; Shiraha, H.; Kobashi, M.; Horiguchi, S.; Okada, H. Laminin 511-E8 Fragment Offers Superior Adhesion Properties for Gastric Cancer Cells Compared with Full-Length Laminin 511. Curr. Issues Mol. Biol. 2022, 44, 1539-1551. https://doi.org/10.3390/cimb44040105
Iwamuro M, Shiraha H, Kobashi M, Horiguchi S, Okada H. Laminin 511-E8 Fragment Offers Superior Adhesion Properties for Gastric Cancer Cells Compared with Full-Length Laminin 511. Current Issues in Molecular Biology. 2022; 44(4):1539-1551. https://doi.org/10.3390/cimb44040105
Chicago/Turabian StyleIwamuro, Masaya, Hidenori Shiraha, Mayu Kobashi, Shigeru Horiguchi, and Hiroyuki Okada. 2022. "Laminin 511-E8 Fragment Offers Superior Adhesion Properties for Gastric Cancer Cells Compared with Full-Length Laminin 511" Current Issues in Molecular Biology 44, no. 4: 1539-1551. https://doi.org/10.3390/cimb44040105
APA StyleIwamuro, M., Shiraha, H., Kobashi, M., Horiguchi, S., & Okada, H. (2022). Laminin 511-E8 Fragment Offers Superior Adhesion Properties for Gastric Cancer Cells Compared with Full-Length Laminin 511. Current Issues in Molecular Biology, 44(4), 1539-1551. https://doi.org/10.3390/cimb44040105





