Chrysophanol Suppresses Cell Growth via mTOR/PPAR-α Regulation and ROS Accumulation in Cultured Human Tongue Squamous Carcinoma SAS Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Western Blotting
2.5. Measuring Intracellular ROS Generation
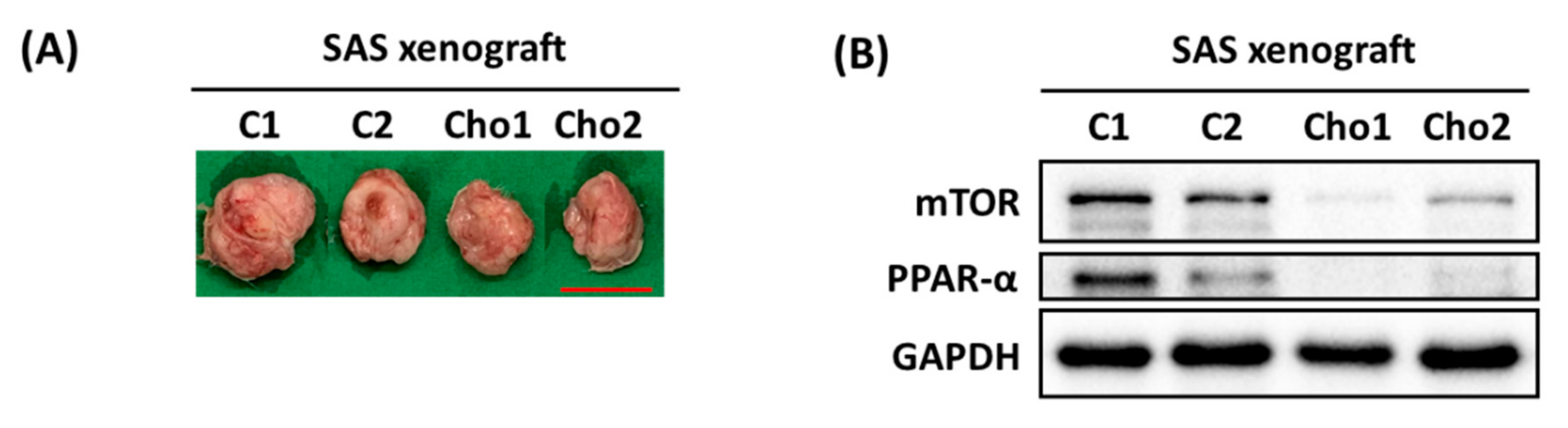
2.6. In Vivo Mice Xenografts
2.7. Statistical Analysis
3. Results
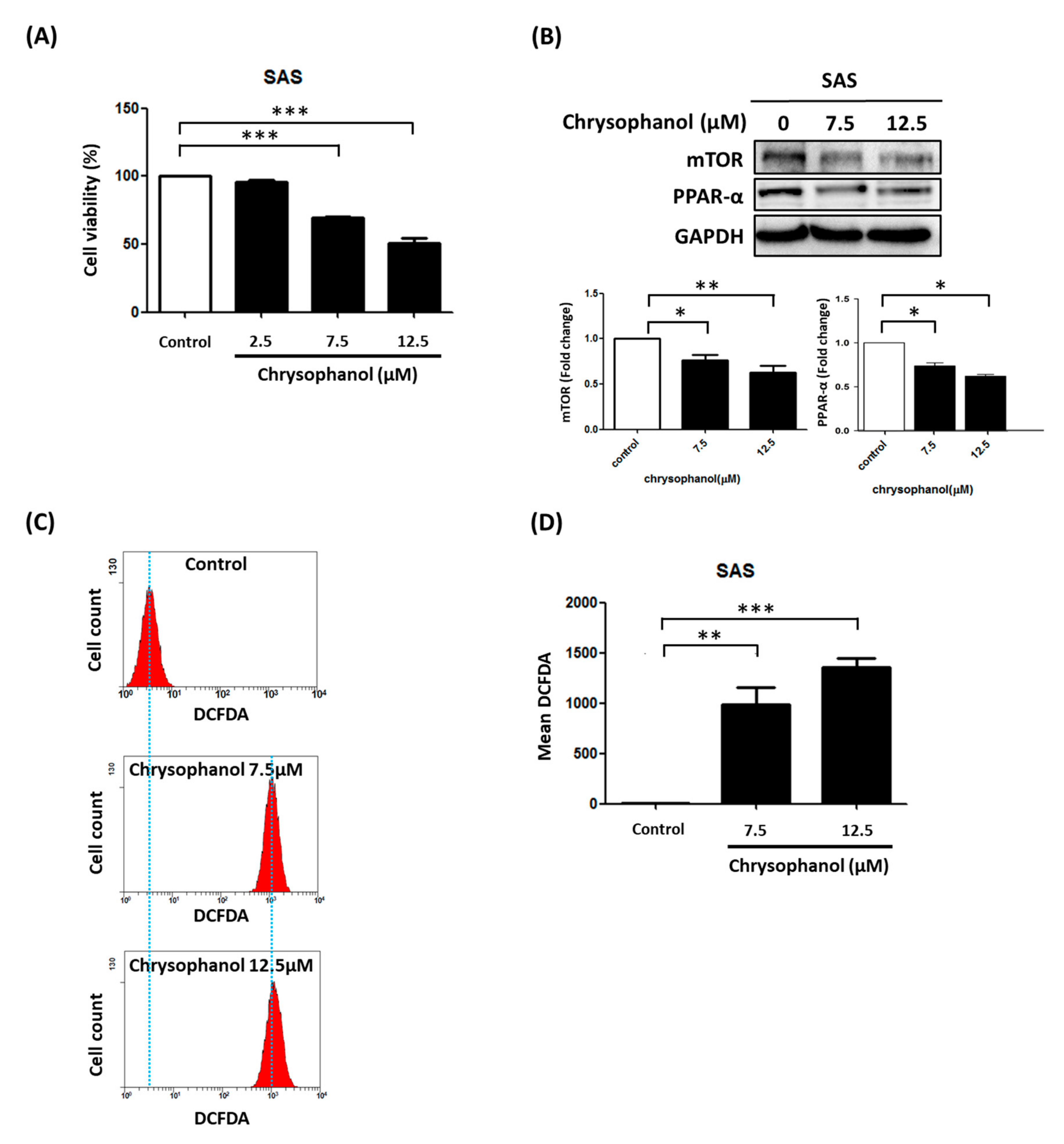
3.1. Chrysophanol Causes Cell Death, Downregulates mTOR/PPAR-α, and Increases ROS Accumulation
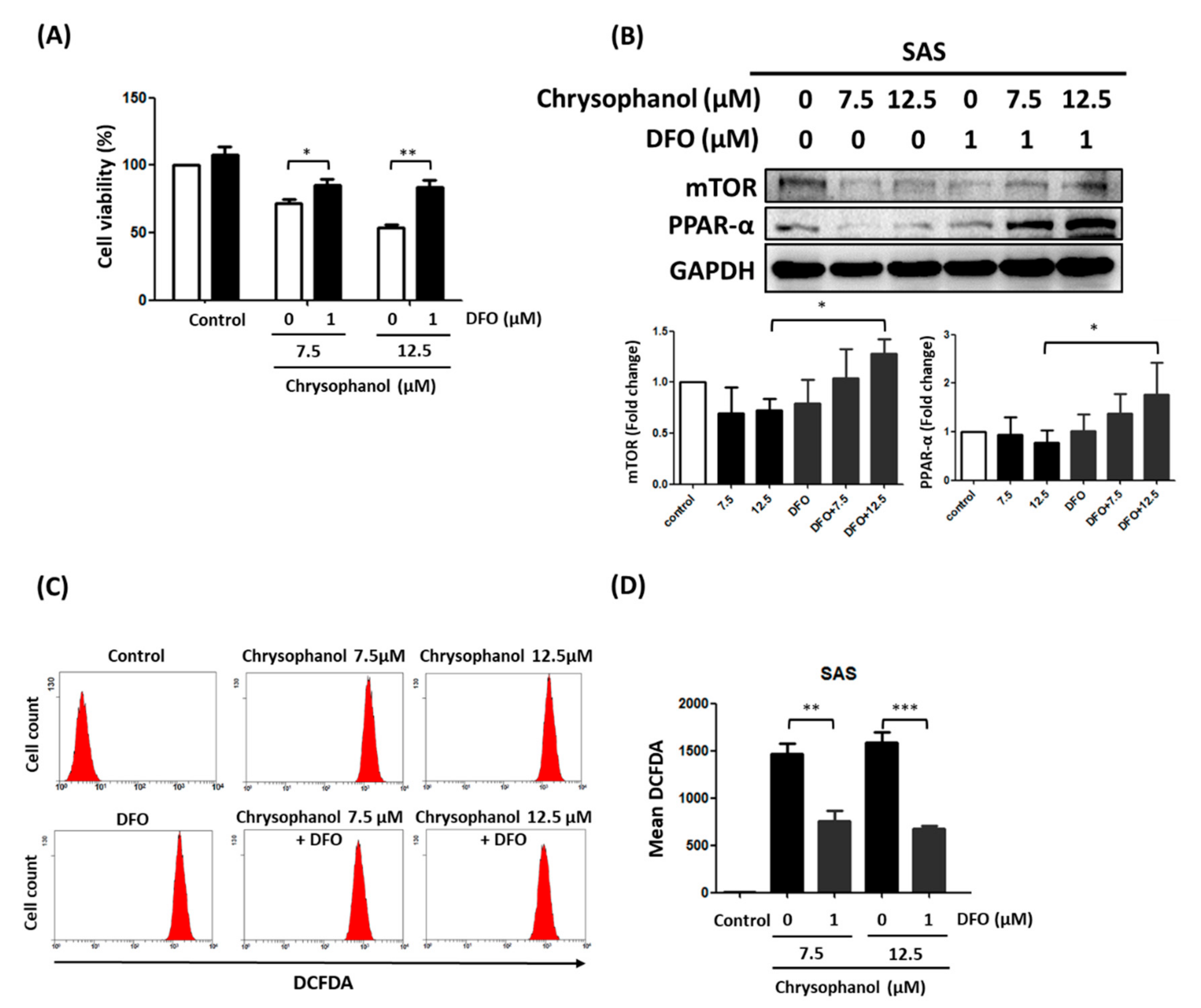
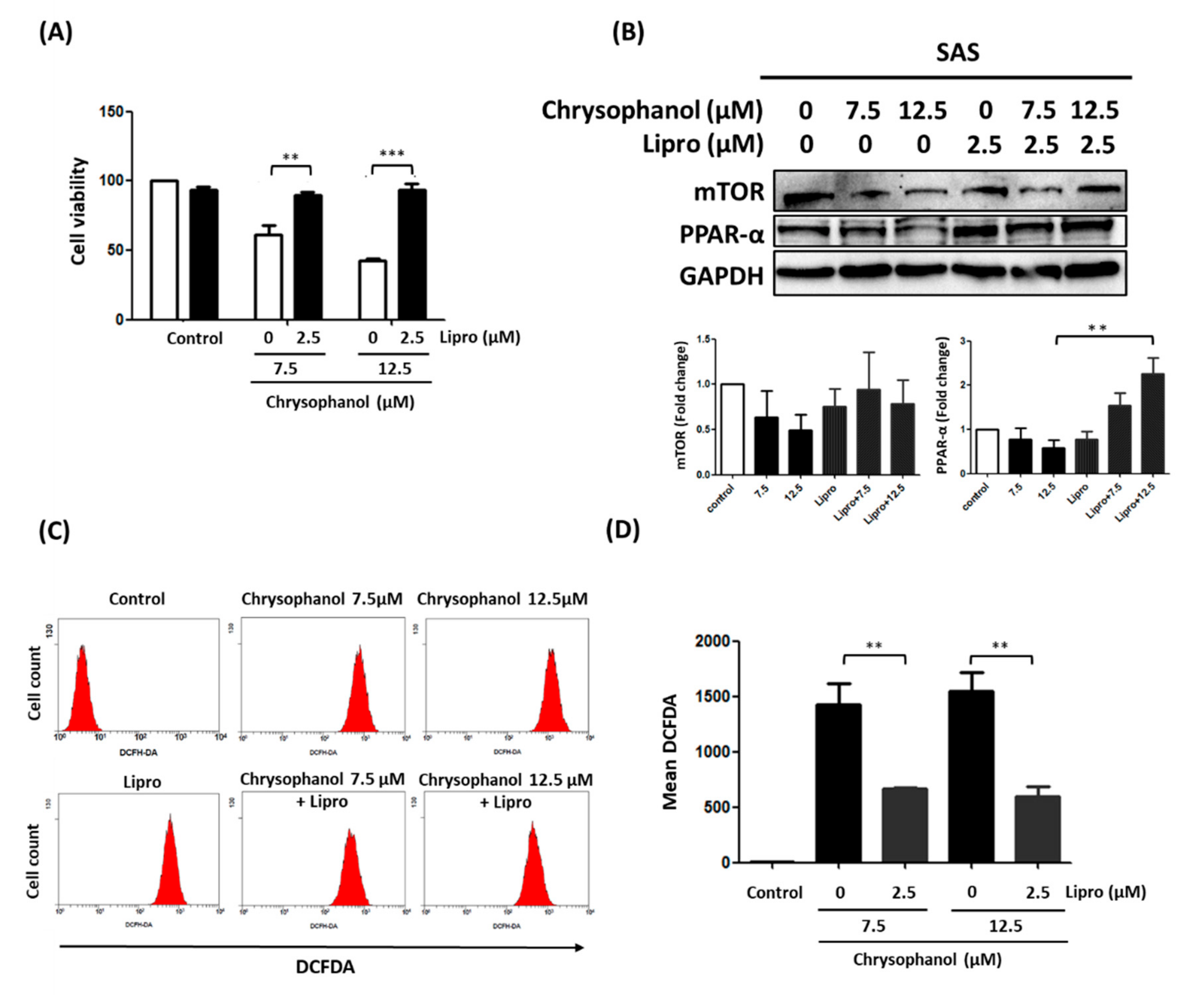
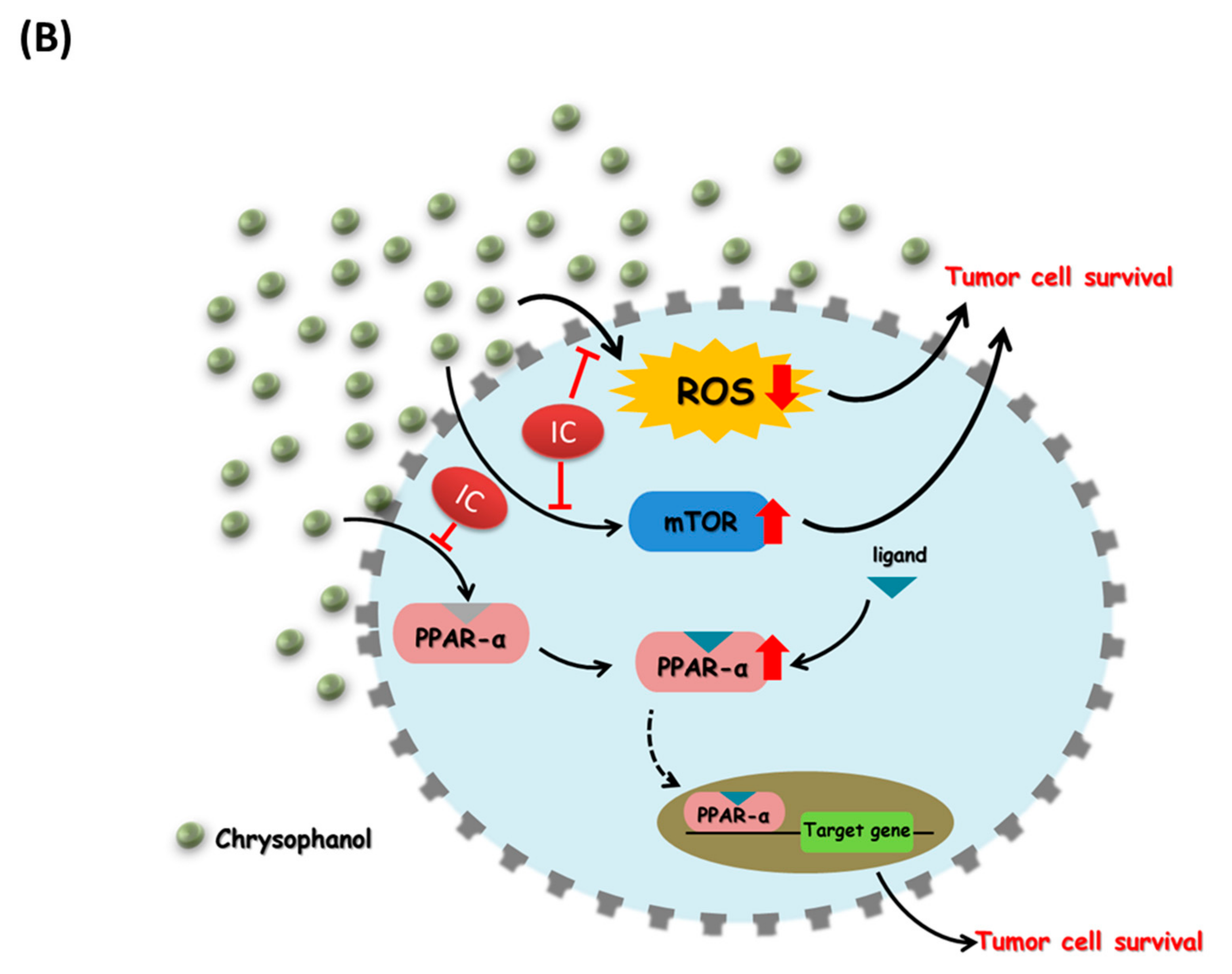
3.2. Chrysophanol Regulates Cell Death, mTOR/PPAR-α Expression, and ROS Accumulation in an Iron-Dependent Manner
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Lin, S.C.; Kao, T.; Chang, C.S.; Hong, P.S.; Shieh, T.M.; Chang, K.W. Genome-wide profiling of oral squamous cell carcinoma. J. Pathol. 2004, 204, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Ganguly, M.; Rao, B.S.; Mukherjee, S.; Mahato, B.; Chaudhuri, K. Clinico-epidemiological profile of oral potentially malignant and malignant conditions among areca nut, tobacco and alcohol users in Eastern India: A hospital based study. J. Oral. Maxillofac. Pathol. 2013, 17, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Living with oral cancer: Epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral. Oncol. 2010, 46, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Miguelanez-Medran, B.C.; Pozo-Kreilinger, J.J.; Cebrian-Carretero, J.L.; Martinez-Garcia, M.A.; Lopez-Sanchez, A.F. Oral squamous cell carcinoma of tongue: Histological risk assessment. A pilot study. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e603–e609. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wu, J.; Gao, Y.; Luo, Y.; Yang, D.; Wang, P. The pharmacological properties of chrysophanol, the recent advances. Biomed. Pharmacother. 2020, 125, 110002. [Google Scholar] [CrossRef]
- Prateeksha; Yusuf, M.A.; Singh, B.N.; Sudheer, S.; Kharwar, R.N.; Siddiqui, S.; Abdel-Azeem, A.M.; Fernandes Fraceto, L.; Dashora, K.; Gupta, V.K. Chrysophanol: A Natural Anthraquinone with Multifaceted Biotherapeutic Potential. Biomolecules 2019, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Hsu, P.C.; Cheng, C.F.; Hsieh, P.C.; Chen, Y.H.; Kuo, C.Y.; Sytwu, H.K. Chrysophanol Regulates Cell Death, Metastasis, and Reactive Oxygen Species Production in Oral Cancer Cell Lines. Evid.-Based Complement. Alternat. Med. 2020, 2020, 5867064. [Google Scholar] [CrossRef]
- Chung, P.C.; Hsieh, P.C.; Lan, C.C.; Hsu, P.C.; Sung, M.Y.; Lin, Y.H.; Tzeng, I.S.; Chiu, V.; Cheng, C.F.; Kuo, C.Y. Role of Chrysophanol in Epithelial-Mesenchymal Transition in Oral Cancer Cell Lines via a Wnt-3-Dependent Pathway. Evid.-Based Complement. Alternat. Med. 2020, 2020, 8373715. [Google Scholar] [CrossRef]
- Hsu, P.C.; Chen, Y.H.; Cheng, C.F.; Kuo, C.Y.; Sytwu, H.K. Interleukin-6 and Interleukin-8 Regulate STAT3 Activation Migration/Invasion and EMT in Chrysophanol-Treated Oral Cancer Cell Lines. Life 2021, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.P.; Lambrecht, M.; Hanin, F.X.; Duprez, T.; Gregoire, V.; Schmitz, S.; Hamoir, M. Advances in the management of squamous cell carcinoma of the head and neck. F1000Prime Rep. 2014, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.M.; Neves, T.J.; Lima, L.G.C.A.; Alves, F.A.; Begnami, M.D. Prognostic implications of the phosphatidylinositol 3-kinase/Akt signaling pathway in oral squamous cell carcinoma: Overexpression of p-mTOR indicates an adverse prognosis. Appl. Cancer Res. 2017, 37, 41. [Google Scholar] [CrossRef] [Green Version]
- Day, T.A.; Shirai, K.; O’Brien, P.E.; Matheus, M.G.; Godwin, K.; Sood, A.J.; Kompelli, A.; Vick, J.A.; Martin, D.; Vitale-Cross, L.; et al. Inhibition of mTOR Signaling and Clinical Activity of Rapamycin in Head and Neck Cancer in a Window of Opportunity Trial. Clin. Cancer Res. 2019, 25, 1156–1164. [Google Scholar] [CrossRef] [Green Version]
- Harsha, C.; Banik, K.; Ang, H.L.; Girisa, S.; Vikkurthi, R.; Parama, D.; Rana, V.; Shabnam, B.; Khatoon, E.; Kumar, A.P.; et al. Targeting AKT/mTOR in Oral Cancer: Mechanisms and Advances in Clinical Trials. Int. J. Mol. Sci. 2020, 21, 3285. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, M.; Yang, K.; Chi, T.; Liao, Z.; Wei, P. PPAR-alpha Modulators as Current and Potential Cancer Treatments. Front. Oncol. 2021, 11, 599995. [Google Scholar] [CrossRef]
- Ligasova, A.; Vydrzalova, M.; Burianova, R.; Bruckova, L.; Vecerova, R.; Janostakova, A.; Koberna, K. A New Sensitive Method for the Detection of Mycoplasmas Using Fluorescence Microscopy. Cells 2019, 8, 1519. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Tseng, H.F.; Hsieh, P.C.; Chiu, V.; Lin, T.Y.; Lan, C.C.; Tzeng, I.S.; Chao, H.N.; Hsu, C.C.; Kuo, C.Y. Nephroprotective Role of Chrysophanol in Hypoxia/Reoxygenation-Induced Renal Cell Damage via Apoptosis, ER Stress, and Ferroptosis. Biomedicines 2021, 9, 1283. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chiu, V.; Huang, C.Y.; Tzeng, I.S.; Hsieh, P.C.; Kuo, C.Y. Promotion of Ferroptosis in Oral Cancer Cell Lines by Chrysophanol. Curr. Top. Nutraceutical Res. 2020, 18, 4. [Google Scholar]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, B.Y.; Pang, Y.L.; Li, W.X.; Zhao, C.X.; Zhang, Y.; Wang, X.; Ning, G.Z.; Kong, X.H.; Liu, C.; Yao, X.; et al. Liproxstatin-1 is an effective inhibitor of oligodendrocyte ferroptosis induced by inhibition of glutathione peroxidase 4. Neural Regen. Res. 2021, 16, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Brockton, N.T.; Dort, J.C. Head and neck cancer: From anatomy to biology. Int. J. Cancer 2013, 133, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Hsiao, S.Y.; Chang, K.Y.; Chang, J.Y. New Insights into Oral Squamous Cell Carcinoma: From Clinical Aspects to Molecular Tumorigenesis. Int. J. Mol. Sci. 2021, 22, 2252. [Google Scholar] [CrossRef]
- Kujan, O.; van Schaijik, B.; Farah, C.S. Immune Checkpoint Inhibitors in Oral Cavity Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. Cancers 2020, 12, 1937. [Google Scholar] [CrossRef]
- Corvo, R. Evidence-based radiation oncology in head and neck squamous cell carcinoma. Radiother. Oncol. 2007, 85, 156–170. [Google Scholar] [CrossRef]
- Hutchinson, M.N.D.; Mierzwa, M.; D’Silva, N.J. Radiation resistance in head and neck squamous cell carcinoma: Dire need for an appropriate sensitizer. Oncogene 2020, 39, 3638–3649. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.C.; Huang, H.B.; Hung, S.K.; Liao, H.F.; Lee, C.C.; Lin, H.Y.; Li, S.C.; Ho, H.C.; Hung, C.L.; Su, Y.C. AZD2014 Radiosensitizes Oral Squamous Cell Carcinoma by Inhibiting AKT/mTOR Axis and Inducing G1/G2/M Cell Cycle Arrest. PLoS ONE 2016, 11, e0151942. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Dou, Z.C.; Ren, W.H.; Li, S.M.; Liang, X.; Zhi, K.Q. CircCDR1as upregulates autophagy under hypoxia to promote tumor cell survival via AKT/ERK(1/2)/mTOR signaling pathways in oral squamous cell carcinomas. Cell Death Dis. 2019, 10, 745. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chen, D.; Liu, H.; Yang, K. Increased expression of lncRNA CASC9 promotes tumor progression by suppressing autophagy-mediated cell apoptosis via the AKT/mTOR pathway in oral squamous cell carcinoma. Cell Death Dis. 2019, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Wu, Y.; Liu, H.; Xie, C. Genipin Induces Autophagy and Suppresses Cell Growth of Oral Squamous Cell Carcinoma via PI3K/AKT/MTOR Pathway. Drug Des. Dev. Ther. 2020, 14, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Ni, C.H.; Yu, C.S.; Lu, H.F.; Yang, J.S.; Huang, H.Y.; Chen, P.Y.; Wu, S.H.; Ip, S.W.; Chiang, S.Y.; Lin, J.G.; et al. Chrysophanol-induced cell death (necrosis) in human lung cancer A549 cells is mediated through increasing reactive oxygen species and decreasing the level of mitochondrial membrane potential. Environ. Toxicol. 2014, 29, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Yang, J.S.; Huang, A.C.; Hsia, T.C.; Chou, S.T.; Kuo, C.L.; Lu, H.F.; Lee, T.H.; Wood, W.G.; Chung, J.G. Chrysophanol induces necrosis through the production of ROS and alteration of ATP levels in J5 human liver cancer cells. Mol. Nutr. Food Res. 2010, 54, 967–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.S. Chrysophanic Acid Induces Necrosis but not Necroptosis in Human Renal Cell Carcinoma Caki-2 Cells. J. Cancer Prev. 2016, 21, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.S.; Cha, E.Y.; Sul, J.Y.; Song, I.S.; Kim, J.Y. Chrysophanic acid blocks proliferation of colon cancer cells by inhibiting EGFR/mTOR pathway. Phytother. Res. 2011, 25, 833–837. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Kuang, F.; Liu, J.; Tang, D.; Kang, R. Oxidative Damage and Antioxidant Defense in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 586578. [Google Scholar] [CrossRef]
- Bayeva, M.; Khechaduri, A.; Puig, S.; Chang, H.C.; Patial, S.; Blackshear, P.J.; Ardehali, H. mTOR regulates cellular iron homeostasis through tristetraprolin. Cell Metab. 2012, 16, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Shang, C.; Zhou, H.; Liu, W.; Shen, T.; Luo, Y.; Huang, S. Iron chelation inhibits mTORC1 signaling involving activation of AMPK and REDD1/Bnip3 pathways. Oncogene 2020, 39, 5201–5213. [Google Scholar] [CrossRef]
- Watson, A.; Lipina, C.; McArdle, H.J.; Taylor, P.M.; Hundal, H.S. Iron depletion suppresses mTORC1-directed signalling in intestinal Caco-2 cells via induction of REDD1. Cell Signal. 2016, 28, 412–424. [Google Scholar] [CrossRef] [Green Version]
- Ohyashiki, J.H.; Kobayashi, C.; Hamamura, R.; Okabe, S.; Tauchi, T.; Ohyashiki, K. The oral iron chelator deferasirox represses signaling through the mTOR in myeloid leukemia cells by enhancing expression of REDD1. Cancer Sci. 2009, 100, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.; Lee, S.K.; Lee, S.K.; Kim, E.C. Differential regulation of iron chelator-induced IL-8 synthesis via MAP kinase and NF-kappaB in immortalized and malignant oral keratinocytes. BMC Cancer 2007, 7, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, P.-C.; Hsu, C.-C.; Hsia, Y.-J.; Kuo, C.-Y. Chrysophanol Suppresses Cell Growth via mTOR/PPAR-α Regulation and ROS Accumulation in Cultured Human Tongue Squamous Carcinoma SAS Cells. Curr. Issues Mol. Biol. 2022, 44, 1528-1538. https://doi.org/10.3390/cimb44040104
Hsu P-C, Hsu C-C, Hsia Y-J, Kuo C-Y. Chrysophanol Suppresses Cell Growth via mTOR/PPAR-α Regulation and ROS Accumulation in Cultured Human Tongue Squamous Carcinoma SAS Cells. Current Issues in Molecular Biology. 2022; 44(4):1528-1538. https://doi.org/10.3390/cimb44040104
Chicago/Turabian StyleHsu, Po-Chih, Chia-Chen Hsu, Yi-Jan Hsia, and Chan-Yen Kuo. 2022. "Chrysophanol Suppresses Cell Growth via mTOR/PPAR-α Regulation and ROS Accumulation in Cultured Human Tongue Squamous Carcinoma SAS Cells" Current Issues in Molecular Biology 44, no. 4: 1528-1538. https://doi.org/10.3390/cimb44040104
APA StyleHsu, P.-C., Hsu, C.-C., Hsia, Y.-J., & Kuo, C.-Y. (2022). Chrysophanol Suppresses Cell Growth via mTOR/PPAR-α Regulation and ROS Accumulation in Cultured Human Tongue Squamous Carcinoma SAS Cells. Current Issues in Molecular Biology, 44(4), 1528-1538. https://doi.org/10.3390/cimb44040104






