Molecular, Viral and Clinical Features of Alcohol- and Non-Alcohol-Induced Liver Injury
Abstract
1. Microsomal Ethanol Oxidizing System Due to Cytochrome (CYP) 2E1 Rather than Catalase or ADH
1.1. The Discovery of MEOS
1.2. Initial Studies on MEOS Isolation
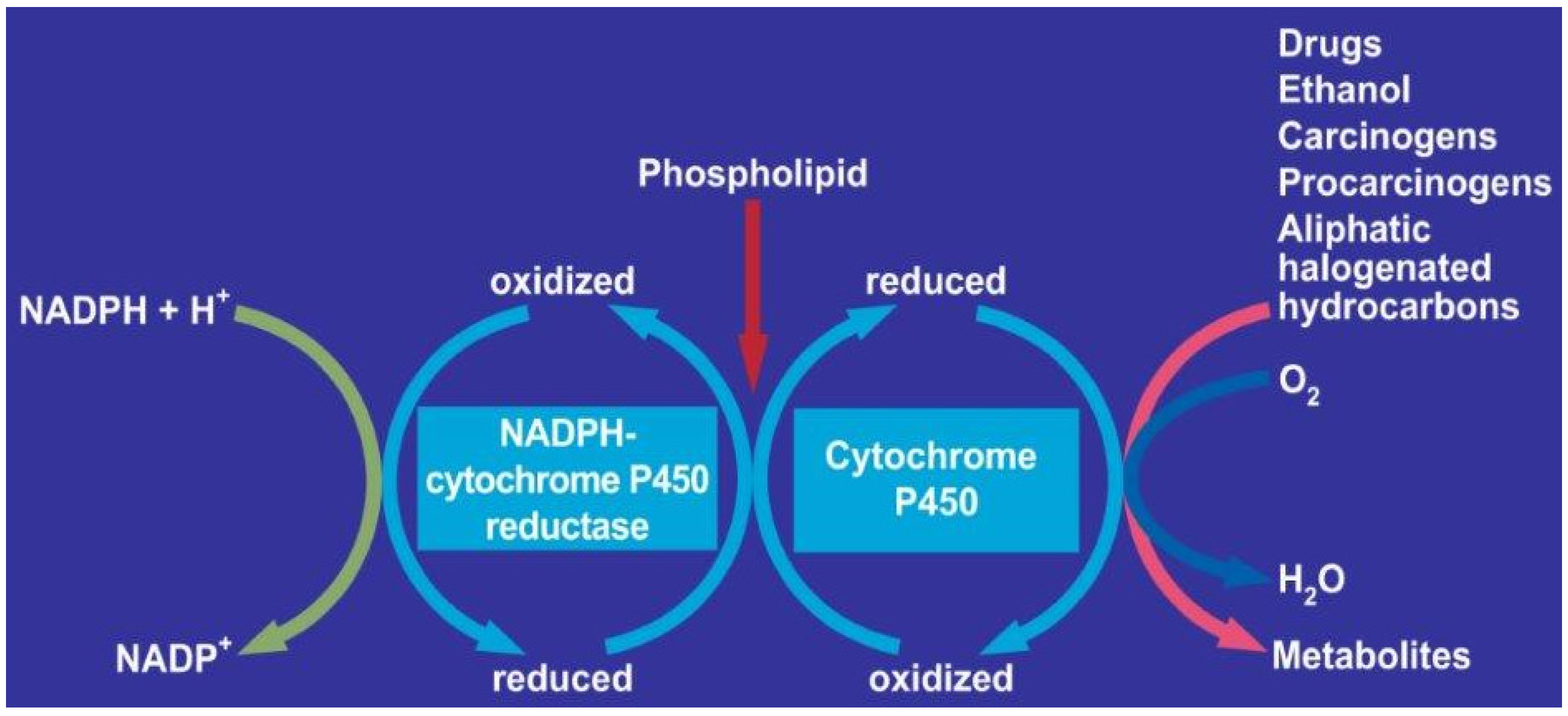
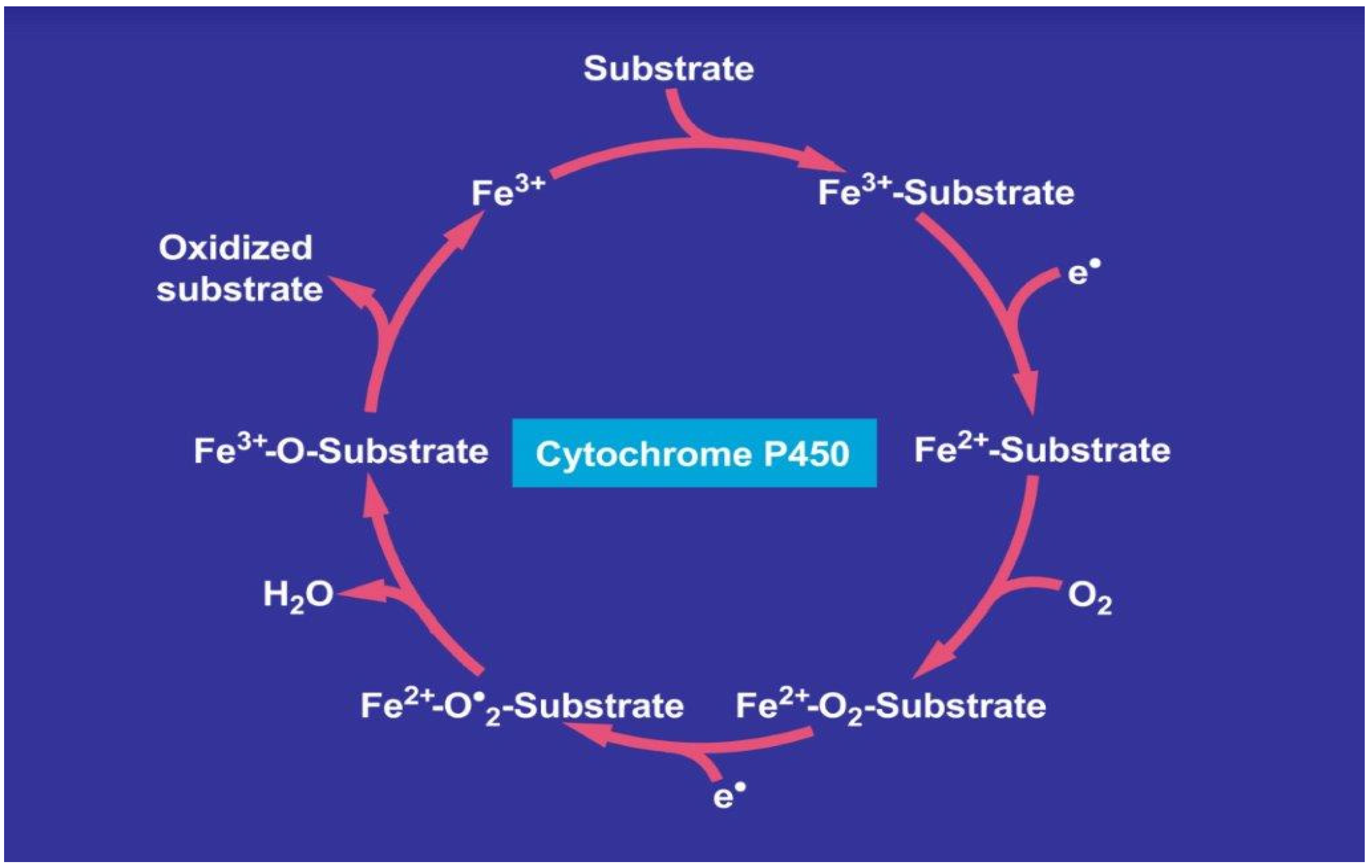
1.3. Components of MEOS: CYP, Reductase and Phospholipids
1.4. MEOS Oxidized Higher Aliphatic Alcohols
1.5. Cellular Proteins
1.6. Role of CYP 2E1 for Ethanol in MEOS and Other Exogenous Substrates
1.7. CYPs Inducers of Other Substances
1.8. Various Aspects
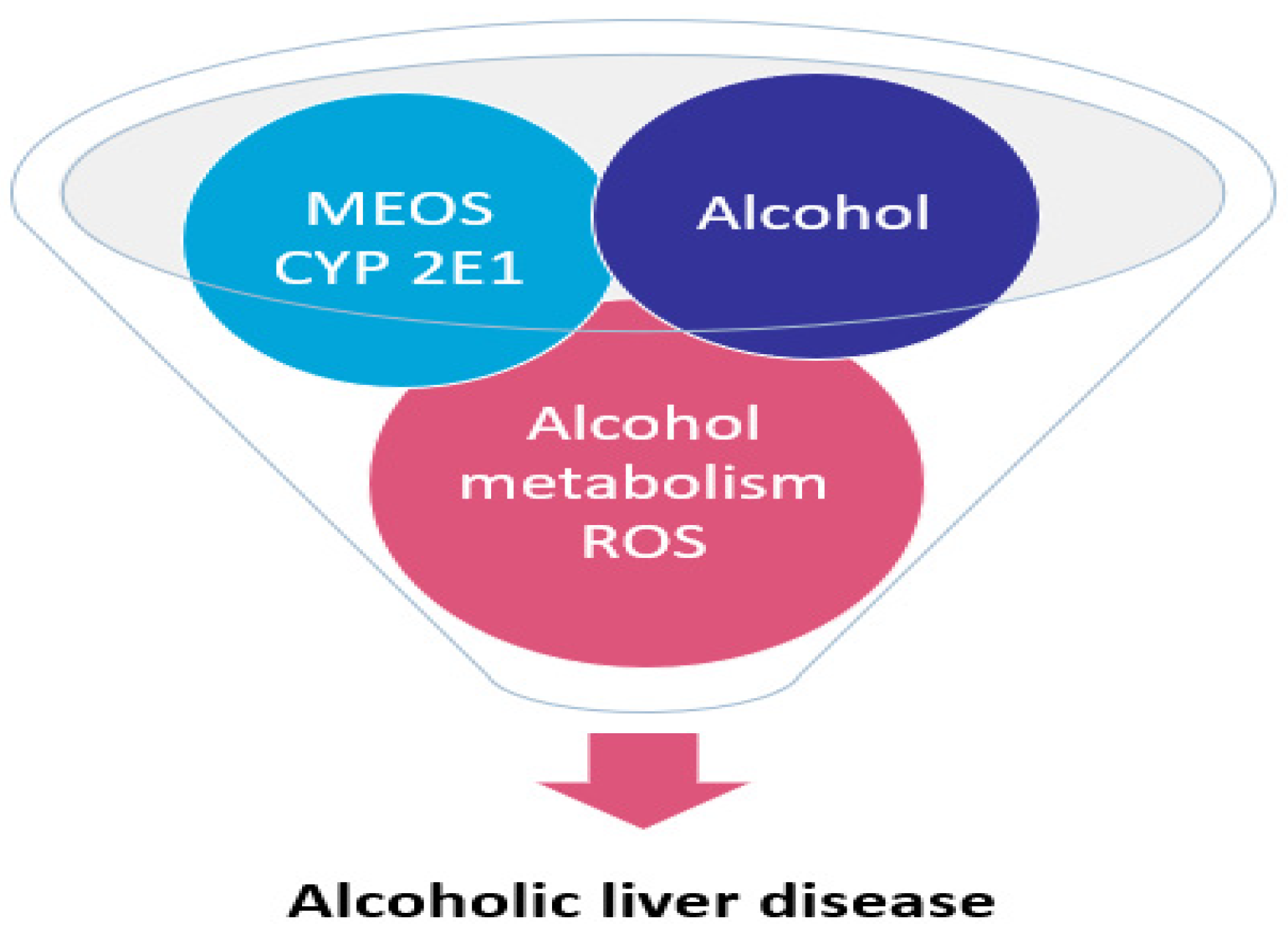
1.9. Alcoholic Liver Disease: The CYP2E1
1.9.1. Hydroxy-Nonenal Which Binds to DNA and Etheno-DNA-Adducts
1.9.2. The Randomized Controlled Clinical Trial with CMZ
2. Biochemical Laboratory Testing for Biomarkers of Alcohol Exposure
2.1. The Need for Biomarkers of Alcohol Exposure
2.2. Biomarkers
3. Liver—Coronavirus Disease 2019—COVID-19—A Review and Clinical Approach
3.1. Coronavirus Disease 2019
3.2. Mechanisms of Liver Function Test Abnormalities
3.3. The Effect of COVID-19 Infection on Patients with Pre-Existing Liver Disease
3.3.1. MAFLD
3.3.2. Hepatitis
3.3.3. Cirrhosis and Hepatocellular Carcinoma
3.3.4. Alcoholic Liver Disease
3.3.5. Liver Transplant
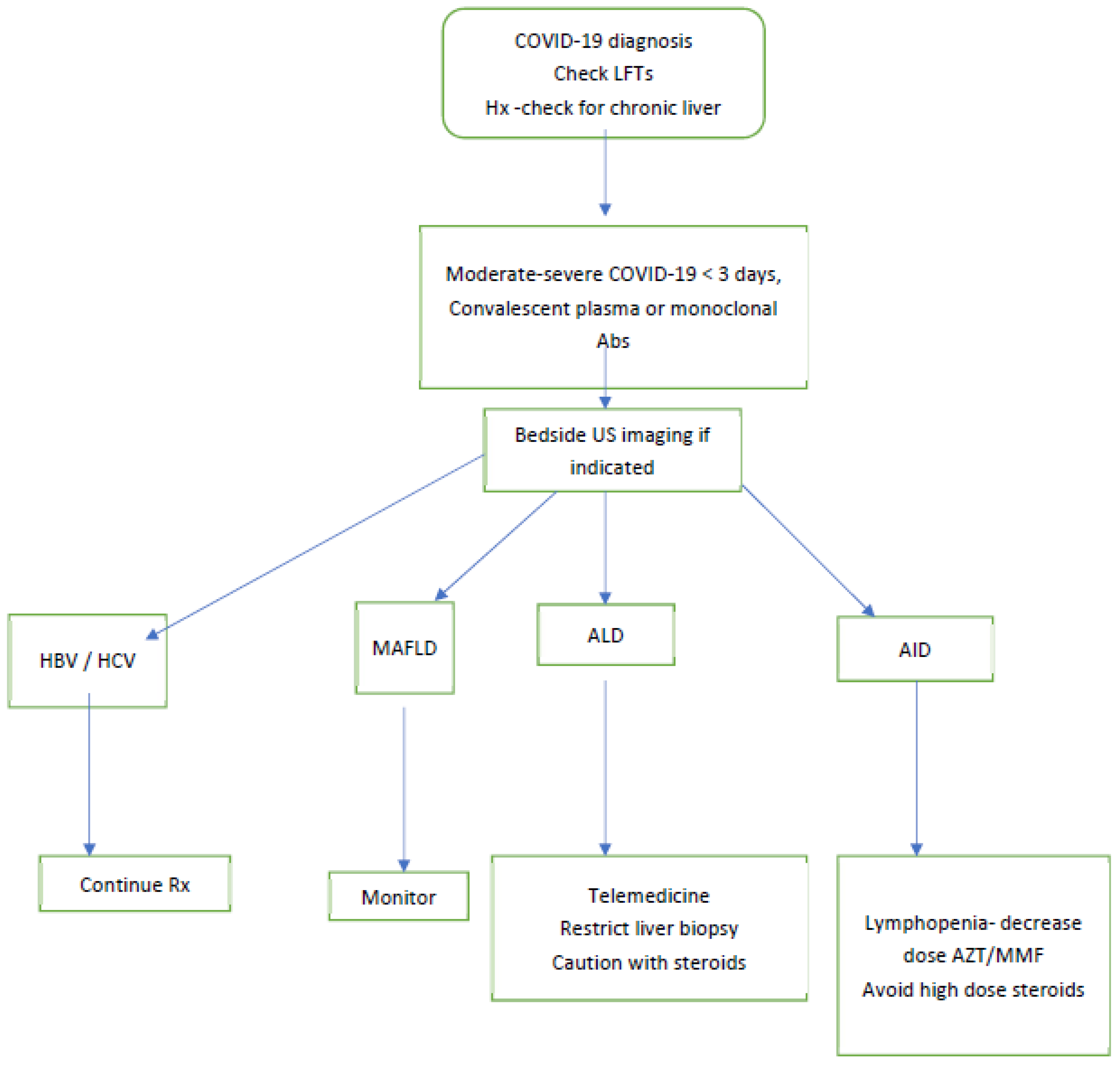
3.4. Kaplan Medical Center Experience with COVID-19
3.5. Suggested Clinical Approach
4. Personalized, Precision Medicine and Non-Invasive Biomarkers to Determine Severity of Alcohol-Induced Hepatocytotoxicity in Human Alcoholic and Non-Alcoholic Liver Disease
4.1. Non-Invasive Biomarkers
4.2. Apoptosis
4.3. Alcohol Misuse and Cancer
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE-2 | angiotensin-converting enzyme 2 |
| AH | alcoholic hepatitis |
| AIH | autoimmune hepatitis |
| ALD | alcohol-related liver disease |
| ALI | acute liver injury |
| ALT | alanine aminotransferase (glutamic-pyruvic transaminase, GPT) |
| ALP | alkaline phosphatase |
| AMA | anti-mitochondrial antibody |
| ANA | antinuclear antibody |
| AST | aspartate aminotransferase (glutamic-oxalic- transaminase, GOT) |
| ASH | alcoholic steatohepatitis |
| BMI | body mass index |
| CCK | caspase cleaved cytokeratin (8 & 18)- M30-M 65 |
| COVID | Corona virus disease |
| DAA | direct-acting anti-virals |
| DAMPs | danger-associated molecular patterns |
| DNA | deoxyribonucleic acid |
| EGF | epithelial growth factor |
| ER | endoplasmic reticulum |
| EtG | ethanol glucuronide |
| EtS | ethanol sulphate |
| FDA | Food and Drug Administration |
| GGT | γ-glutamyl-transferase |
| GSH | glutathione |
| HBV | hepatitis virus B |
| HCV | hepatitis virus C |
| HER | hydroxyethyl radical |
| HGF | hepatocyte growth factor |
| HSC | hepatic stellate cells |
| IL | Interleukin: IL-6, IL-8 |
| INR | International Normalized Ratio |
| KC | Kupffer cells |
| LPS | lypo-poli-saccharides |
| MAA | malon-dialdehyde |
| MAFLD | metabolic associated fatty liver disease, |
| Mean ± SD | mean ± standard deviation |
| MEOS | microsomal ethanol oxidizing system |
| NAD+ | nicotinamide adenine dinucleotide, |
| NADH | reduced nicotinamide adenine dinucleotide |
| NADP+ | nicotinamide adenine dinucleotide phosphate, |
| NADPH | reduced nicotinamide adenine dinucleotide phosphate, |
| PAMPs | pathogen-associated molecular patterns |
| PhET | phosphatidyl ethanol |
| POEMS | Polyneuropathy, organomegaly, endocrinopathy, M-protein, skin changes syndrome |
| PPAR alpha | peroxisome proliferator—activated receptor alpha, |
| PRRs | pattern-recognition receptors |
| RBC | red blood cell |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| SOCS | suppressor of cytokine signaling |
| SREBP-1 | sterol regulatory element binding protein-1 |
| TGF-β | transforming growth factor beta |
| TNF-alpha | Tumor necrosis factor alpha |
| TLR4 | toll-like receptor 4 |
| U/L | Unit/Liter |
| ULN | Upper the limit of normal |
| VEGF | vascular endothelial growth factor |
References
- Lieber, C.S.; DeCarli, L.M. Ethanol Oxidation by Hepatic Microsomes: Adaptive Increase after Ethanol Feeding. Science 1968, 162, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; DeCarli, L.M. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J. Biol. Chem. 1970, 245, 2505–2512. [Google Scholar] [CrossRef]
- Lieber, C.S.; DeCarli, L.M. The role of the hepatic microsomal ethanol oxidizing system (MEOS) for ethanol metabolism in vivo. J. Pharmacol. Exp. Ther. 1972, 181, 279–287. [Google Scholar]
- Lieber, C.S. Microsomal Ethanol-Oxidizing System (MEOS): The First 30 Years (1968-1998)-A Review. Alcohol. Clin. Exp. Res. 1999, 23, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; DeCarli, L.M.; Matsuzaki, S.; Ohnishi, K.; Teschke, R. The Microsomal ethanol oxidizing systems (MEOS). In Methods in Enzymology; Elsevier BV: Amsterdam, The Netherlands, 1978; Volume 52, pp. 355–367. [Google Scholar]
- Teschke, R. Biochemical Aspects of the Hepatic Microsomal Ethanol-oxidizing System (MEOS): Resolved Initial Controversies and Updated Molecular Views. Biochem. Pharmacol. 2019, 8, 1–13. [Google Scholar] [CrossRef][Green Version]
- Lu, A.Y.; Coon, M.J. Role of hemoprotein P-450 in fatty acid omega-hydroxylation in a soluble enzyme system from liver microsomes. J. Biol. Chem. 1968, 243, 1331–1332. [Google Scholar] [CrossRef]
- Teschke, R.; Hasumura, Y.; Joly, J.-G.; Ishii, H.; Lieber, C. Microsomal ethanol-oxidizing system (MEOS): Purification and properties of a rat liver system free of catalase and alcohol dehydrogenase. Biochem. Biophys. Res. Commun. 1972, 49, 1187–1193. [Google Scholar] [CrossRef]
- Teschke, R.; Hasumura, Y.; Lieber, C.S. NADPH-dependent oxidation of methanol, ethanol, propanol and butanol by hepatic microsomes. Biochem. Biophys. Res. Commun. 1974, 60, 851–857. [Google Scholar] [CrossRef]
- Teschke, R.; Hasumura, Y.; Lieber, C.S. Hepatic microsomal ethanol-oxidizing system: Solubilization, isolation, and characterization. Arch. Biochem. Biophys. 1974, 163, 404–415. [Google Scholar] [CrossRef]
- Teschke, R.; Hasumura, Y.; Lieber, C.S. Hepatic microsomal alcohol-oxidizing system. Affinity for methanol, ethanol, propanol, and butanol. J. Biol. Chem. 1975, 250, 7397–7404. [Google Scholar] [CrossRef]
- Teschke, R.; Ohnishi, K.; Hasumura, Y.; Lieber, C.S. Hepatic microsomal ethanol oxidizing system: Isolation and reconstitution. In Microsomes and Drug Oxidations; Ullrich, V., Roots, I., Hildebrandt, A., Estabrook, R.W., Conney, A.H., Eds.; Pergamon Press: Oxford, UK, 1977; pp. 103–110. [Google Scholar]
- Teschke, R.; Neuman, M.G.; Liangpunsakul, S.; Seitz, H.-K. Alcoholic Liver Disease and the co-triggering Role of MEOS with Its CYP 2E1 Catalytic Cycle and ROS. Arch. Gastroenterol. Res. 2021, 2, 9–25. [Google Scholar] [CrossRef]
- Meijers, R.; Morris, R.; Adolph, H.W.; Merli, A.; Lamzin, V.; Cedergren-Zeppezauer, E.S. On the Enzymatic Activation of NADH. J. Biol. Chem. 2001, 276, 9316–9321. [Google Scholar] [CrossRef] [PubMed]
- Setshedi, M.; Wands, J.R.; De La Monte, S.M. Acetaldehyde adducts in alcoholic liver disease. Oxidat. Med. Cell. Longev. 2010, 3, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Van Vleet, T.R.; Philip, B.K. Acetaldehyde. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Lieber, C.S. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 2004, 34, 9–19. [Google Scholar] [CrossRef]
- Wu, D.; I Cederbaum, A. Oxidative Stress and Alcoholic Liver Disease. Semin. Liver Dis. 2009, 29, 141–154. [Google Scholar] [CrossRef]
- Damgaard, S.E. TheD(V/K) Isotope Effect of the Cytochrome P-450-Mediated Oxidation of Ethanol and Its Biological Applications. JBIC J. Biol. Inorg. Chem. 1982, 125, 593–603. [Google Scholar] [CrossRef]
- Ohnishi, K.; Lieber, C.S. Reconstitution of the microsomal ethanol-oxidizing system. Qualitative and quantitative changes of cy-tochrome P-450 after chronic ethanol consumption. J. Biol. Chem. 1977, 52, 7124–7131. [Google Scholar] [CrossRef]
- Teschke, R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef]
- Teschke, R. Alcoholic Liver Disease: Current Mechanistic Aspects with Focus on Their Clinical Relevance. Biomedicines 2019, 7, 68. [Google Scholar] [CrossRef]
- Hasumura, Y.; Teschke, R.; Lieber, C.S. Acetaldehyde oxidation by hepatic mitochondria: Its decrease after chronic ethanol con-sumption. Science 1975, 189, 727–729. [Google Scholar] [CrossRef]
- Asai, H.; Imaoka, S.; Kuroki, T.; Monna, T.; Funae, Y. Microsomal ethanol oxidizing system activity by human hepatic cytochrome P450s. J. Pharmacol. Exp. Ther. 1996, 277, 1004–1009. [Google Scholar] [PubMed]
- García-Bañuelos, J.; Panduro, A.; Gordillo-Bastidas, D.; Gordillo-Bastidas, E.; Muñoz-Valle, J.F.; Gurrola-Díaz, C.M.; Sánchez-Enríquez, S.; Ruiz-Madrigal, B.; Bastidas-Ramírez, B.E. Genetic Polymorphisms of Genes Coding to Alcohol-Metabolizing Enzymes in Western Mexicans: Association of CYP2E1*c2/CYP2E1*5B Allele with Cirrhosis and Liver Function. Alcohol. Clin. Exp. Res. 2012, 36, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Gaviria-Calle, M.; Jaramillo, A.D.; Aranzazu, M.; Di Filippo, D.; Montoya, M.; Roldán, I.; Jaramillo, S.; Restrepo-Gutiérrez, J.C.; Hoyos, S.; Navas, M.C. Polimorfismos en los genes de la enzima alcohol deshidrogenasa (ADH1) y la citocromo P450 2E1 (CYP2E1) en pacientes con cirrosis y carcinoma hepatocelular. Biomédica 2018, 38, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P450 2E1 and its roles in disease. Chem. Interact. 2020, 322, 109056. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.; Liangpunsakul, S. Alcohol Metabolizing Enzymes, Microsomal Ethanol Oxidizing System, Cytochrome P450 2E1, Catalase, and Aldehyde Dehydrogenase in Alcohol-Associated Liver Disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef]
- Neuman, M.G.; Mueller, J.; Mueller, S. Non-invasive Biomarkers of Liver Inflammation and Cell Death in Response to Alcohol Detoxification. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef]
- Kubiak-Tomaszewska, G.; Tomaszewski, P.; Pachecka, J.; Struga, M.; Olejarz, W.; Mielczarek-Puta, M.; Nowicka, G. Molecular mechanisms of ethanol biotransformation: Enzymes of oxidative and nonoxidative metabolic pathways in human. Xenobiotica 2020, 50, 1180–1201. [Google Scholar] [CrossRef]
- Lieber, C.S. Alcohol and the liver: 1994 update. Gastroenterology 1994, 106, 1085–1105. [Google Scholar] [CrossRef]
- Lieber, C.S. Cytochrome P-4502E1: Its physiological and pathological role. Physiol. Rev. 1997, 77, 517–544. [Google Scholar] [CrossRef]
- Lieber, C.S. A commentary. Milestones in liver disease. The unexpected outcomes of medical research: Serendipity and the mi-crosomal ethanol oxidizing system. J. Hepatol. 2004, 40, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cederbaum, A.I. Cytochrome P450s and alcoholic liver disease. Curr. Pharm. Des. 2018, 24, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015, 4, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, A.C.; Lucas, D.; Menez, J.F.; Seitz, H.K. Chlormethiazole inhibition of cytochrome P4502E1 as assessed by chlorzoxazone hydroxylation in humans. Hepatology 1997, 26, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Seitz, H.K.; French, S.W.; Malnick, S.; Tsukamoto, H.; Cohen, L.B.; Hoffman, P.; Tabakoff, B.; Fasullo, M.; Nagy, L.E.; et al. Alcoholic-Hepatitis, Links to Brain and Microbiome: Mechanisms, Clinical and Experimental Research. Biomedicines 2020, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Banerjee, A.; Jang, S.; Yoo, S.-H.; Yun, J.-W.; Gonzalez, F.J.; Keshavarzian, A.; Song, B.-J. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic. Biol. Med. 2013, 65, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Voigt, R.M.; Keshavarzian, A. Intestinal CYP2E1: A mediator of alcohol-induced gut leakiness. Redox Biol. 2014, 3, 40–46. [Google Scholar] [CrossRef]
- Vera-Barajas, A.; Abenavoli, L.; Scarpellini, E.; Méndez-Sánchez, N.; Valencia-Rodríguez, A.; Ponciano-Rodríguez, G.; Wang, D.Q.-H. The mechanism of dysbiosis in alcoholic liver disease leading to liver cancer. Hepatoma Res. 2020, 2020, 5. [Google Scholar] [CrossRef]
- Neuman, M.G.; French, S.W.; Zakhari, S.; Malnick, S.; Seitz, H.K.; Cohen, L.B.; Salaspuro, M.; Voinea-Griffin, A.; Barasch, A.; Kirpich, I.A.; et al. Alcohol, microbiome, life style influence alcohol and non-alcoholic organ damage. Exp. Mol. Pathol. 2017, 102, 162–180. [Google Scholar] [CrossRef]
- Leo, M.A.; Lieber, C.S. Hepatic Fibrosis After Long-Term Administration of Ethanol and Moderate Vitamin A Supplementation in the Rat. Hepatology 2007, 3, 1–11. [Google Scholar] [CrossRef]
- Seitz, H.K. Alcohol an retinoid metabolism (Editorial). Gut 2000, 47, 748–750. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, C.; Russell, R.M.; Seitz, H.K.; Wang, X.-D. Ethanol enhances retinoic acid metabolism into polar metabolites in rat liver via induction of cytochrome P4502E1. Gastroenterology 2001, 120, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Dan, Z.; Popov, Y.; Patsenker, E.; Preimel, D.; Liu, C.; Wang, X.; Seitz, H.K.; Schuppan, D.; Stickel, F. Hepatotoxicty of alcohol–induced polar retinol metabolites involves apoptosis via loss of mitochondrial membrane potential. FASEB J. 2005, 19, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Seitz, H.K. Alcohol and Retinoid Interaction. In Nutrition and Alcohol: Linking Nutrient Interactions and Dietary Intake; Watson, R.R., Preedy, V.R., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2004; pp. 313–321. [Google Scholar]
- Liu, C.; Chung, J.; Seitz, H.K.; Russell, R.M.; Wang, X.-D. Chlormethiazole treatment prevents reduced hepatic vitamin A levels in ethanol-fed rats. Alcohol. Clin. Exp. Res. 2002, 26, 1703–1709. [Google Scholar] [CrossRef]
- Jaurigue, M.M.; Cappell, M.S. Therapy for alcoholic liver disease. World J. Gastroenterol. 2014, 20, 2143–2158. [Google Scholar] [CrossRef]
- Hohmann, N.; Schröder, F.; Moreira, B.; Teng, H.; Burhenne, J.; Bruckner, T.; Mueller, S.; Haefeli, W.E.; Seitz, H.K. Clomethiazole inhibits cytochrome P450 2E1 and improves alcoholic liver disease. Gut 2021. [Google Scholar] [CrossRef]
- Avila, M.A.; Dufour, J.F.; Gerbes, A.L.; Zoulim, F.; Bataller, R.; Burra, P.; Cortez-Pinto, H.; Gao, B.; Gilmore, I.; Mathurin, P.; et al. Recent advances in alcohol-related liver disease (ALD): Summary of a Gut round table meeting. Gut 2020, 69, 764–780. [Google Scholar] [CrossRef] [PubMed]
- Witkiewitz, K.; Litten, R.Z.; Leggio, L. Advances in the science and treatment of alcohol use disorder. Sci. Adv. 2019, 5, eaax4043. [Google Scholar] [CrossRef]
- Finn, D.A. The Endocrine System and Alcohol Drinking in Females. Alcohol Res. Curr. Rev. 2020, 40, 2. [Google Scholar] [CrossRef]
- Denny, L.A.; Coles, S.; Blitz, R. Fetal Alcohol Syndrome and Fetal Alcohol Spectrum Disorders. Am. Fam. Physician 2017, 96, 515–522. [Google Scholar]
- Weinmann, W.; Schaefer, P.; Thierauf, A.; Schreiber, A.; Wurst, F.M. Confirmatory analysis of ethylglucuronide in urine by liq-uid-chromatography/electrospray ionization/tandem mass spectrometry according to forensic guidelines. J. Am. Soc. Mass Spectrom. 2004, 15, 188–193. [Google Scholar] [CrossRef]
- Viel, G.; Boscolo-Berto, R.; Cecchetto, G.; Fais, P.; Nalesso, A.; Ferrara, S.D. Phosphatidylethanol in Blood as a Marker of Chronic Alcohol Use: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2012, 13, 14788–14812. [Google Scholar] [CrossRef]
- Bager, H.; Christensen, L.P.; Husby, S.; Bjerregaard, L.B.S. Biomarkers for the Detection of Prenatal Alcohol Exposure: A Review. Alcohol. Clin. Exp. Res. 2017, 41, 251–261. [Google Scholar] [CrossRef]
- Baselt, R.C. Disposition of Toxic Drugs and Chemicals in Man, 9th ed.; Biomedical Publications: Seal Beach, CA, USA, 2011; Volume xxviii, p. 1877. [Google Scholar]
- Fagan, K.J.; Irvine, K.M.; McWhinney, B.C.; Fletcher, L.M.; Horsfall, L.U.; Johnson, L.; O’Rourke, P.; Martin, J.; Scott, I.; Pretorius, C.J.; et al. Diagnostic sensitivity of carbohydrate deficient transferrin in heavy drinkers. BMC Gastroenterol. 2014, 14, 97. [Google Scholar] [CrossRef]
- Zühlsdorf, A.; Park, J.H.; Wada, Y.; Rust, S.; Reunert, J.; DuChesne, I.; Grüneberg, M.; Marquardt, T. Transferrin variants: Pitfalls in the diagnostics of Congenital disorders of glycosylation. Clin. Biochem. 2015, 48, 11–13. [Google Scholar] [CrossRef]
- Veronesi, A.; Rota, C.; Trenti, T.; Cariani, E. Carbohydrate-Deficient Transferrin Determination in a Clinical Setting: Consistency Between Capillary Electrophoresis Assays and Utility of HPLC as a Confirmatory Test. J. Clin. Lab. Anal. 2015, 30, 494–499. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Nardo, A.D.; Schneeweiss-Gleixner, M.; Bakail, M.; Dixon, E.D.; Lax, S.F.; Trauner, M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021, 41, 20–32. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Liu, L.-G.; Wang, Y.-B.; Zhang, T.; Li, M.-H.; Xu, Y.-L.; Gao, G.-J.; Xiong, H.-F.; Fan, Y.; et al. Pattern of liver injury in adult patients with COVID-19: A retrospective analysis of 105 patients. Mil. Med Res. 2020, 7, 1–8. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Phipps, M.M.; Barraza, L.H.; Lasota, E.D.; Sobieszczyk, M.E.; Pereira, M.R.; Zheng, E.X.; Fox, A.N.; Zucker, J.; Verna, E.C. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology 2020, 72, 807–817. [Google Scholar] [CrossRef]
- Cai, Q.; Huang, D.; Yu, H.; Zhu, Z.; Xia, Z.; Su, Y.; Li, Z.; Zhou, G.; Gou, J.; Qu, J.; et al. COVID-19: Abnormal liver function tests. J. Hepatol. 2020, 73, 566–574. [Google Scholar] [CrossRef]
- Anastasiou, O.E.; Korth, J.; Herbstreit, F.; Witzke, O.; Lange, C.M. Mild versus Severe Liver Injury in SARS-CoV-2 Infection. Dig. Dis. 2020, 39, 52–57. [Google Scholar] [CrossRef]
- Lei, F.; Liu, Y.; Zhou, F.; Qin, J.; Zhang, P.; Zhu, L.; Zhang, X.; Cai, J.; Lin, L.; Ouyang, S.; et al. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology 2020, 72, 389–398. [Google Scholar] [CrossRef]
- Kovalic, A.J.; Satapathy, S.K.; Thuluvath, P.J. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: A systematic review and meta-analysis. Hepatol. Int. 2020, 14, 612–620. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, L.; Li, J.; Cheng, X.; Yang, J.; Tian, C.; Zhang, Y.; Huang, S.; Liu, Z.; Cheng, J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin. Gastroenterol. Hepatol. 2020, 18, 1561–1566. [Google Scholar] [CrossRef]
- Bangash, M.N.; Patel, J.; Parekh, D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol. Hepatol. 2020, 5, 529–530. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-l.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Xu, L.; Liu, J.; Lu, M.; Yang, D.; Zheng, X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020, 40, 998–1004. [Google Scholar] [CrossRef]
- Chai, X.; Hu, L.; Zhang, Y.; Han, W. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-NCoV Infection. biorxiv 2020. [Google Scholar] [CrossRef]
- Zhao, C.L.; Rapkiewicz, A.; Maghsoodi-Deerwester, M.; Gupta, M.; Cao, W.; Palaia, T.; Zhou, J.; Ram, B.; Vo, D.; Rafiee, B.; et al. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID-19). Hum. Pathol. 2021, 109, 59–68. [Google Scholar] [CrossRef]
- Bertolini, A.; Van De Peppel, I.P.; Bodewes, F.A.; Moshage, H.; Fantin, A.; Farinati, F.; Fiorotto, R.; Jonker, J.W.; Strazzabosco, M.; Verkade, H.J.; et al. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology 2020, 72, 1864–1872. [Google Scholar] [CrossRef]
- Elsoukkary, S.S.; Mostyka, M.; Dillard, A.; Berman, D.R.; Ma, L.X.; Chadburn, A.; Yantiss, R.K.; Jessurun, J.; Seshan, S.V.; Borczuk, A.C.; et al. Autopsy Findings in 32 Patients with COVID-19: A Single-Institution Experience. Pathobiology 2020, 88, 56–68. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, L.; Li, J.; Tian, C.; Zhang, Y.; Huang, S.; Liu, Z.; Cheng, J. Clinical Features of COVID-19-Related Liver Damage. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020, 383. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M.; Longo, M.; Fracanzani, A.L. MAFLD in COVID-19 patients: An insidious enemy. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 867–872. [Google Scholar] [CrossRef]
- Ji, D.; Qin, E.; Xu, J.; Zhang, D.; Cheng, G.; Wang, Y.; Lau, G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J. Hepatol. 2020, 73, 451–453. [Google Scholar] [CrossRef]
- Mushtaq, K.; Khan, M.U.; Iqbal, F.; Alsoub, D.H.; Chaudhry, H.S.; Ata, F.; Iqbal, P.; Elfert, K.; Balaraju, G.; Almaslamani, M.; et al. NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression – The debate continues. J. Hepatol. 2021, 74, 482–484. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Zheng, K.; Wang, X.-B.; Yan, H.-D.; Sun, Q.-F.; Pan, K.-H.; Wang, T.-Y.; Ma, H.-L.; Chen, Y.-P.; George, J.; et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J. Hepatol. 2020, 73, 719–721. [Google Scholar] [CrossRef]
- Anugwom, C.M.; Aby, E.S.; Debes, J.D. Inverse Association Between Chronic Hepatitis B Infection and Coronavirus Disease 2019 (COVID-19): Immune Exhaustion or Coincidence? Clin. Infect. Dis. 2021, 72, 180–182. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Cai, Q.; Sun, L.; Huang, D.; Zhou, G.; He, Q.; Wang, F.; Liu, L.; Chen, J. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol. Res. 2020, 50, 1211–1221. [Google Scholar] [CrossRef]
- Crawford, A.; Angelosanto, J.M.; Kao, C.; Doering, T.A.; Odorizzi, P.M.; Barnett, B.E.; Wherry, E.J. Molecular and Transcriptional Basis of CD4+ T Cell Dysfunction during Chronic Infection. Immunity 2014, 40, 289–302. [Google Scholar] [CrossRef]
- Mangia, A.; Cenderello, G.; Verucchi, G.; Ciancio, A.; Fontana, A.; Piazzolla, V.; Minerva, N.; Squillante, M.M.; Copetti, M. Is positivity for hepatitis C virus antibody predictive of lower risk of death in COVID-19 patients with cirrhosis? World J. Clin. Cases 2020, 8, 5831–5834. [Google Scholar] [CrossRef]
- Marjot, T.; Buescher, G.; Sebode, M.; Barnes, E.; Barritt, A.S., IV; Armstrong, M.J.; Baldelli, L.; Kennedy, J.; Mercer, C.; Ozga, A.-K.; et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J. Hepatol. 2021, 74, 1335–1343. [Google Scholar] [CrossRef]
- Iavarone, M.; D’Ambrosio, R.; Soria, A.; Triolo, M.; Pugliese, N.; Del Poggio, P.; Perricone, G.; Massironi, S.; Spinetti, A.; Buscarini, E.; et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J. Hepatol. 2020, 73, 1063–1071. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Garcia-Tsao, G.; Biggins, S.W.; Kamath, P.S.; Wong, F.; McGeorge, S.; Shaw, J.; Pearson, M.; Chew, M.; Fagan, A.; et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: Multicentre matched cohort. Gut 2021, 70, 531–536. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Garcia-Tsao, G.; Wong, F.; Biggins, S.W.; Kamath, P.S.; McGeorge, S.; Chew, M.; Pearson, M.; Shaw, J.; Kalluri, A.; et al. Cirrhosis Is Associated with High Mortality and Readmissions Over 90 Days Regardless of COVID-19: A Multicenter Cohort. Liver Transpl. 2021, 27, 1343–1347. [Google Scholar] [CrossRef]
- Da, B.L.; Im, G.Y.; Schiano, T.D. Coronavirus Disease 2019 Hangover: A Rising Tide of Alcohol Use Disorder and Alcohol-Associated Liver Disease. Hepatology 2020, 72, 1102–1108. [Google Scholar] [CrossRef]
- Kushner, T.; Cafardi, J. Chronic Liver Disease and COVID-19: Alcohol Use Disorder/Alcohol-Associated Liver Disease, Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Autoimmune Liver Disease, and Compensated Cirrhosis. Clin. Liver Dis. 2020, 15, 195–199. [Google Scholar] [CrossRef]
- Mansoor, E.; Perez, A.; Abou-Saleh, M.; Sclair, S.N.; Cohen, S.; Cooper, G.S.; Mills, A.; Schlick, K.; Khan, A. Clinical Characteristics, Hospitalization, and Mortality Rates of Coronavirus Disease 2019 Among Liver Transplant Patients in the United States: A Multicenter Research Network Study. Gastroenterology 2021, 160, 459–462.e1. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, J.; Rodríguez-Perálvarez, M.; Salcedo, M.; Arias-Milla, A.; Muñoz-Serrano, A.; Graus, J.; Nuño, J.; Gastaca, M.; Bustamante-Schneider, J.; Cachero, A.; et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J. Hepatol. 2021, 74, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.L.; Alin, P.; Malnick, S. The Evidence for High-Titer Convalescent Plasma in SARS-CoV-2. SN Compr. Clin. Med. 2021, 3, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.L.; German, A.; Mousa, S.; Gahnnam, W.; Malnick, D.H.S. Liver and COVID-19 Experience of a Regional Hospital. Arch. Clin. Biomed. Res. 2021, 5, 808–816. [Google Scholar] [CrossRef]
- Mueller, S.; Englert, S.; Seitz, H.K.; Badea, R.I.; Erhardt, A.; Bozaari, B.; Beaugrand, M.; Lupsor-Platon, M. Inflammation-adapted liver stiffness values for improved fibrosis staging in patients with hepatitis C virus and alcoholic liver disease. Liver Int. 2015, 35, 2514–2521. [Google Scholar] [CrossRef]
- Bannaga, A.S.; Metzger, J.; Kyrou, I.; Voigtländer, T.; Book, T.; Melgarejo, J.; Latosinska, A.; Pejchinovski, M.; Staessen, J.A.; Mischak, H.; et al. Discovery, validation and sequencing of urinary peptides for diagnosis of liver fibrosis—A multicentre study. EBioMedicine 2020, 62, 103083. [Google Scholar] [CrossRef]
- Neuman, M.G.; Schmilovitz-Weiss, H.; Hilzenrat, N.; Bourliere, M.; Marcellin, P.; Trepo, C.; Mazulli, T.; Moussa, G.; Patel, A.; Baig, A.A.; et al. Markers of Inflammation and Fibrosis in Alcoholic Hepatitis and Viral Hepatitis C. Int. J. Hepatol. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Neuman, M.G.; Brenner, D.A.; Rehermann, B.; Taieb, J.; Chollet-Martin, S.; Cohard, M.; Garaud, J.J. Mechanisms of alcoholic liver disease: Cytokines. Alcohol. Clin. Exp. Res. 2001, 25, 251s–253s. [Google Scholar] [CrossRef]
- Neuman, M.G.; Cohen, L.; Zakhari, S.; Nanau, R.M.; Mueller, S.; Schneider, M.; Parry, C. Alcoholic liver disease: A synopsis of the Charles Lieber’s Memorial Symposia 2009–2012. Alcohol Alcohol. 2014, 49, 373–380. [Google Scholar] [CrossRef]
- Neuman, M.G.; Katz, G.G.; Malkiewicz, I.M.; Mathurin, P.; Tsukamoto, H.; Adachi, M.; Ishii, H.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C.; et al. Conference article: Alcoholic liver injury and apoptosis—synopsis of the symposium held at ESBRA 2001: 8th Congress of the European Society for Biomedical Research on Alcoholism, Paris, September 16, 2001. Alcohol 2002, 28, 117–128. [Google Scholar] [CrossRef]
- Neuman, M.G. Cytokine–chemokines modulate fibrosis in alcoholic hepatitis. Rom. J. Hepatol. 2007, 3, 19–34. [Google Scholar]
- Neuman, M.G.; French, S.W.; French, B.A.; Seitz, H.K.; Cohen, L.B.; Mueller, S.; Osna, N.A. Alcoholic and non-alcoholic steato-hepatitis. Exp. Mol. Pathol. 2014, 97, 492–510. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Seitz, H.K.; Tuma, P.L.; Osna, N.A.; Casey, C.A.; Karbanda, K.K.; Cohen, L.B.; Malnick, S.D.; Adhikari, R.; Mitra, R.; et al. Alcohol basic and translational research:15th charles lieber—1st Samuel French satellite symposium. Exp. Mol. Patol. 2022, in press. [Google Scholar] [CrossRef]
- Moreau, R. The Pathogenesis of ACLF: The Inflammatory Response and Immune Function. Semin. Liver Dis. 2016, 36, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Ahmad, M.F.; Nagy, L.E.; Tsukamoto, H. Inflammatory pathways in alcoholic steatohepatitis. J. Hepatol. 2019, 70, 249–259. [Google Scholar] [CrossRef]
- Gaul, S.; Leszczynska, A.; Alegre, F.; Kaufmann, B.; Johnson, C.D.; Adams, L.A.; Wree, A.; Damm, G.; Seehofer, D.; Calvente, C.J.; et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. 2021, 74, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.V.; Kroemer, G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef]
- Neuman, M.G.; Maor, Y.; Nanau, R.M.; Melzer, E.; Mell, H.; Opris, M.; Cohen, L.; Malnick, S. Alcoholic Liver Disease: Role of Cytokines. Biomolecules 2015, 5, 2023–2034. [Google Scholar] [CrossRef]
- Neuman, M.G.; Cohen, L.B. Inflammation and Liver Cell Death in Patients with Hepatitis C Viral Infection. Curr. Issues Mol. Biol. 2021, 43, 2022–2035. [Google Scholar] [CrossRef]
- Katz, G.G.; Shear, N.H.; Malkiewicz, I.M.; Valentino, K.; Neuman, M.G. Signaling for ethanol-induced apoptosis and repair in vitro. Clin. Biochem. 2001, 34, 219–227. [Google Scholar] [CrossRef]
- Mehal, W.; Imaeda, A. Cell Death and Fibrogenesis. Semin. Liver Dis. 2010, 30, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Leers, M.P.; Kolgen, W.; Bjorklund, V.; Bergman, T.; Tribbick, G.; Persson, B.; Bjorklund, P. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J. Pathol. 1999, 187, 567–572. [Google Scholar] [CrossRef]
- Lavallard, V.J.; Bonnafous, S.; Patouraux, S.; Saint-Paul, M.-C.; Rousseau, D.; Anty, R.; Le Marchand-Brustel, Y.; Tran, A.; Gual, P. Serum Markers of Hepatocyte Death and Apoptosis Are Non Invasive Biomarkers of Severe Fibrosis in Patients with Alcoholic Liver Disease. PLoS ONE 2011, 6, e17599. [Google Scholar] [CrossRef] [PubMed]
- Bantel, H.; Ruck, P.; Schulze-Osthoff, K. In situ monitoring of caspase activation in hepatobiliary diseases. Cell Death Differ. 2000, 7, 504–505. [Google Scholar] [CrossRef] [PubMed]
- Van Eyken, P.; Desmet, V.J. Cytokeratins and the liver. Liver 1993, 13, 113–122. [Google Scholar] [CrossRef]
- Atkinson, S.R.; Grove, J.I.; Liebig, S.; Astbury, S.; Vergis, N.; Goldin, R.; Quaglia, A. In Severe Alcoholic Hepatitis, Serum Keratin-18 Fragments Are Diagnostic, Prognostic, and Theragnostic Biomarkers. Am. J. Gastroenterol. 2020, 115, 1857–1868. [Google Scholar] [CrossRef]
- Neuman, M.G.; Cohen, L.B.; Malnick, S. Familial non-alcoholic steatohepatitis leading to hepatocellular carcinoma. Chem. Interact. 2020, 323, 109054. [Google Scholar] [CrossRef]
- Neary, J.; Goodwin, S.E.; Cohen, L.B.; Neuman, M.G. Alcohol Misuse Link to POEMS Syndrome in a Patient. Cancers 2017, 9, 129. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver EASL clinical practice guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [CrossRef]
- Antar, R.; Wong, P.; Ghali, P. A meta-analysis of nutritional supplementation for management of hospitalized alcoholic hepatitis. Can. J. Gastroenterol. 2012, 26, 463–467. [Google Scholar] [CrossRef]
- Cabré, E.; Rodríguez-Iglesias, P.; Caballería, J. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: A multicenter randomized trial. Hepatology 2000, 32, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Deltenre, P.; Senterre, C. Intensive enteral nutrition is ineffective for patients with severe alcoholic hepatitis treated with corticosteroids. Gastroenterology 2016, 150, 903–910.e8. [Google Scholar] [CrossRef] [PubMed]

| Acetaldehyde C2H4O | Singlet radical 1O2 | Alkoxyl radical RO. |
| Ethoxy radical CH3CH2OS | Superoxide radical HO.2 | Peroxyl radical ROO• |
| Hydroxyethyl radical CH3C.HOH | Hydrogen peroxide H2O2 | Lipidperoxides |
| Acetyl radical CH3CHO | Hydroxyl radical HO• |
| Acetaldehyde | Chloroform | 1,2-Dibromoethane | Methyl t-butyl ether |
| Sevoflurane | |||
| Acetol | 1-Chloropropane | Diethylether | Methoxyflurane |
| Styrene | |||
| Acetone | Chlorzoxazone | Dimethylformamide | Monochlorobenzene |
| 1,1,1,2-Tetrachloroethane | |||
| Acetaminophen | 1,1-Dichloroethane | Enflurane | 4-Nitrophenol |
| 1,1,2,2-Tetrachloroethane | |||
| Aniline | 1,2-Dichloroethane | Ethanol | Nitrosamines |
| Tetrachloroethylene | |||
| Benzene | 1,1-Dichloroethylene | Ethylbenzene | |
| N-nitrosodimethylamine | Trichloroethylene | ||
| Bromobenzene | cis-1,2-Dichloroethylene | Halothane | n- Pentane |
| Toluol | |||
| n- Butanol | trans-1,2-Dichloroethylene | n-Hexane | Phenol |
| 1,1,1-Trichloroethane | |||
| Caffeine | Dichloromethane | Isoflurane | n-Propanol |
| 1,1,2-Trichloroethane | |||
| Carbon tetrachloride | 1,2-Dichloropropane | Methanol | Propylbenzene |
| Vinylchloride |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuman, M.G.; Seitz, H.K.; Teschke, R.; Malnick, S.; Johnson-Davis, K.L.; Cohen, L.B.; German, A.; Hohmann, N.; Moreira, B.; Moussa, G.; et al. Molecular, Viral and Clinical Features of Alcohol- and Non-Alcohol-Induced Liver Injury. Curr. Issues Mol. Biol. 2022, 44, 1294-1315. https://doi.org/10.3390/cimb44030087
Neuman MG, Seitz HK, Teschke R, Malnick S, Johnson-Davis KL, Cohen LB, German A, Hohmann N, Moreira B, Moussa G, et al. Molecular, Viral and Clinical Features of Alcohol- and Non-Alcohol-Induced Liver Injury. Current Issues in Molecular Biology. 2022; 44(3):1294-1315. https://doi.org/10.3390/cimb44030087
Chicago/Turabian StyleNeuman, Manuela G., Helmut K. Seitz, Rolf Teschke, Stephen Malnick, Kamisha L. Johnson-Davis, Lawrence B. Cohen, Anit German, Nicolas Hohmann, Bernhardo Moreira, George Moussa, and et al. 2022. "Molecular, Viral and Clinical Features of Alcohol- and Non-Alcohol-Induced Liver Injury" Current Issues in Molecular Biology 44, no. 3: 1294-1315. https://doi.org/10.3390/cimb44030087
APA StyleNeuman, M. G., Seitz, H. K., Teschke, R., Malnick, S., Johnson-Davis, K. L., Cohen, L. B., German, A., Hohmann, N., Moreira, B., Moussa, G., & Opris, M. (2022). Molecular, Viral and Clinical Features of Alcohol- and Non-Alcohol-Induced Liver Injury. Current Issues in Molecular Biology, 44(3), 1294-1315. https://doi.org/10.3390/cimb44030087









