An Exploratory In Vivo Study on the Effect of Annurca Apple Extract on Hair Growth in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design
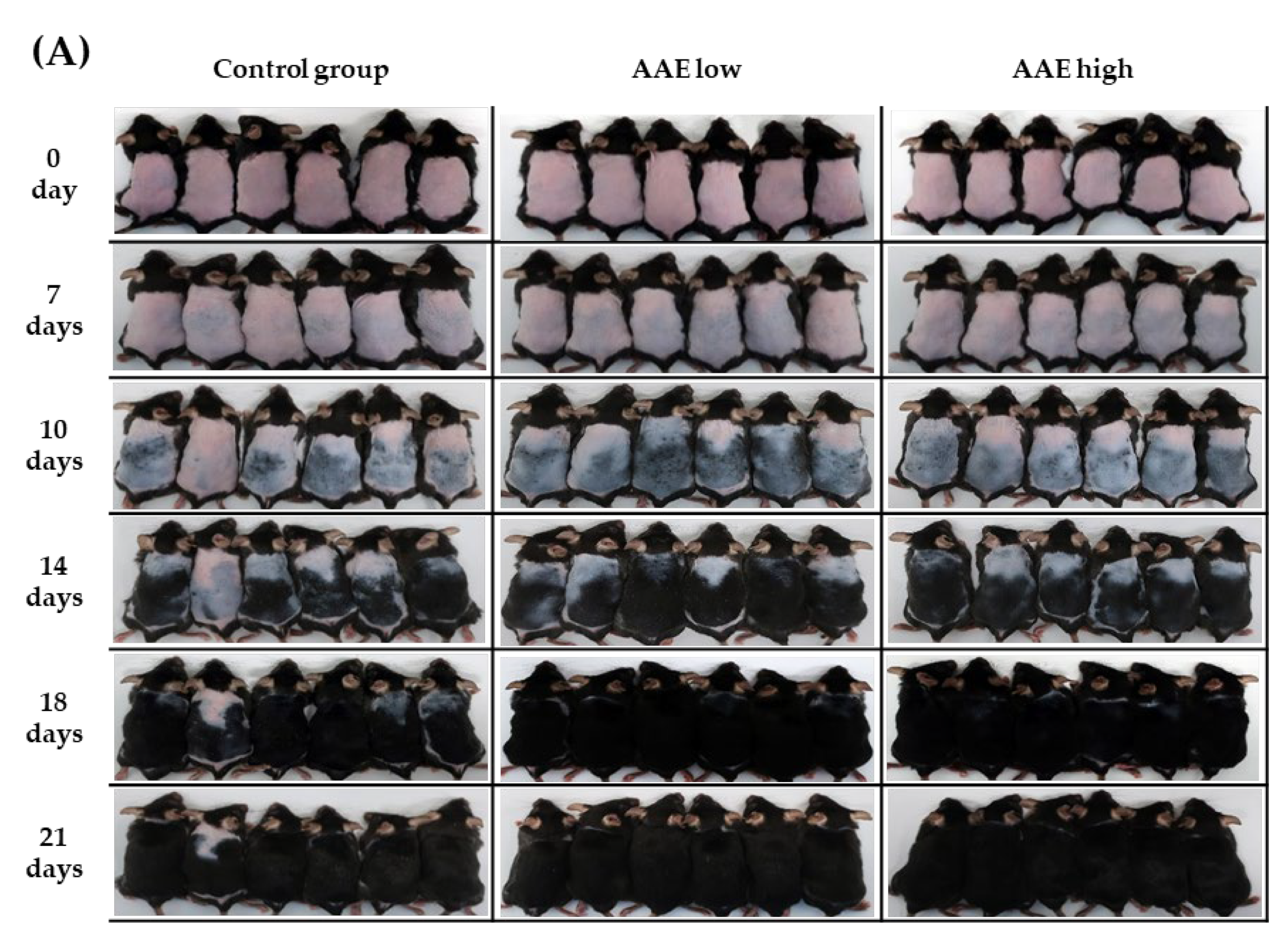
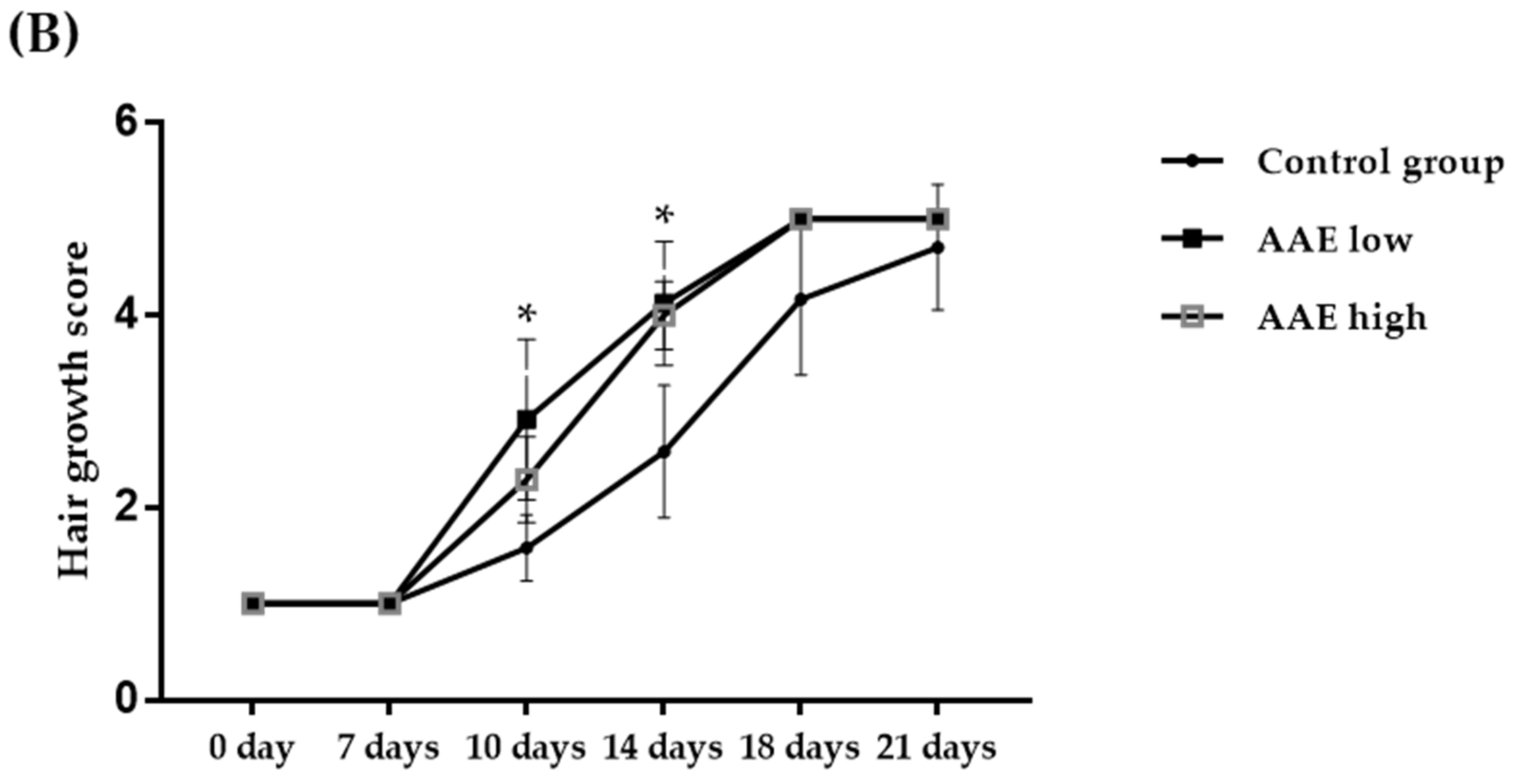
2.3. Visual Analysis of Improvement in Hair Growth
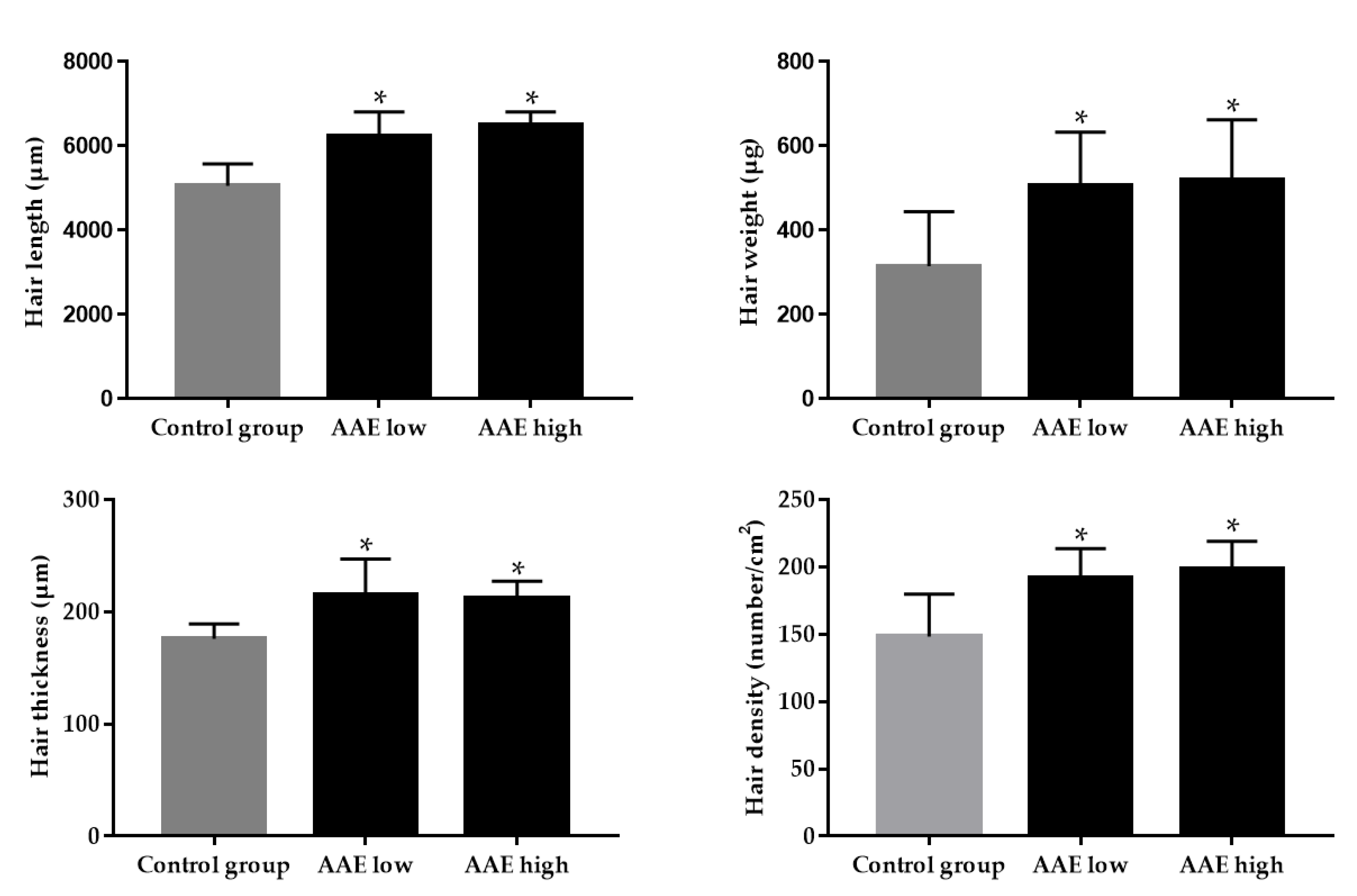
2.4. Measurements of Hair Growth
2.5. Histological Analysis
2.6. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
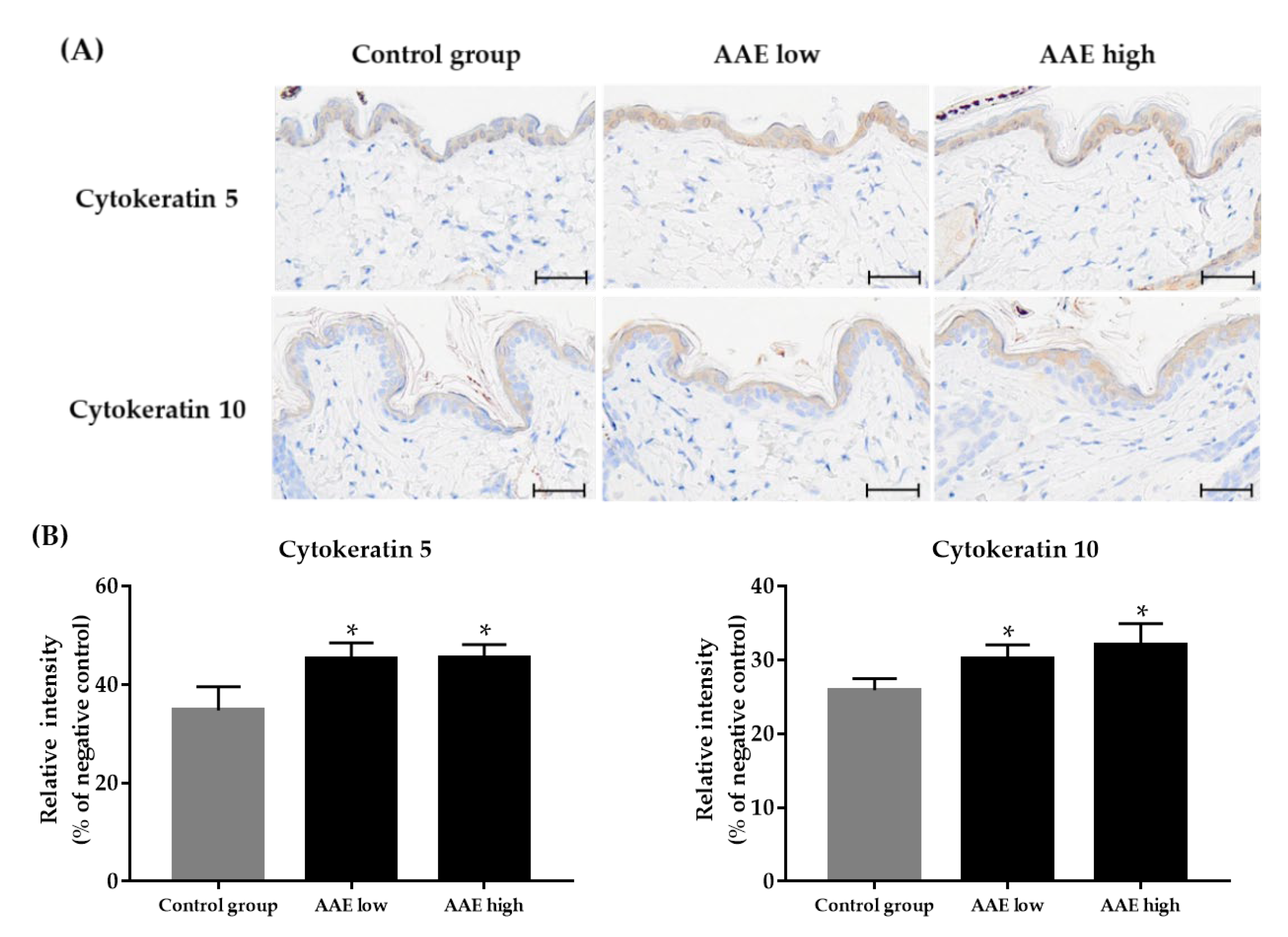
2.7. Immunohistochemistry
2.8. Statistical Analysis
3. Results
3.1. Hair Growth-Promoting Effect of AAE in C57BL/6 Mice
3.2. Effect of AAE on Gene Expression of Growth Factors in the Skin Tissue of C57BL/6 Mice
3.3. Effect of AAE on Type 1 5α-Reductase Activity in the Skin Tissue of C57BL/6 Mice
3.4. Effect of AAE on Expression of Epidermal Cytokeratins in the Skin Tissue of C57BL/6 Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juchaux, F.; Sellathurai, T.; Perrault, V.; Boirre, F.; Delannoy, P.; Bakkar, K.; Albaud, J.; Gueniche, A.; Cheniti, A.; Dal Belo, S.; et al. A combination of pyridine-2, 4-dicarboxylic acid diethyl ester and resveratrol stabilizes hypoxia-inducible factor 1-alpha and improves hair density in female volunteers. Int. J. Cosmet. Sci. 2020, 42, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Clinic, A.; Asper, A.; Mittal, A.; Shome, D.; Parbhoo, D.; Thanzama, J.; Doshi, K.; Sachde, N.; Gaunkar, R.; Kapoor, R.; et al. Evaluation of the safety and effectiveness of intradermal administration of QR678 Neo® hair growth factor formulation: A phase-IV, open-label, single-arm multi-ethnicity clinical trial. J. Cosmet. Dermatol. 2022, 21, 580–589. [Google Scholar] [CrossRef]
- Piccolo, M.; Ferraro, M.G.; Maione, F.; Maisto, M.; Stornaiuolo, M.; Tenore, G.C.; Santamaria, R.; Irace, C.; Novellino, E. Induction of Hair Keratins Expression by an Annurca Apple-Based Nutraceutical Formulation in Human Follicular Cells. Nutrients 2019, 11, 3041. [Google Scholar] [PubMed] [Green Version]
- Badolati, N.; Sommella, E.; Riccio, G.; Salviati, E.; Heintz, D.; Bottone, S.; Di Cicco, E.; Dentice, M.; Tenore, G.; Campiglia, P.; et al. Annurca Apple Polyphenols Ignite Keratin Production in Hair Follicles by Inhibiting the Pentose Phosphate Pathway and Amino Acid Oxidation. Nutrients 2018, 10, E1406. [Google Scholar] [CrossRef] [Green Version]
- Bassino, E.; Gasparri, F.; Munaron, L. Protective Role of Nutritional Plants Containing Flavonoids in Hair Follicle Disruption: A Review. Int. J. Mol. Sci. 2020, 21, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamimura, A.; Takahashi, T. Procyanidin B-2, extracted from apples, promotes hair growth: A laboratory study. Br. J. Dermatol. 2002, 146, 41–51. [Google Scholar] [CrossRef]
- Kamimura, A.; Takahashi, T.; Watanabe, Y. Investigation of topical application of procyanidin B-2 from apple to identify its potential use as a hair growing agent. Phytomedicine 2000, 7, 529–536. [Google Scholar] [CrossRef]
- Takahashi, T.; Kamiya, T.; Hasegawa, A.; Yokoo, Y. Procyanidin oligomers selectively and intensively promote proliferation of mouse hair epithelial cells in vitro and activate hair follicle growth in vivo. J. Investig. Derm. 1999, 112, 310–316. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Avino, M.; Santamaria, R.; Irace, C.; Piccolo, M.; Maisto, M.; Novellino, E. Annurca Apple Nutraceutical Formulation Enhances Keratin Expression in a Human Model of Skin and Promotes Hair Growth and Tropism in a Randomized Clinical Trial. J. Med. Food 2018, 21, 90–103. [Google Scholar] [CrossRef] [Green Version]
- Hyun, J.; Im, J.; Kim, S.-W.; Kim, H.Y.; Seo, I.; Bhang, S.H. Morus alba Root Extract Induces the Anagen Phase in the Human Hair Follicle Dermal Papilla Cells. Pharmaceutics 2021, 13, 1155. [Google Scholar] [CrossRef]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwabuchi, T.; Ogura, K.; Tamba, K.; Tsunekawa, Y.; Sugano, M.; Hagiwara, K.; Kiso, A. Cepharanthine induces the proliferation of human dermal papilla cells and stimulates vascular endothelial growth factor expression through increased intracellular calcium mobilization and hypoxia-inducible factor activation. Clin. Exp. Dermatol. 2021, 46, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, D.M.; Ring, B.D.; Yanagihara, D.; Benson, W.; Wiemann, B.; Starnes, C.O.; Pierce, G.F. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am. J. Pathol. 1995, 147, 145–154. [Google Scholar]
- Gentile, P.; Scioli, M.G.; Bielli, A.; De Angelis, B.; De Sio, C.; De Fazio, D.; Ceccarelli, G.; Trivisonno, A.; Orlandi, A.; Cervelli, V.; et al. Platelet-Rich Plasma and Micrografts Enriched with Autologous Human Follicle Mesenchymal Stem Cells Improve Hair Re-Growth in Androgenetic Alopecia. Biomolecular Pathway Analysis and Clinical Evaluation. Biomedicines 2019, 7, 27. [Google Scholar] [PubMed] [Green Version]
- Han, M.; Li, C.; Zhang, C.; Song, C.; Xu, Q.; Liu, Q.; Guo, J.; Sun, Y. Single-cell transcriptomics reveals the natural product Shi-Bi-Man promotes hair regeneration by activating the FGF pathway in dermal papilla cells. Phytomedicine 2022, 104, 154260. [Google Scholar] [CrossRef]
- Booth, C.; Potten, C.S. Keratinocyte Growth Factor Increases Hair Follicle Survival Following Cytotoxic Insult. J. Investig. Dermatol. 2000, 114, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Danilenko, D.M.; Ring, B.D.; Pierce, G.F. Growth factors and cytokines in hair follicle development and cycling: Recent insights from animal models and the potentials for clinical therapy. Mol. Med. Today 1996, 2, 460–467. [Google Scholar] [CrossRef]
- Seo, H.-S.; Lee, D.-J.; Chung, J.-h.; Lee, C.-H.; Kim, H.R.; Kim, J.E.; Kim, B.J.; Jung, M.H.; Ha, K.-T.; Jeong, H.-S. Hominis placenta facilitates hair re-growth by upregulating cellular proliferation and expression of fibroblast growth factor-7. BMC Complement. Altern. Med. 2016, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Erdogan, S.; Hwang, D.; Hwang, S.; Han, E.H.; Lim, Y.-H. Bee Venom Promotes Hair Growth in Association with Inhibiting 5α-Reductase Expression. Biol. Pharm. Bull. 2016, 39, 1060–1068. [Google Scholar] [CrossRef] [Green Version]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, M.G.; Piccolo, M.; Pezzella, A.; Guerra, F.; Maione, F.; Tenore, G.C.; Santamaria, R.; Irace, C.; Novellino, E. Promelanogenic Effects by an Annurca Apple-Based Natural Formulation in Human Primary Melanocytes. Clin. Cosmet. Investig. Derm. 2021, 14, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Barker, N. The canonical Wnt/β-catenin signalling pathway. Wnt Signal. 2008, 468, 5–15. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.I.; Ham, S.; Lee, S.G.; Jung, I.; Suk, J.; Yoo, J.; Choi, S.-Y.; Lee, J.H. An Exploratory In Vivo Study on the Effect of Annurca Apple Extract on Hair Growth in Mice. Curr. Issues Mol. Biol. 2022, 44, 6280-6289. https://doi.org/10.3390/cimb44120428
Lee YI, Ham S, Lee SG, Jung I, Suk J, Yoo J, Choi S-Y, Lee JH. An Exploratory In Vivo Study on the Effect of Annurca Apple Extract on Hair Growth in Mice. Current Issues in Molecular Biology. 2022; 44(12):6280-6289. https://doi.org/10.3390/cimb44120428
Chicago/Turabian StyleLee, Young In, Seoyoon Ham, Sang Gyu Lee, Inhee Jung, Jangmi Suk, Jinhee Yoo, Su-Young Choi, and Ju Hee Lee. 2022. "An Exploratory In Vivo Study on the Effect of Annurca Apple Extract on Hair Growth in Mice" Current Issues in Molecular Biology 44, no. 12: 6280-6289. https://doi.org/10.3390/cimb44120428
APA StyleLee, Y. I., Ham, S., Lee, S. G., Jung, I., Suk, J., Yoo, J., Choi, S.-Y., & Lee, J. H. (2022). An Exploratory In Vivo Study on the Effect of Annurca Apple Extract on Hair Growth in Mice. Current Issues in Molecular Biology, 44(12), 6280-6289. https://doi.org/10.3390/cimb44120428





