Abstract
Esculetin is an antioxidant and anti-inflammatory compound derived from coumarin. Oxidative stress can cause overproduction of reactive oxygen species (ROS), which can lead to the development of chronic kidney failure. In this study, human embryonic kidney 293 (HEK293) cells were treated with tert-butyl hydroperoxide (t-BHP) to determine the antioxidant effects of esculetin. HEK293 cells were treated with t-BHP to validate changes in cell viability, ROS production, and apoptosis, and then treated with esculetin to evaluate the changes. Changes in mRNA and protein levels were analyzed using a proteome kit, PCR, and Western blotting. Esculetin improved HEK293 cell viability and reduced apoptosis caused by t-BHP-induced oxidative stress. At the mRNA and protein levels, esculetin decreased pro-apoptotic factor expression as well as increased anti-apoptotic factor expression. The antioxidant efficacy of esculetin was validated when it inhibited the apoptosis caused by t-BHP-induced oxidative stress in HEK293 cells.
1. Introduction
Kidneys play an important role in blood pressure and volume regulation by maintaining electrolyte balance in body fluids and removing waste products from the body, thereby maintaining homeostasis. However, renal function decline can occur as a result of diabetic nephropathy (with symptoms such as glomerular hypertrophy, mesangial expansion, and basement membrane thickening) [1,2], kidney dysfunction (due to obesity-induced hypertension) [3], and chronic kidney disease-induced renal failure [4].
Reactive oxygen species (ROS) generated excessively as a result of oxidative stress is linked to kidney dysfunction diseases, such as diabetic nephropathy [5], chronic kidney disease [6], and hypertension [7]. It also causes glomerular podocyte loss [8] and endoplasmic reticulum (ER) stress injury to human renal glomerular endothelial cells [9], both of which contribute to kidney dysfunction. In addition, one study found that oxidative stress increases the proliferation of mesangial cells [10] and is linked to glomerulonephritis [11].
Numerous antioxidant drugs that inhibit ROS generation have been studied to treat kidney dysfunction [12,13]. Natural products and their derivatives are gaining popularity as antioxidant drugs. Because of their chemical structure, drugs derived from natural products have greater chemical diversity, higher stereochemical content, and lower hydrophobicity, making them ideal drugs and sources of drugs [14,15,16,17].
In animal models of acute kidney injury and renal cells, antioxidants have shown to alleviate ROS-induced oxidative damage [18,19]. In addition, antioxidants have been shown in in vitro and in vivo experiments to prevent oxidative stress caused by high glucose levels in podocytes [20] as well as in clinical studies to treat diabetic nephropathy [21].
Coumarin is a naturally occurring benzopyrone that acts as an antioxidant and ROS scavenger. Esculetin, a coumarin-derived compound, is a natural plant product found in Atremisia capillaris, Citrus limonia, and Euphorbia lathyris, as well as Cortex Fraxini. It is a synthetic drug with antioxidant, antitumor, and anti-inflammatory properties. Because esculetin has higher antioxidant activity than other coumarins, studies on its use as an antioxidant to combat ROS are being conducted [22,23,24,25,26,27].
Using tert-butyl hydroperoxide (t-BHP) as an oxidative stress inducer, we investigated the antioxidant efficacy of esculetin against renal dysfunction in a human embryonic kidney 293 (HEK293) cell injury model.
2. Materials and Methods
2.1. Cell Culture and MTT Assay
After growing HEK293 to 70 percent confluence using DMEM (WELGENE, Daegu, Republic of Korea) supplemented with 10% FBS (WELGENE) in 96-well plates, esculetin and t-BHP were added at the indicated concentrations for the indicated times. To evaluate cell viability, MTT reagent was used (Sigma Aldrich, St. Louis, MO, USA).
2.2. Measurement of ROS
2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA; Sigma Aldrich, St. Louis, MO, USA) was used to detect intracellularly active oxygen. Following 3 h of treatment with t-BHP and esculetin, HEK293 cells were treated with 3 μM DCF-DA for 30 min and washed with HBSS (WELGENE). The DCF-DA-positive cells were validated using a fluorescence microscope (BX51, Oylmpus, Tokyo, Japan). Fluorescence intensity was measured by imageJ software (NIH, Bethesda, MD, USA)
2.3. Apoptosis Assay
A one-step TUNEL staining kit (Promega, Madison, WI, USA) was used for the detection of apoptotic cells. Apoptotic cells were assessed under a fluorescence microscope. In this assessment, TUNEL-positive apoptotic cells were identified using green fluorescence emitted by the cells under fluorescence microscope (BX51, Oylmpus, Tokyo, Japan). ImageJ software (NIH, Bethesda, MD, USA) was used to count TUNEL-positive cells.
2.4. Proteome Assay Kit
The apoptosis-related signaling pathways were analyzed using Proteome Profiler™ (R&D Systems, Wiesbaden, Germany). Protein expression levels were determined using an image analyzer (ATTO, Tokyo, Japan).
2.5. Real-Time PCR
TRIzolTM reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA from the collected cells. The primers used were as follows: Bax, 5′-AAA CTG GTG CTC AAG GCC C-3′ and 5′-CTT CAG TGA CTC GGC CA GG-3′; Bcl2, 5′-GAT AAC GGA GGC TGG GAT GC-3′ and 5′-TCA CTT GTG GCC CAG ATA GG-3′; Caspase-3, 5′-TTG GAC TGT GGG ATT GAG ACG-3′ and 5′-CGC TGC ACA AAG TGA CTG GA-3′; PARP, 5′-GCT TCA GCC TCC TTG CTA CA-3′ and 5′-TTC GCC ACT TCA TCC ACT CC-3′; β-actin, 5′-CTC ACC CTG AAG TAC CCC ATC-3′ and 5′-GGA TAG CAC AGC CTG GAT AGC A-3′. PCRs were performed using a MiniOpticon™ Real-Time PCR System (Bio-Rad, Hercules, CA, USA) and 2xSYBR® Green PCR Master Mix (Enzo, New York, NY, USA). The results were normalized to β-actin levels. Three independent experiments were conducted in triplicate.
2.6. Western Blot Analysis
Western blotting was used to evaluate the expression levels of apoptosis-related proteins in HEK293 cells. The antibodies were anti-Bax, anti-Bcl2, anti-PARP, and anti-caspase-3 (Abcam, Waltham, MA, USA). Finally, a Western blotting detection kit (SuperSignal™ West Dura Extended Duration Substrate, ThermoFisher Scientific, Waltham, MA, USA) was used to observe the protein bands after treatment with an HRP-conjugated secondary antibody (Advansta, San Jose, CA, USA). Protein expression levels were determined using an image analyzer (ATTO, Tokyo, Japan).
2.7. Statistical Analysis
The mean and standard deviation are calculated from the data. Prism 8.0 (GraphPad, San Diego, CA, USA) was used to perform an ANOVA followed by a Tukey’s post-hoc test.
3. Results
3.1. Esculetin Inhibits t-BHP-Induced HEK293 Cell Injury
The effects of esculetin and t-BHP on HEK293 cells were confirmed using an MTT assay. Treatment with esculetin alone confirmed the concentration that did not affect cell viability, and there was no effect up to 50 μM (Figure 1A). t-BHP was found to decrease cell viability in a concentration-dependent manner, with cell viability being approximately 40% at 300 µM (Figure 1B). In addition, the combination of t-BHP and esculetin was found to increase cell viability in a concentration-dependent manner up to 50 μM (Figure 1C).
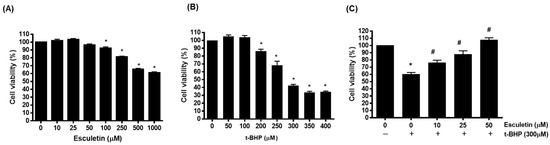
Figure 1.
Effect of esculetin on t-BHP-induced HEK293 cell injury. The cell viability of HEK293 cells exposed to different concentrations of esculetin (A), t-BHP (B) and t-BHP with esculetin (C). In the bar graphs, the values represent means ± SD, n = 5. * p < 0.05 vs. untreated control group, # p < 0.05 vs. t-BHP-treated control group.
3.2. Esculetin Inhibits t-BHP-Induced ROS Generation in HEK293 Cells
The effects of esculetin and t-BHP on HEK293 cells were confirmed using a DCF-DA assay. It was confirmed that t-BHP at 100 µM concentration promoted ROS generation in HEK293 cells, whereas esculetin inhibited it in a dose-dependent manner (Figure 2).
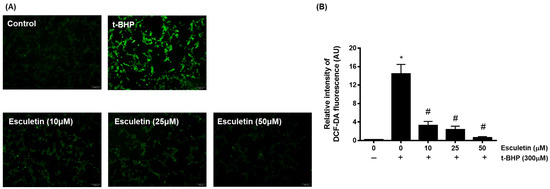
Figure 2.
Effect of esculetin on t-BHP-induced ROS generation in HEK293 cells. (A) The changes in ROS levels in HEK253 cells exposed to t-BHP with esculetin were detected using DCFH-DA dye. (B) In the bar graphs, the values represent means ± SD, n = 5. * p < 0.05 vs. untreated control group, # p < 0.05 vs. t-BHP-treated group.
3.3. Esculetin Inhibits t-BHP-Induced Apoptosis of HEK293 Cells
TUNEL staining was used to confirm apoptosis by detecting cell death-associated DNA fragmentation by endonucleases [28]. It was confirmed that t-BHD promoted apoptosis, whereas esculetin inhibited it in a dose-dependent manner (Figure 3).
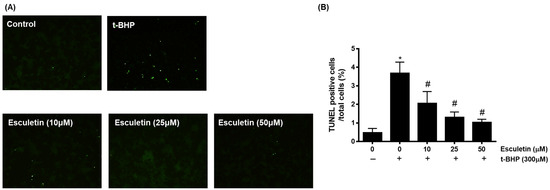
Figure 3.
Effect of esculetin on t-BHP-induced apoptosis in HEK293 cells. (A) Apoptosis of HEK253 cells exposed to t-BHP with esculetin were detected using TUNEL staining. (B) In the bar graphs, the values represent means ± SD, n = 5. * p < 0.05 vs. untreated control group, # p < 0.05 vs. t-BHP-treated group.
3.4. Esculetin Regulates Apoptosis-Related Signaling Pathways in HEK293 Cells
The Proteome Profiler Human Apoptosis Array Kit was used to validate the enriched signaling pathways. Cytochrome c, cleaved caspase-3, Bax, and phospho-Rad17 levels were increased by t-BHP and decreased by 10 µM esculetin (Figure 4).
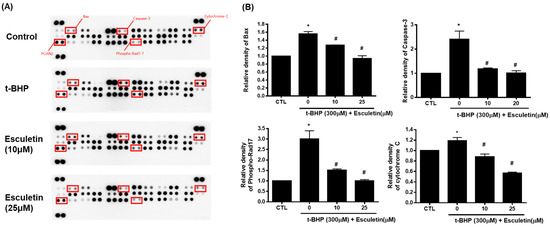
Figure 4.
Effect of esculetin on apoptosis-related signaling pathways in HEK293 cells. (A) Apoptosis-related protein assay. (B) In the bar graphs, the values represent means ± SD, n = 5. * p < 0.05 vs. untreated control group, # p < 0.05 vs. t-BHP-treated group.
3.5. Esculetin Regulates the Expression of Apoptosis-Related mRNA in HEK293 Cells
The mRNA expression of t-BHP and esculetin-affected apoptosis markers in HEK293 cells were investigated to validate the results of the protein array. t-BHP increased and decreased the mRNA levels of pro-apoptosis markers (Bax, PARP, and Caspase-3) and anti-apoptosis marker (Bcl-2), respectively. Esculetin was confirmed to decrease the mRNA expression of apoptosis markers in a dose-dependent manner (Figure 5).
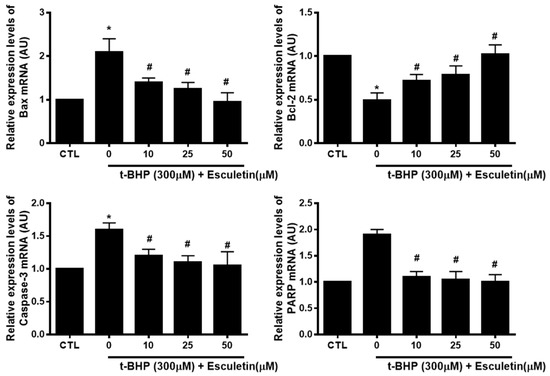
Figure 5.
Effect of esculetin on the expression of apoptosis-related mRNA in HEK293 cells. The mRNA expression levels of Bax, bcl-2, caspase-3 and PARP. In the bar graphs, the values represent means ± SD, n = 5. * p < 0.05 vs. untreated control group, # p < 0.05 vs. t-BHP-treated group.
3.6. Esculetin Regulates the Expression of Apoptosis-Related Proteins in HEK293 Cells
The effects of t-BHP and esculetin on HEK293 cells were also confirmed at the protein level. t-BHP increased and decreased the protein expression of pro-apoptosis markers (Bax, PARP, and Caspase-3) and anti-apoptosis marker (Bcl-2), respectively. Esculetin was confirmed to decrease the protein expression of apoptosis markers in a dose-dependent manner (Figure 6).
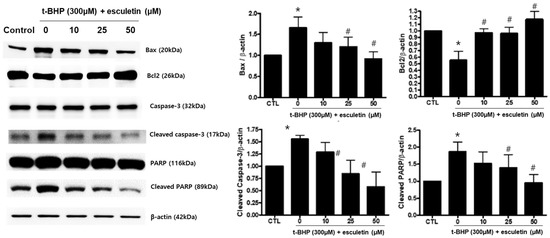
Figure 6.
Effect of esculetin on the expression of apoptosis-related proteins in HEK293 cells. The protein expression levels of Bax, bcl-2, cleaved caspase-3 and cleaved PARP. In the bar graphs, the values represent means ± SD, n = 5. * p < 0.05 vs. untreated control group, # p < 0.05 vs. t-BHP-treated group.
4. Discussion
It was examined in the present study whether esculetin inhibits apoptosis induced by oxidative stress in HEK294 cells. There are several major pathogenic mechanisms involved in chronic kidney disease, such as damaged DNA repair systems, inflammation, increased apoptosis, and increased oxidative stress.
There are very low levels of ROS generated from the mitochondria of renal cells, but these levels can be significantly increased in response to certain factors, such as angiotensin II, TNF-α, LDL, and NADPH oxidase, as well as pathological conditions, such as diabetes [5,29].
Due to the presence of simian virus 40 large T antigen, HEK293 cells express a very high level of protein [30]. Human renal epithelial cells are frequently used to study oxidative stress, renal function, and renal diseases resulting from injury to renal epithelial cells [31].
Organic peroxide t-BHP induces oxidative stress via the peroxyl and alkoxyl radical pathways as well as the glutathione peroxidase pathway via cytochrome P450. It is generally used to investigate the effects of oxidative stress on cells and tissues. It has been used instead of H2O2 in oxidative stress studies [32,33]. In addition, t-BHP, a pro-oxidant compound, induces the production of free radicals via cytochrome P-450 and induces the generation of OH-radicals, such as lipid peroxides. ROS also inhibits cell proliferation by promoting oxidative stress-induced apoptosis [32,34,35,36].
Esculetin has been shown to be a potent antioxidant in various cells [37]. It reduces oxidative stress by inhibiting neutrophil-dependent superoxide anion production and lipid peroxidation, and by scavenging free radicals [38,39,40,41]. It has been demonstrated that esculetin reduces liver lesions induced by t-BHP, including hepatocellular edema, leukocyte infiltration, and necrosis in rats [41]. A hamster lung fibroblast cell (V79-4) treated with esculetin prevented lipid peroxidation, protein carbonylation, and DNA damage caused by H2O2 [42]. In oxidation-induced H9c2 cells, after esculetin treatment, Bcl-2 expression is up-regulated and Bax expression is down-regulated. Moreover, it inhibited the activity of caspase-3. As a result, esculetin improved viability in hypoxia/reoxygenation-stimulated H9c2 cells, suppressed oxidative stress, and inhibited cell death [43]. Furthermore, esculetin exerts anti-apoptosis activity in the mouse model of middle cerebral artery occlusion by up-regulating Bcl-2 expression and down-regulating Bax expression and downstream cleaving caspase-3 [44]. Based on these previous results, we confirmed that esculetin restores the proliferation of HEK293 cells that have been subjected to t-BHP-induced oxidative stress.
ROS-induced oxidative stress promotes endothelial changes that induce vascular remodeling (by promoting inflammation and enhancing cytokine production and expression of surface adhesion molecules) [45,46], induce apoptosis (via angiotensin II induction in renal proximal tubule epithelial cells (RPTECs) and mesangial cells), induce podocyte autophagy, and enhance ROS generation by promoting feedback loops [47,48,49]. Here, it was demonstrated that esculetin inhibits t-BHP-induced apoptosis of HEK293 cells. In addition, alterations in the apoptotic pathway caused by t-BHP-induced oxidative stress in HEK293 cells were confirmed at the mRNA and protein levels.
Anti-apoptotic proteins Bcl-2 and Bcl-XL inhibit cytochrome c release, whereas pro-apoptotic proteins, such as Bcl-2-associated X protein (Bax), Bcl-2 homologue antagonist/killer (Bak), and BH3 interacting-domain death agonist (Bid) promote cytochrome c release. The binding of cytochrome c to deoxyadenosine triphosphate (dATP) activates apoptotic protease activating factor (Apaf)-1 and procaspase-9. As a result, apoptosis is induced by caspase-3 activation [50,51,52].
Cytochrome c release is inhibited by anti-apoptotic proteins Bcl-2 and Bcl-XL, but promoted by pro-apoptotic proteins, such as Bax, Bak, and Bid. When cytochrome c binds deoxyadenosine triphosphate (dATP), apoptotic protease activating factor (Apaf)-1 and procaspase-9 are activated. Consequently, caspase-3 activation induces apoptosis [50,51,52].
Caspases are cysteine proteases that destroy cellular proteins and cause cell death. These caspase enzymes are classified as either initiators or effectors. Caspases-2,-8,-9, and-10 are apoptosis initiator caspases, while caspase-3,-6, and 7 are apoptosis effector caspases. In many tissues, caspase-3 promotes DNA fragmentation and cell death, and PARP-1 maintains genomic integrity and DNA repair. The caspase-3 enzyme cleaves PARP-1 and inactivates it during apoptosis. [53,54,55,56].
Studies have shown that the phenolic compound esculetin is an excellent antioxidant capable of reducing oxidative damage [57,58]. Another study found that esculetin reduced the oxidative stress marker increased by t-BHP in rat hepatocytes [41]. Furthermore, esculetin reduced apoptosis caused by H2O2-induced oxidative stress in C2C12 mouse myoblasts [59]. Similarly, in this study, esculetin inhibited pro-apoptotic proteins Bax, Caspase-3, and PARP-1, while activating anti-apoptotic protein Bcl2 in oxidative stress-induced apoptosis.
Therefore, esculetin was confirmed to inhibit apoptosis in HEK293 cells by reducing the t-BHP-induced oxidative stress.
As a result, esculetin mediated the cytoprotective effect of HEK293 cells under t-BHP-induced oxidative stress and reduced apoptosis, suggesting that it could be used as an antioxidant to alleviate renal dysfunction. However, while the efficacy of esculetin was demonstrated in vitro in this study, in vivo conditions could be altered by more complex mechanisms, necessitating additional in vivo studies.
Author Contributions
W.K.J. performed the experiments and wrote the manuscript; S.-B.P., H.Y.Y. and Y.H.K. performed the experiments and analyzed the data; J.K. designed and supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) funded by the Ministry of Agriculture, Food and Rural Affairs (grant number 1210400202HD020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qi, C.; Mao, X.; Zhang, Z.; Wu, H. Classification and Differential Diagnosis of Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 8637138. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Jo, K.; Kim, J.S.; Pyo, M.K.; Kim, J. GS-E3D, a new pectin lyase-modified red ginseng extract, inhibited diabetes-related renal dysfunction in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2017, 17, 430. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity, kidney dysfunction and hypertension: Mechanistic links. Nat. Rev. Nephrol. 2019, 15, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.D.; Blakeman, T. Chronic kidney disease: Identification and management in primary care. Pragmat. Obs. Res. 2016, 7, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.V.; Gao, S.; Li, H.; Goligorsky, M.S. Hyperglycemic switch from mitochondrial nitric oxide to superoxide production in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2130–H2139. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Loperena, R.; Harrison, D.G. Oxidative Stress and Hypertensive Diseases. Med. Clin. N. Am. 2017, 101, 169–193. [Google Scholar] [CrossRef]
- Zhou, L.L.; Hou, F.F.; Wang, G.B.; Yang, F.; Xie, D.; Wang, Y.P.; Tian, J.W. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int. 2009, 76, 1148–1160. [Google Scholar] [CrossRef]
- Liang, X.; Duan, N.; Wang, Y.; Shu, S.; Xiang, X.; Guo, T.; Yang, L.; Zhang, S.; Tang, X.; Zhang, J. Advanced oxidation protein products induce endothelial-to-mesenchymal transition in human renal glomerular endothelial cells through induction of endoplasmic reticulum stress. J. Diabetes Its Complicat. 2016, 30, 573–579. [Google Scholar] [CrossRef]
- Zhao, D.; Guo, J.; Liu, L.; Huang, Y. Rosiglitazone attenuates high glucose-induced proliferation, inflammation, oxidative stress and extracellular matrix accumulation in mouse mesangial cells through the Gm26917/miR-185-5p pathway. Endocr. J. 2021, 68, 751–762. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, H.; Hu, J.; Zhao, X. Rhein Inhibits NF-kappaB Signaling Pathway to Alleviate Inflammatory Response and Oxidative Stress of Rats with Chronic Glomerulonephritis. Appl. Bionics Biomech. 2022, 2022, 9671759. [Google Scholar] [CrossRef]
- Ostergaard, J.A.; Cooper, M.E.; Jandeleit-Dahm, K.A.M. Targeting oxidative stress and anti-oxidant defence in diabetic kidney disease. J. Nephrol. 2020, 33, 917–929. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Stratton, C.F.; Newman, D.J.; Tan, D.S. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorg. Med. Chem. Lett. 2015, 25, 4802–4807. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Dennis, J.M.; Witting, P.K. Protective Role for Antioxidants in Acute Kidney Disease. Nutrients 2017, 9, 718. [Google Scholar] [CrossRef]
- Khazim, K.; Gorin, Y.; Cavaglieri, R.C.; Abboud, H.E.; Fanti, P. The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am. J. Physiol. Renal. Physiol. 2013, 305, F691–F700. [Google Scholar] [CrossRef]
- Kandhare, A.D.; Mukherjee, A.; Bodhankar, S.L. Antioxidant for treatment of diabetic nephropathy: A systematic review and meta-analysis. Chem. Biol. Interact. 2017, 278, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Jang, J.Y.; Shim, J.H.; Myung, P.K.; Chae, J.I. Esculetin, a Coumarin Derivative, Exhibits Anti-proliferative and Pro-apoptotic Activity in G361 Human Malignant Melanoma. J. Cancer Prev. 2015, 20, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Masamoto, Y.; Ando, H.; Murata, Y.; Shimoishi, Y.; Tada, M.; Takahata, K. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Biosci. Biotechnol. Biochem. 2003, 67, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anticancer Agents 2005, 5, 29–46. [Google Scholar] [CrossRef]
- Liang, C.; Ju, W.; Pei, S.; Tang, Y.; Xiao, Y. Pharmacological Activities and Synthesis of Esculetin and Its Derivatives: A Mini-Review. Molecules 2017, 22, 387. [Google Scholar] [CrossRef]
- Rubio, V.; García-Pérez, A.I.; Tejedor, M.C.; Herráez, A.; Diez, J.C. Esculetin Neutralises Cytotoxicity of t-BHP but Not of H(2)O(2) on Human Leukaemia NB4 Cells. Biomed. Res. Int. 2017, 2017, 9491045. [Google Scholar] [CrossRef]
- Moore, C.L.; Savenka, A.V.; Basnakian, A.G. TUNEL Assay: A Powerful Tool for Kidney Injury Evaluation. Int. J. Mol. Sci. 2021, 22, 412. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Pedraza-Chaverri, J. Mitochondrial Redox Signaling and Oxidative Stress in Kidney Diseases. Biomolecules 2021, 11, 1144. [Google Scholar] [CrossRef]
- Iuchi, K.; Oya, K.; Hosoya, K.; Sasaki, K.; Sakurada, Y.; Nakano, T.; Hisatomi, H. Different morphologies of human embryonic kidney 293T cells in various types of culture dishes. Cytotechnology 2020, 72, 131–140. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, Y.; Wang, Y.; Lei, Q.; Zhao, X.; Yi, R.; Zhang, X. Improvement of Flavonoids in Lemon Seeds on Oxidative Damage of Human Embryonic Kidney 293T Cells Induced by H2O2. Oxid. Med. Cell. Longev. 2020, 2020, 3483519. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, H.; Sun, W.; Liu, K.; Lu, J.J.; Chen, X. Tert-butyl hydroperoxide (t-BHP) induced apoptosis and necroptosis in endothelial cells: Roles of NOX4 and mitochondrion. Redox Biol. 2017, 11, 524–534. [Google Scholar] [CrossRef]
- Kučera, O.; Endlicher, R.; Roušar, T.; Lotková, H.; Garnol, T.; Drahota, Z.; Cervinková, Z. The effect of tert-butyl hydroperoxide-induced oxidative stress on lean and steatotic rat hepatocytes in vitro. Oxid. Med. Cell. Longev. 2014, 2014, 752506. [Google Scholar] [CrossRef]
- Pober, J.S.; Min, W.; Bradley, J.R. Mechanisms of endothelial dysfunction, injury, and death. Annu. Rev. Pathol. 2009, 4, 71–95. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Liu, T.-J.; Lai, H.-C. Pathobiological Mechanisms of Endothelial Dysfunction Induced by tert-Butyl Hydroperoxide via Apoptosis, Necrosis and Senescence in a Rat Model. Int. J. Med. Sci. 2020, 17, 368–382. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.I.; Jang, M.; Namkoong, S.; Park, R.; Ju, H.; Choi, I.; Oh, W.K.; Park, J. Conessine Interferes with Oxidative Stress-Induced C2C12 Myoblast Cell Death through Inhibition of Autophagic Flux. PLoS ONE 2016, 11, e0157096. [Google Scholar] [CrossRef]
- Sriset, Y.; Chatuphonprasert, W.; Jarukamjorn, K. Optimized models of xenobiotic-induced oxidative stress in HepG2 cells. Trop. J. Pharm. Res. 2019, 18, 1001. [Google Scholar] [CrossRef]
- Tien, Y.C.; Liao, J.C.; Chiu, C.S.; Huang, T.H.; Huang, C.Y.; Chang, W.T.; Peng, W.H. Esculetin ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. Int. J. Mol. Sci. 2011, 12, 4053–4067. [Google Scholar] [CrossRef]
- Turkekul, K.; Colpan, R.D.; Baykul, T.; Ozdemir, M.D.; Erdogan, S. Esculetin Inhibits the Survival of Human Prostate Cancer Cells by Inducing Apoptosis and Arresting the Cell Cycle. J. Cancer Prev. 2018, 23, 10–17. [Google Scholar] [CrossRef]
- Park, S.S.; Park, S.K.; Lim, J.H.; Choi, Y.H.; Kim, W.J.; Moon, S.K. Esculetin inhibits cell proliferation through the Ras/ERK1/2 pathway in human colon cancer cells. Oncol. Rep. 2011, 25, 223–230. [Google Scholar]
- Lin, W.L.; Wang, C.J.; Tsai, Y.Y.; Liu, C.L.; Hwang, J.M.; Tseng, T.H. Inhibitory effect of esculetin on oxidative damage induced by t-butyl hydroperoxide in rat liver. Arch. Toxicol. 2000, 74, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Chae, S.W.; Kang, S.S.; Lee, K.H.; Kang, H.K.; et al. Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta Pharmacol. Sin. 2008, 29, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, C.; Ma, Q.; Chen, S. Esculetin inhibits oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Biochem. Biophys. Res. Commun. 2018, 501, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pei, A.; Chen, J.; Yu, H.; Sun, M.L.; Liu, C.F.; Xu, X. A natural coumarin derivative esculetin offers neuroprotection on cerebral ischemia/reperfusion injury in mice. J. Neurochem. 2012, 121, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Shah, A.M. Endothelial cell superoxide generation: Regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1014–R1030. [Google Scholar] [CrossRef]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Reddy, K.; Radhakrishanan, N.; Franki, N.; Ding, G.; Singhal, P.C. Angiotensin II induces apoptosis in renal proximal tubular cells. Am. J. Physiol. Ren. Physiol. 2003, 284, F955–F965. [Google Scholar] [CrossRef]
- Lodha, S.; Dani, D.; Mehta, R.; Bhaskaran, M.; Reddy, K.; Ding, G.; Singhal, P.C. Angiotensin II-induced mesangial cell apoptosis: Role of oxidative stress. Mol. Med. 2002, 8, 830–840. [Google Scholar] [CrossRef]
- Yadav, A.; Vallabu, S.; Arora, S.; Tandon, P.; Slahan, D.; Teichberg, S.; Singhal, P.C. ANG II promotes autophagy in podocytes. Am. J. Physiol. Cell. Physiol. 2010, 299, C488–C496. [Google Scholar] [CrossRef]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Weinberg, A.M.; Al-Wasiyah, M.K.; Alqahtani, M.H.; Mobasheri, A. Biomarkers of Chondrocyte Apoptosis and Autophagy in Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 20560–20575. [Google Scholar] [CrossRef]
- Galanti, C.; Musumeci, G.; Valentino, J.; Giunta, S.; Castorina, S. A role for apoptosis in temporomandibularjoint disc degeneration. A contemporary review. Ital. J. Anat. Embryol. 2013, 118, 151–158. [Google Scholar]
- Loreto, C.; Rapisarda, V.; Carnazza, M.L.; Musumeci, G.; D’Agata, V.; Valentino, M.; Martinez, G. Bitumen products alter bax, bcl-2 and cytokeratin expression: An in vivo study of chronically exposed road pavers. J. Cutan. Pathol. 2007, 34, 699–704. [Google Scholar] [CrossRef]
- Musumeci, G.; Castrogiovanni, P.; Mazzone, V.; Szychlinska, M.A.; Castorina, S.; Loreto, C. Histochemistry as a unique approach for investigating normal and osteoarthritic cartilage. Eur. J. Histochem. 2014, 58, 2371. [Google Scholar] [CrossRef]
- Musumeci, G.; Castrogiovanni, P.; Loreto, C.; Castorina, S.; Pichler, K.; Weinberg, A.M. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: A morphological study. Int. J. Mol. Sci. 2013, 14, 15767–15784. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Seifarth, C.; Mobasheri, A. Resveratrol inhibits IL-1 beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann. N. Y. Acad. Sci. 2007, 1095, 554–563. [Google Scholar] [CrossRef]
- Giunta, S.; Castorina, A.; Marzagalli, R.; Szychlinska, M.A.; Pichler, K.; Mobasheri, A.; Musumeci, G. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int. J. Mol. Sci. 2015, 16, 5922–5944. [Google Scholar] [CrossRef]
- Block, G. The data support a role for antioxidants in reducing cancer risk. Nutr. Rev. 1992, 50, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Han, M.H.; Park, C.; Lee, D.S.; Hong, S.H.; Choi, I.W.; Kim, G.Y.; Choi, S.H.; Shim, J.H.; Chae, J.I.; Yoo, Y.H.; et al. Cytoprotective effects of esculetin against oxidative stress are associated with the upregulation of Nrf2-mediated NQO1 expression via the activation of the ERK pathway. Int. J. Mol. Med. 2017, 39, 380–386. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).