Chrysanthemum coronarium L. Protects against Premature Senescence in Human Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Analysis of Chrysanthemum coronarium L. (CC) Extract
2.2. Cell Culture and Materials
2.3. Western Blot Analysis
2.4. Staining for Senescence Associated β-Galactosidase
2.5. Detection of Superoxide and Nitric Oxide Formation
2.6. Statistical Analysis
3. Results
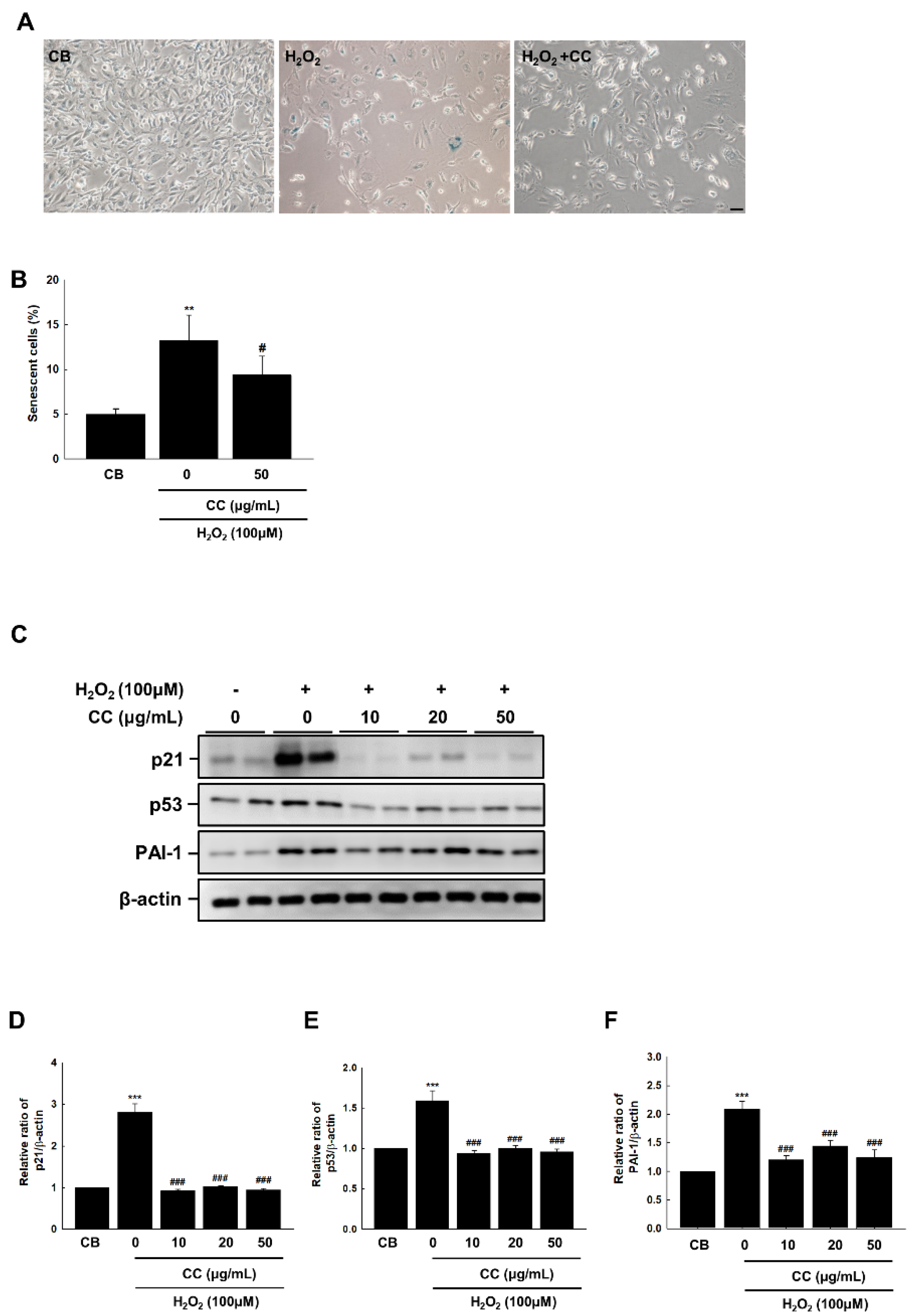
3.1. Chrysanthemum coronarium L. (CC) Extract Reversed Senescence in HUVECs
3.2. Chrysanthemum coronarium L. (CC) Extract Reduces Oxidative Stress-Induced Endothelial Senescence in HUVECs
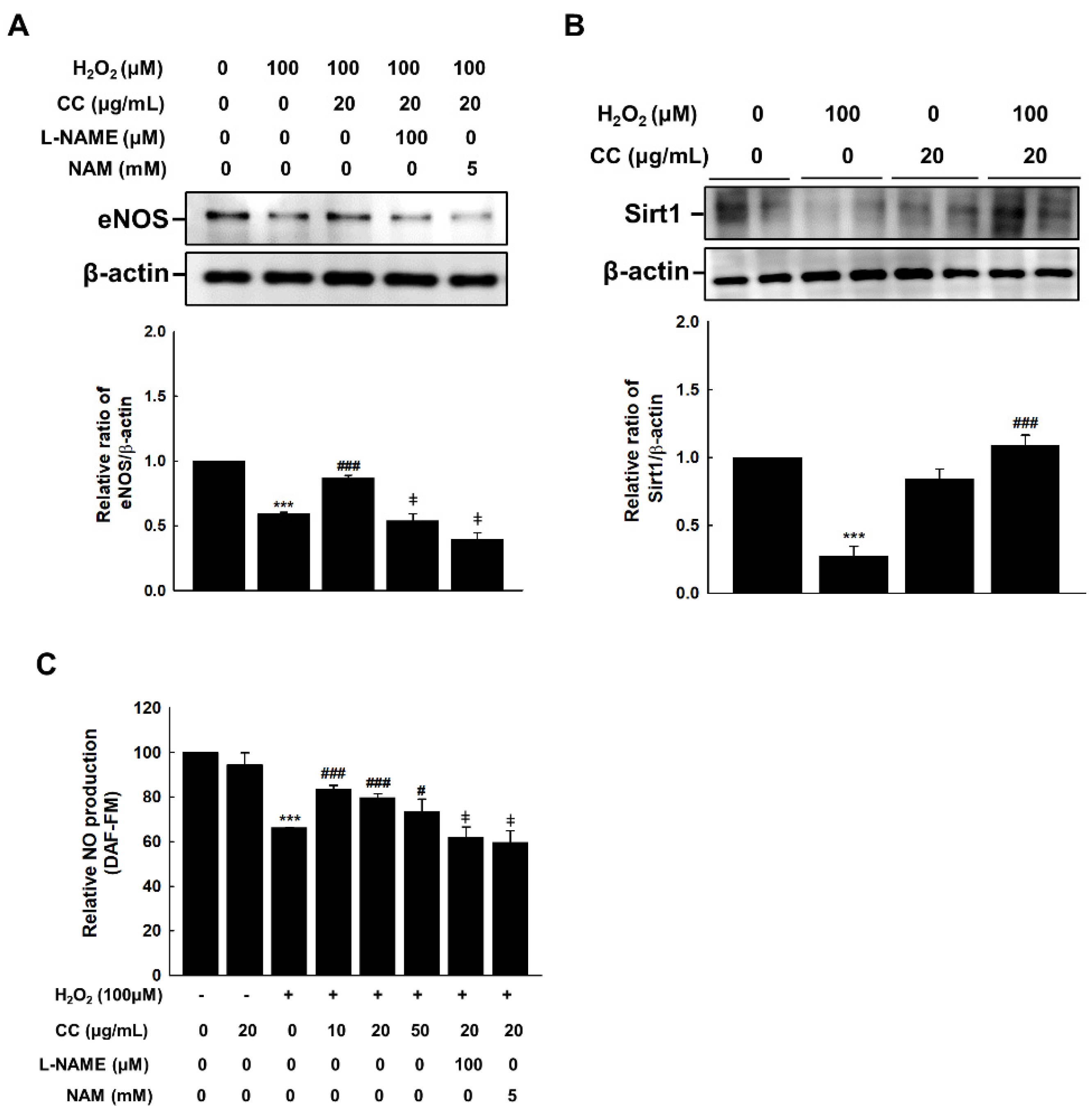
3.3. Chrysanthemum coronarium L. (CC) Extract Reduces Amount of NO Generated from Endothelium to Prevent Senescence in HUVECs
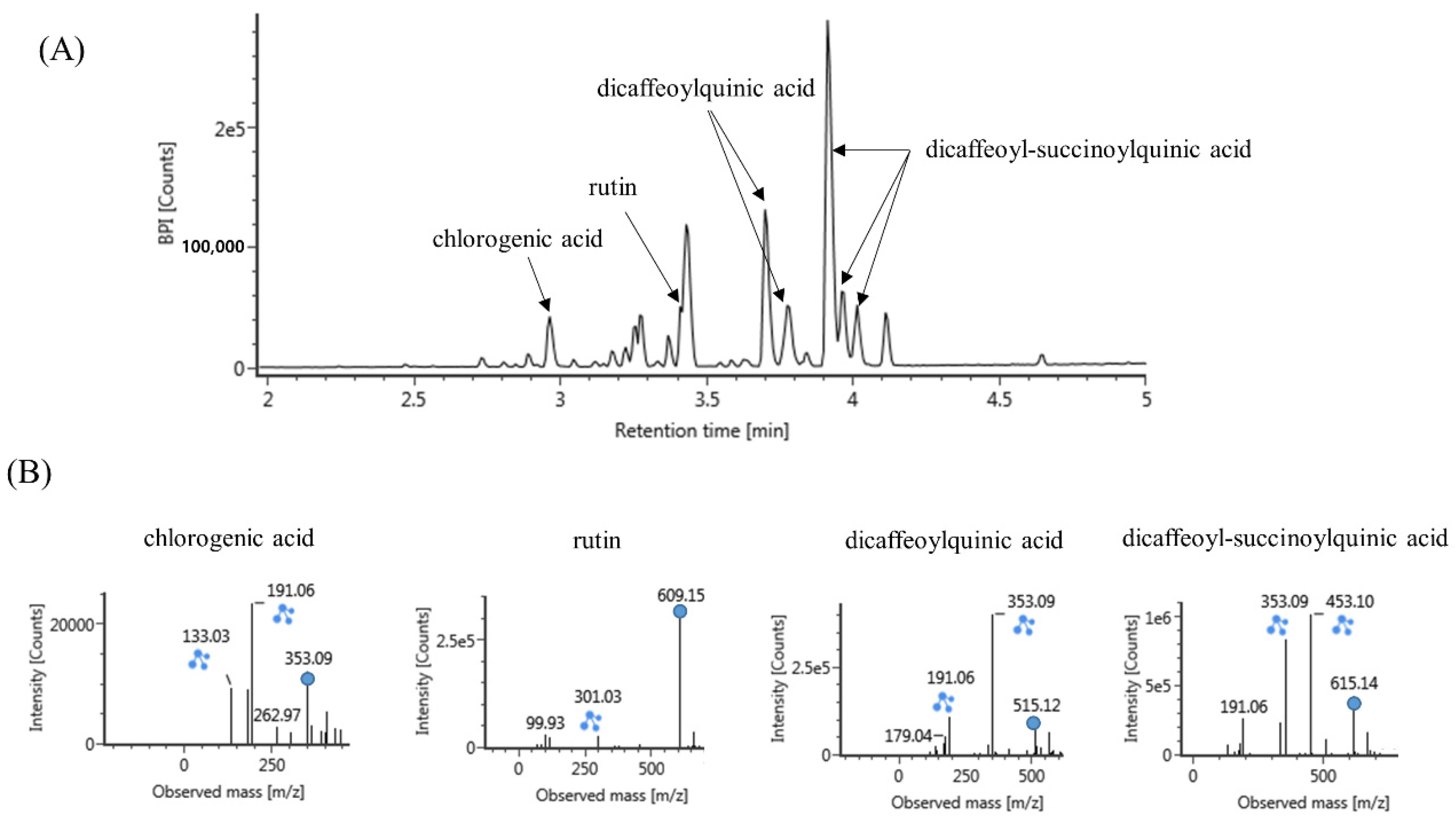
3.4. UPLC-Q-TOF Mass Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharpless, N.E.; Sherr, C.J. Forging a Signature of In Vivo Senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [PubMed]
- Chen, J.; Huang, X.; Halicka, D.; Brodsky, S.; Avram, A.; Eskander, J.; Bloomgarden, N.A.; Darzynkiewicz, Z.; Goli-gorsky, M.S. Contribution of p16INK4a and P21Cip1 Pathways to Induction of Premature Senescence of Human En-dothelial Cells: Permissive Role of p53. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1575–H1586. [Google Scholar] [CrossRef] [PubMed]
- Beauséjour, C.M.; Krtolica, A.; Galimi, F.; Narita, M.; Lowe, S.W.; Yaswen, P.; Campisi, J. Reversal of Human Cellular Senescence: Roles of the p53 and p16 Pathways. EMBO J. 2003, 22, 4212–4222. [Google Scholar] [CrossRef] [PubMed]
- Uryga, A.K.; Bennett, M.R. Ageing Induced Vascular Smooth Muscle Cell Senescence in Atherosclerosis. J. Physiol. 2016, 594, 2115–2124. [Google Scholar] [PubMed]
- Jia, G.; Aroor, A.R.; DeMarco, V.G.; Martinez-Lemus, L.A.; Meininger, G.A.; Sowers, J.R. Vascular Stiffness in Insulin Resistance and Obesity. Front. Physiol. 2015, 6, 231. [Google Scholar]
- Chrysanthemum coronarium L. Korea Agency of Education. Promotion and Information Service in Food, Agriculture, Forestry and Fisheries. Available online: http://www.bris.go.kr/portal/resource/book/selectResourceBookD%20tlInfo.do?lfrcMnno=MANUIP3300200004&gubun=1&siteGb=&menuNo= (accessed on 22 September 2022).
- Hayashi, T.; Matsui-Hirai, H.; Miyazaki-Akita, A.; Fukatsu, A.; Funami, J.; Ding, Q.F.; Kamalanathan, S.; Hattori, Y.; Ignarro, L.J.; Iguchi, A. Endothelial Cellular Senescence Is Inhibited by Nitric Oxide: Implications in Atherosclerosis Associated with Menopause and Diabetes. Proc. Natl. Acad. Sci. USA 2006, 103, 17018–17023. [Google Scholar]
- Vasa, M.; Breitschopf, K.; Zeiher, A.M.; Dimmeler, S. Nitric Oxide Activates Telomerase and Delays Endothelial Cell Senescence. Circ. Res. 2000, 87, 540–542. [Google Scholar] [CrossRef]
- Stein, S.; Matter, C.M. Protective Roles of SIRT1 in Atherosclerosis. Cell Cycle 2011, 10, 640–647. [Google Scholar]
- Man, A.W.C.; Li, H.; Xia, N. The Role of Sirtuin1 in Regulating Endothelial Function, Arterial Remodeling and Vascular Aging. Front. Physiol. 2019, 10, 1173. [Google Scholar]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 Promotes Endothelium-Dependent Vascular Relaxation by Activating Endothelial Nitric Oxide Synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [PubMed]
- Ahn, S.Y.; Cho, C.H.; Park, K.G.; Lee, H.J.; Lee, S.; Park, S.K.; Lee, I.K.; Koh, G.Y. Tumor necrosis factor-α in-duces fractalkine expression preferentially in arterial endothelial cells and mithramycin A suppresses TNF-α-induced fractalkine expression. Am. J. Pathol. 2004, 164, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.D.; Wang, H.; Kho, S.H.; Rinkiko, S.; Sheng, X.; Shen, H.M.; Zhu, Y.Z. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS ONE 2013, 8, e53147. [Google Scholar]
- Douglas, E.V.; Rahul, R.; Sadiya, S.K.; Mesut, E.; Asish, K.G. PAI-1 is a Marker and a Mediator of Senescence. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1446–1452. [Google Scholar]
- Chang, L.; Karin, M. Mammalian MAP Kinase Signalling Cascades. Nature 2001, 410, 37–40. [Google Scholar]
- Ben-Porath, I.; Weinberg, R.A. The Signals and Pathways Activating Cellular Senescence. Int. J. Biochem. Cell Biol. 2005, 37, 961–976. [Google Scholar] [CrossRef]
- Mühleder, S.; Fernández-Chacón, M.; Garcia-Gonzalez, I.; Benedito, R. Endothelial Sprouting, Proliferation, or Senescence: Tipping the Balance from Physiology to Pathology. Cell. Mol. Life Sci. 2021, 78, 1329–1354. [Google Scholar]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Ota, H.; Akishita, M.; Eto, M.; Iijima, K.; Kaneki, M.; Ouchi, Y. Sirt1 Modulates Premature Senescence-Like Phenotype in Human Endothelial Cells. J. Mol. Cell. Cardiol. 2007, 43, 571–579. [Google Scholar] [CrossRef]
- Sasaki, T.; Maier, B.; Bartke, A.; Scrable, H. Progressive Loss of SIRT1 with Cell Cycle Withdrawal. Aging Cell 2006, 5, 413–422. [Google Scholar] [CrossRef]
- Ota, H.; Eto, M.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. SIRT1/eNOS Axis as a Potential Target against Vascular Senescence, Dysfunction and Atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 431–435. [Google Scholar]
- Noh, O.J.; Bae, S.J. Antioxidant Activities of Chrysanthemum coronarium L. fractions on the liposomal Phospholipid Membrane. J. Life Sci. 2002, 12, 144–150. [Google Scholar]
- Leonardo, S.; Giacomo, L.P.; Giorgio, P.; Giovanna, P. Bioactive compounds and antioxidants from a Mediterranean garland harvested at two stages of maturity. Nat. Prod. Res. 2017, 31, 2941–2944. [Google Scholar]
- Yoshiko, H.; Haruhito, A.U.; Nozomu, O.; Yasuhiro, O.; Shugo, O.; Mariko, N.; Rika, T.; Hidemi, T.; Jun, W. The Protective Effect of Chlorogenic Acid on Vascular Senescence via the Nrf2/HO-1 Pathway. Int. J. Mol. Sci. 2020, 21, 4527. [Google Scholar]
- Kazunori, S.; Julie, D.; Noelia, G.D.; Sayo, A.; Farhana, F.; Francis, G.S.; Hiroko, I. 3,4,5-Tricaffeoylquinic acid induces adult neurogenesis and improves deficit of learning and memory in aging model senescence-accelerated prone 8 mice. Aging (Albany NY) 2019, 11, 401–422. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, M.J.; Lee, A.S. Chrysanthemum coronarium L. Protects against Premature Senescence in Human Endothelial Cells. Curr. Issues Mol. Biol. 2022, 44, 5839-5847. https://doi.org/10.3390/cimb44120397
Sung MJ, Lee AS. Chrysanthemum coronarium L. Protects against Premature Senescence in Human Endothelial Cells. Current Issues in Molecular Biology. 2022; 44(12):5839-5847. https://doi.org/10.3390/cimb44120397
Chicago/Turabian StyleSung, Mi Jeong, and Ae Sin Lee. 2022. "Chrysanthemum coronarium L. Protects against Premature Senescence in Human Endothelial Cells" Current Issues in Molecular Biology 44, no. 12: 5839-5847. https://doi.org/10.3390/cimb44120397
APA StyleSung, M. J., & Lee, A. S. (2022). Chrysanthemum coronarium L. Protects against Premature Senescence in Human Endothelial Cells. Current Issues in Molecular Biology, 44(12), 5839-5847. https://doi.org/10.3390/cimb44120397





