Aromatic Polyphenol π-π Interactions with Superoxide Radicals Contribute to Radical Scavenging and Can Make Polyphenols Mimic Superoxide Dismutase Activity
Abstract
1. Introduction
2. Materials and Methods
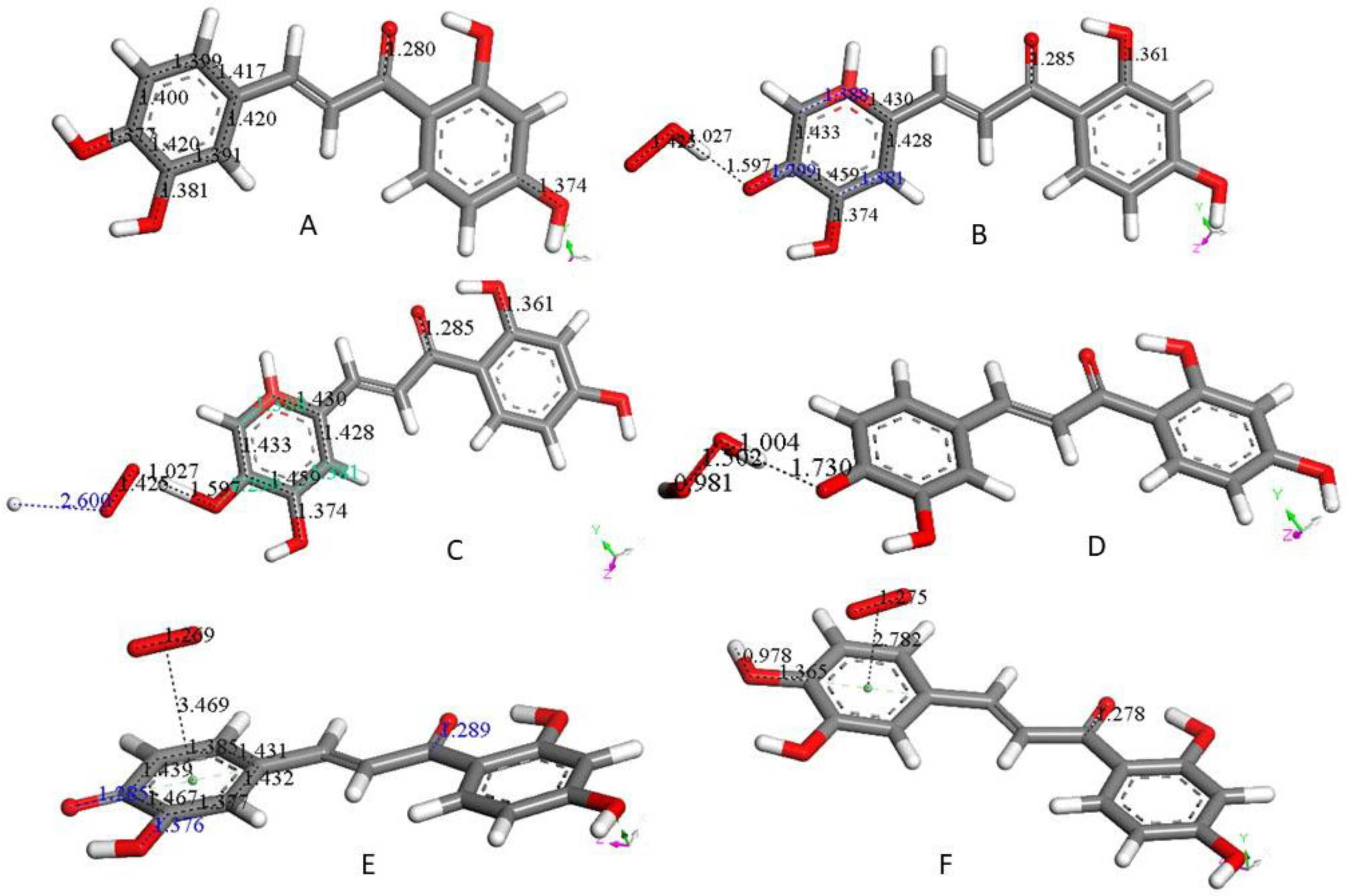
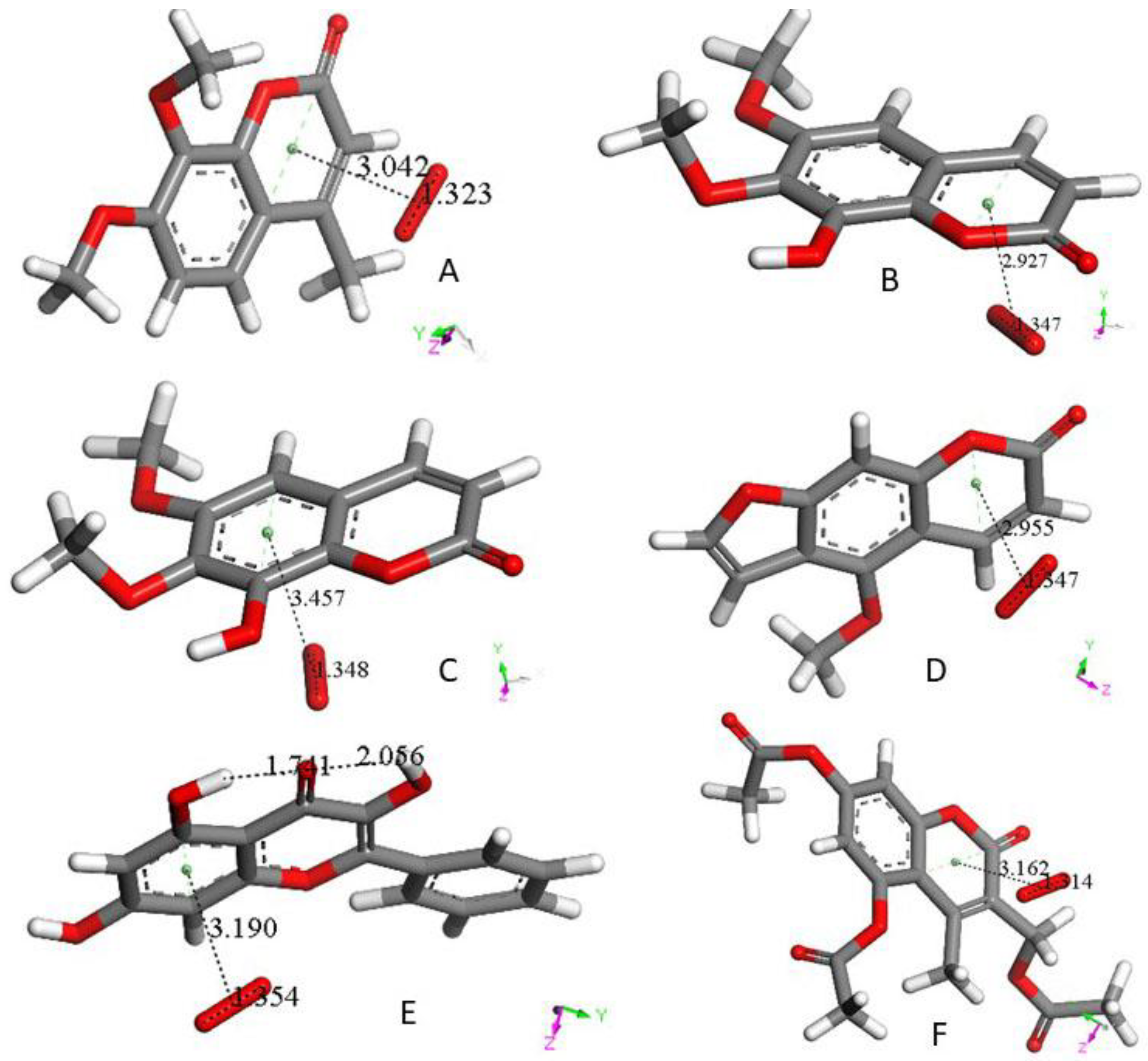
3. Results and Discussion
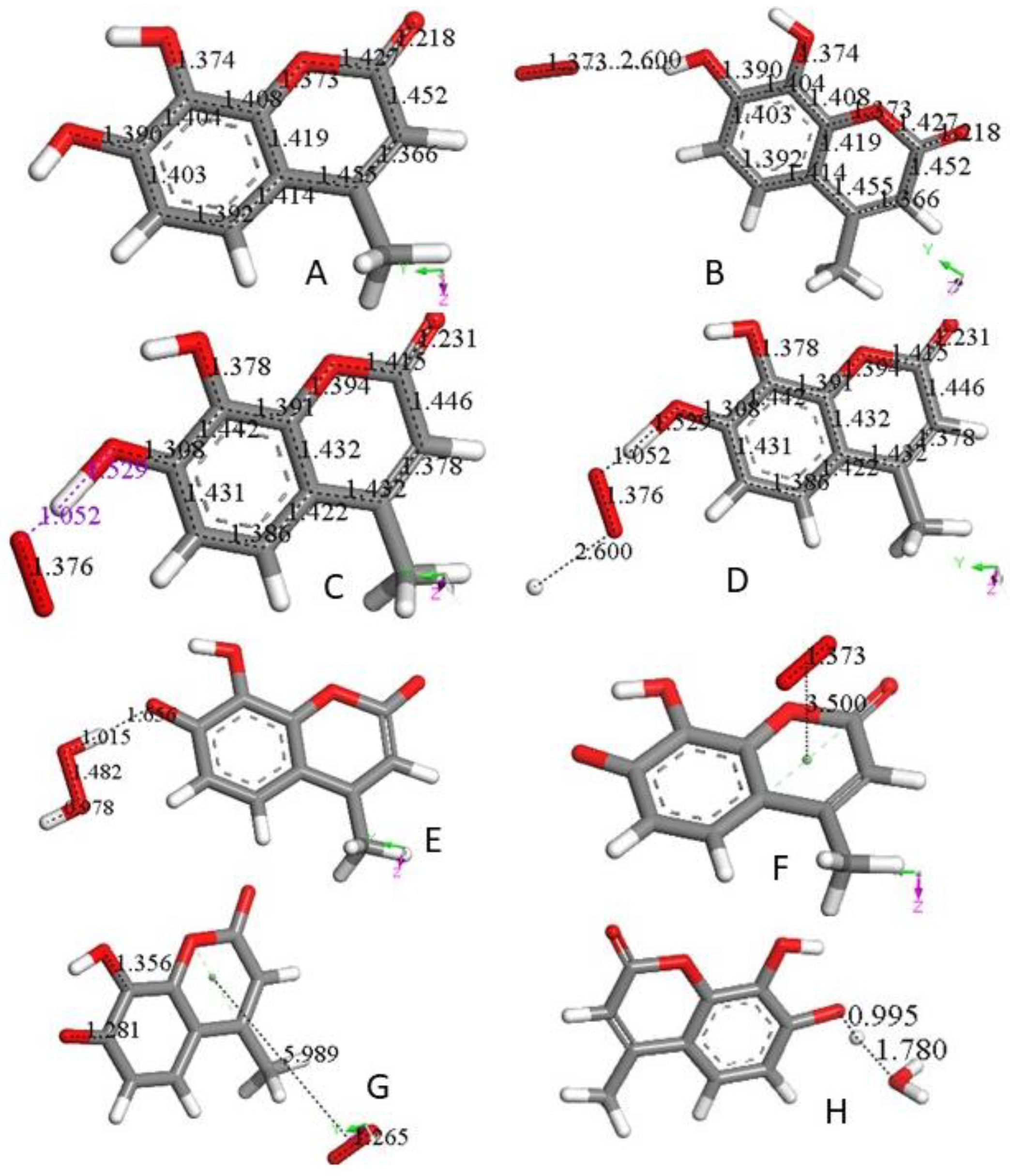
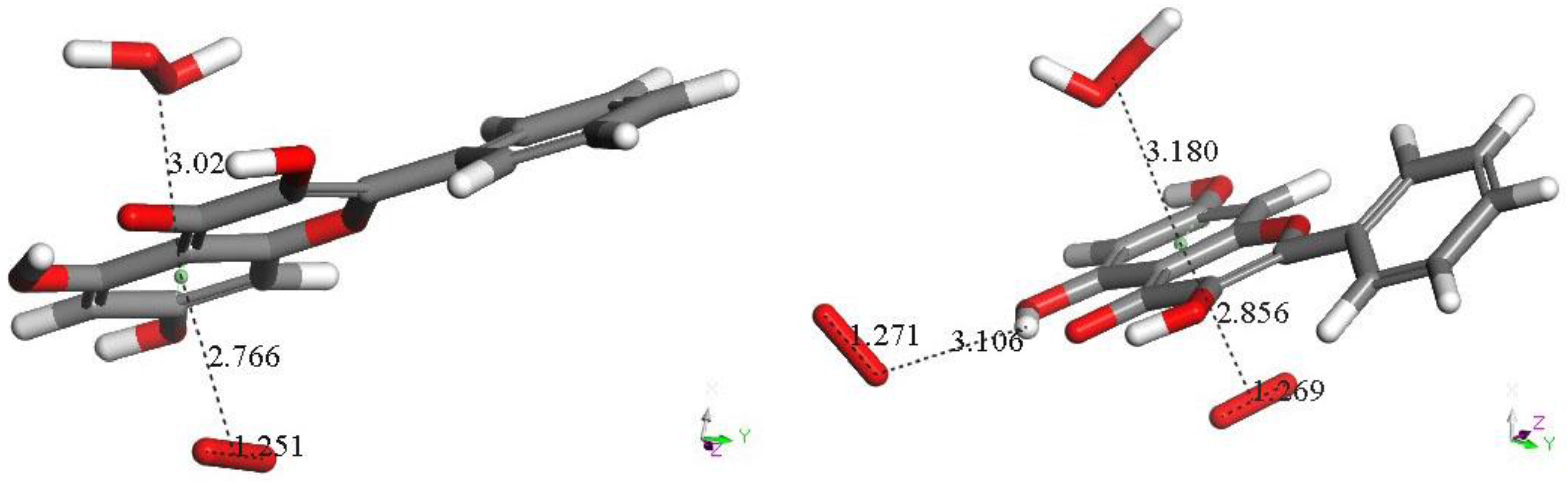
3.1. 4-Methyl-7,8-di-hydroxy Coumarin
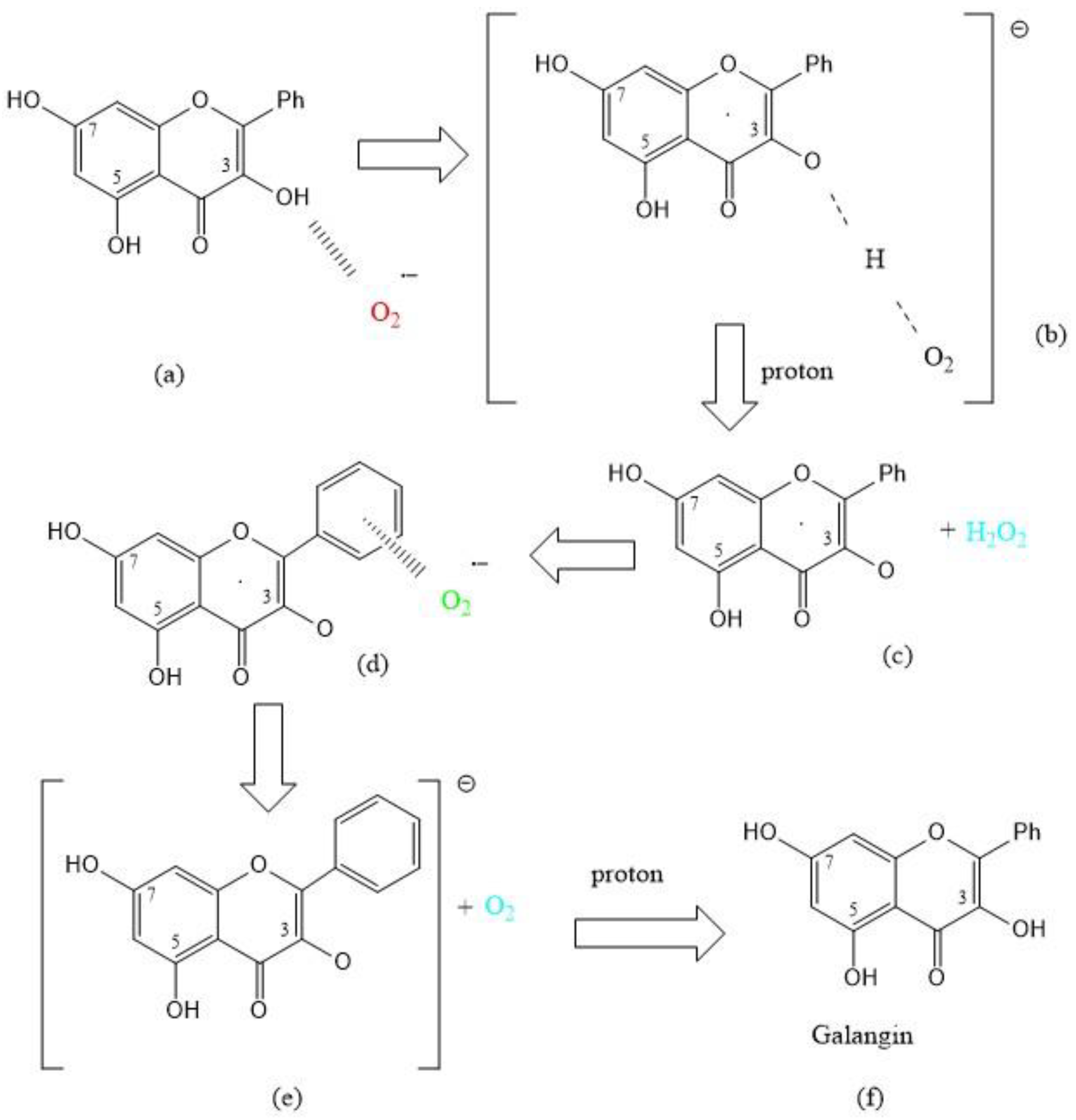
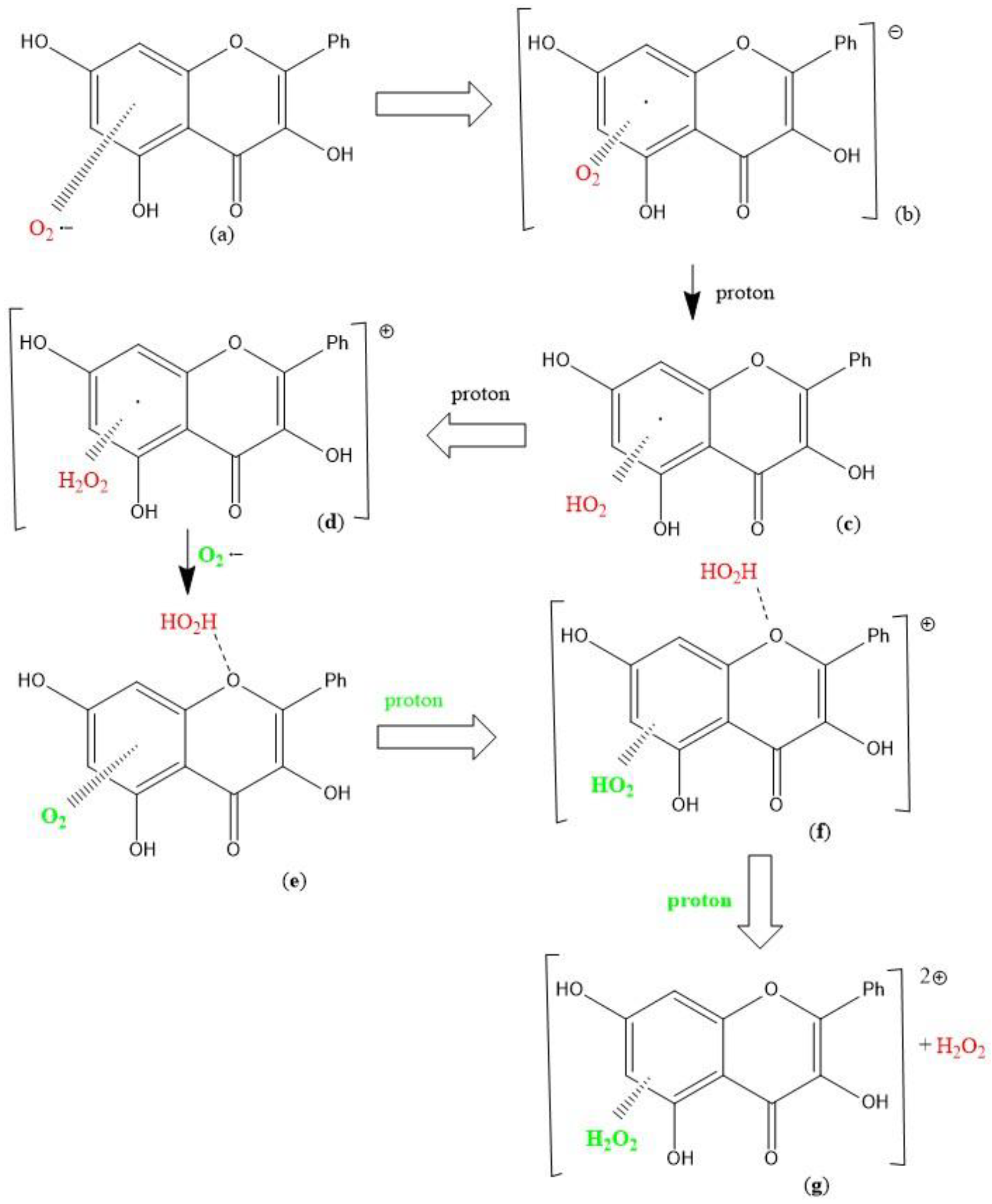
3.2. Galangin
3.3. Butein
3.4. Additional Scavengers
3.5. RRDE Cyclovoltammetry
3.6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Maier, C.M.; Chan, P.H. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist 2002, 8, 323–334. [Google Scholar] [CrossRef]
- Wu, J. Tackle the free radicals damage in COVID-19. Nitric Oxide 2020, 102, 39–41. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochem. Mosc. 2005, 70, 200–214. [Google Scholar] [CrossRef]
- Jadresko, D.; Milicević, A.; Jovanovic, I.N. Reactivity of flavonoids toward superoxide radical: An electrochemical approach. Electrochim. Acta 2022, 421, 140501. [Google Scholar] [CrossRef]
- Belli, S.; Rossi, M.; Molasky, N.; Middleton, L.; Caldwell, C.; Bartow-McKenney, C.; Duong, M.; Chiu, J.; Gibbs, E.; Caldwell, A.; et al. Effective and Novel Application of Hydrodynamic Voltammetry to the Study of Superoxide Radical Scavenging by Natural Phenolic Antioxidants. Antioxidants 2019, 8, 14. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M.; Kaur, S.; Garcia-Villar, E.; Molasky, N.; Belli, S.; Sitek, J.D.; Gionfra, F.; Pedersen, J.Z.; Incerpi, S. Antioxidant Properties of Embelin in Cell Culture. Electrochemistry and Theoretical Mechanism of Scavenging. Potential Scavenging of Superoxide Radical through the Cell Membrane. Antioxidants 2020, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Belli, S.; Caruso, F.; Roy, G.; Rossi, M. Antioxidant studies by hydrodynamic voltammetry and DFT, quantitative analyses by HPLC-DAD of clovamide, a natural phenolic compound found in Theobroma Cacao L. beans. Food Chem. 2021, 341 Pt 2, 128260. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Wen, K.; Caruso, F.; Belli, S. Emodin Scavenging of Superoxide Radical Includes π–π Interaction. X-ray Crystal Structure, Hydrodynamic Voltammetry and Theoretical Studies. Antioxidants 2020, 9, 194. [Google Scholar] [CrossRef]
- Caruso, F.; Paumier, S.; Rossi, M. X-ray Crystal Structure of Embelin and Its DFT Scavenging of Superoxide Radical. J. Comput. Chem. 2018, 39, 1143–1148. [Google Scholar] [CrossRef]
- Ward, M.B.; Scheitler, A.; Yu, M.; Senft, L.; Zillmann, A.S.; Gorden, J.D.; Schwartz, D.D.; Ivanović-Burmazović, I.; Goldsmith, C.R. Superoxide dismutase activity enabled by a redox-active ligand rather than metal. Nat. Chem. 2018, 10, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Senft, L.; Moore, J.L.; Franke, A.; Fisher, K.R.; Scheitler, A.; Zahl, A.; Puchta, R.; Fehn, D.; Ison, S.; Sader, S.; et al. Quinol-containing ligands enable high superoxide dismutase activity by modulating coordination number, charge, oxidation states and stability of manganese complexes throughout redox cycling. Chem. Sci. 2021, 12, 10483–10500. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Delley, B.J. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B Cond. Matter Mat. Phys. 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.Z.; Oliveira, C.; Incerpi, S.; Kumar, V.; Fiore, A.M.; De Vito, P.; Prasad, A.K.; Malhotra, S.V.; Parmar, V.S.; Saso, L. Antioxidant activity of 4-methylcoumarins. J. Pharm. Pharmacol. 2007, 59, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, A.; Davari, M.D.; Chaparzadeh, N.; Zarghami, N.; Pedersen, J.Z.; Incerpi, S.; Saso, L.; Moosavi-Movahedi, A.A. Theoretical and experimental studies on the structure-antioxidant activity relationship of synthetic 4-methylcoumarins. J. Iran. Chem. Soc. 2011, 8, 973–982. [Google Scholar] [CrossRef]
- Caruso, F.; Berinato, M.; Hernandez, M.; Belli, S.; Smart, C.; Rossi, M. Antioxidant properties of bee propolis and an important component, galangin, described by X-ray crystal structure, DFT-D and hydrodynamic voltammetry. PLoS ONE 2022, 17, e0267624. [Google Scholar] [CrossRef]
- Okoye, I.; Yu, S.; Caruso, F.; Rossi, M. X-ray Structure Determination, Antioxidant Voltammetry Studies of Butein and 2,4-Dihydroxy-3,4-dimethoxychalcone. Computational Studies of 4 Structurally Related 2,4-diOH Chalcones to Examine Their Antimalarial Activity by Binding to Falcipain-2. Molecules 2021, 26, 6511. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Aktar, S.; Davis, M.; Hefter Feuss, E.; Roman-Holba, S.; Wen, K.; Gahn, C.; Caruso, F. The Grapefruit Effect: Interaction between Cytochrome P450 and Coumarin Food Components, Bergamottin, Fraxidin and Osthole. X-ray Crystal Structure and DFT Studies. Molecules 2020, 25, 3158. [Google Scholar] [CrossRef] [PubMed]

| BHT | Chrysin | Eriodictyol | DHDM | Butein | Clovamide | Quercetin ** | Galangin |
|---|---|---|---|---|---|---|---|
| −0.16 × 104 [12] | −1.10 × 104 [11] | −2.20 × 104 [11] | −8.0 × 104 [26] | −11.2 × 104 [26] | −12.0 × 104 [13] | −5.30 × 104 [11] −15.4 × 104 [12] −15.5 × 104 [13] | −19.0 × 104 [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, F.; Incerpi, S.; Pedersen, J.; Belli, S.; Kaur, S.; Rossi, M. Aromatic Polyphenol π-π Interactions with Superoxide Radicals Contribute to Radical Scavenging and Can Make Polyphenols Mimic Superoxide Dismutase Activity. Curr. Issues Mol. Biol. 2022, 44, 5209-5220. https://doi.org/10.3390/cimb44110354
Caruso F, Incerpi S, Pedersen J, Belli S, Kaur S, Rossi M. Aromatic Polyphenol π-π Interactions with Superoxide Radicals Contribute to Radical Scavenging and Can Make Polyphenols Mimic Superoxide Dismutase Activity. Current Issues in Molecular Biology. 2022; 44(11):5209-5220. https://doi.org/10.3390/cimb44110354
Chicago/Turabian StyleCaruso, Francesco, Sandra Incerpi, Jens Pedersen, Stuart Belli, Sarjit Kaur, and Miriam Rossi. 2022. "Aromatic Polyphenol π-π Interactions with Superoxide Radicals Contribute to Radical Scavenging and Can Make Polyphenols Mimic Superoxide Dismutase Activity" Current Issues in Molecular Biology 44, no. 11: 5209-5220. https://doi.org/10.3390/cimb44110354
APA StyleCaruso, F., Incerpi, S., Pedersen, J., Belli, S., Kaur, S., & Rossi, M. (2022). Aromatic Polyphenol π-π Interactions with Superoxide Radicals Contribute to Radical Scavenging and Can Make Polyphenols Mimic Superoxide Dismutase Activity. Current Issues in Molecular Biology, 44(11), 5209-5220. https://doi.org/10.3390/cimb44110354








