The Signaling Pathways Induced by Exosomes in Promoting Diabetic Wound Healing: A Mini-Review
Abstract
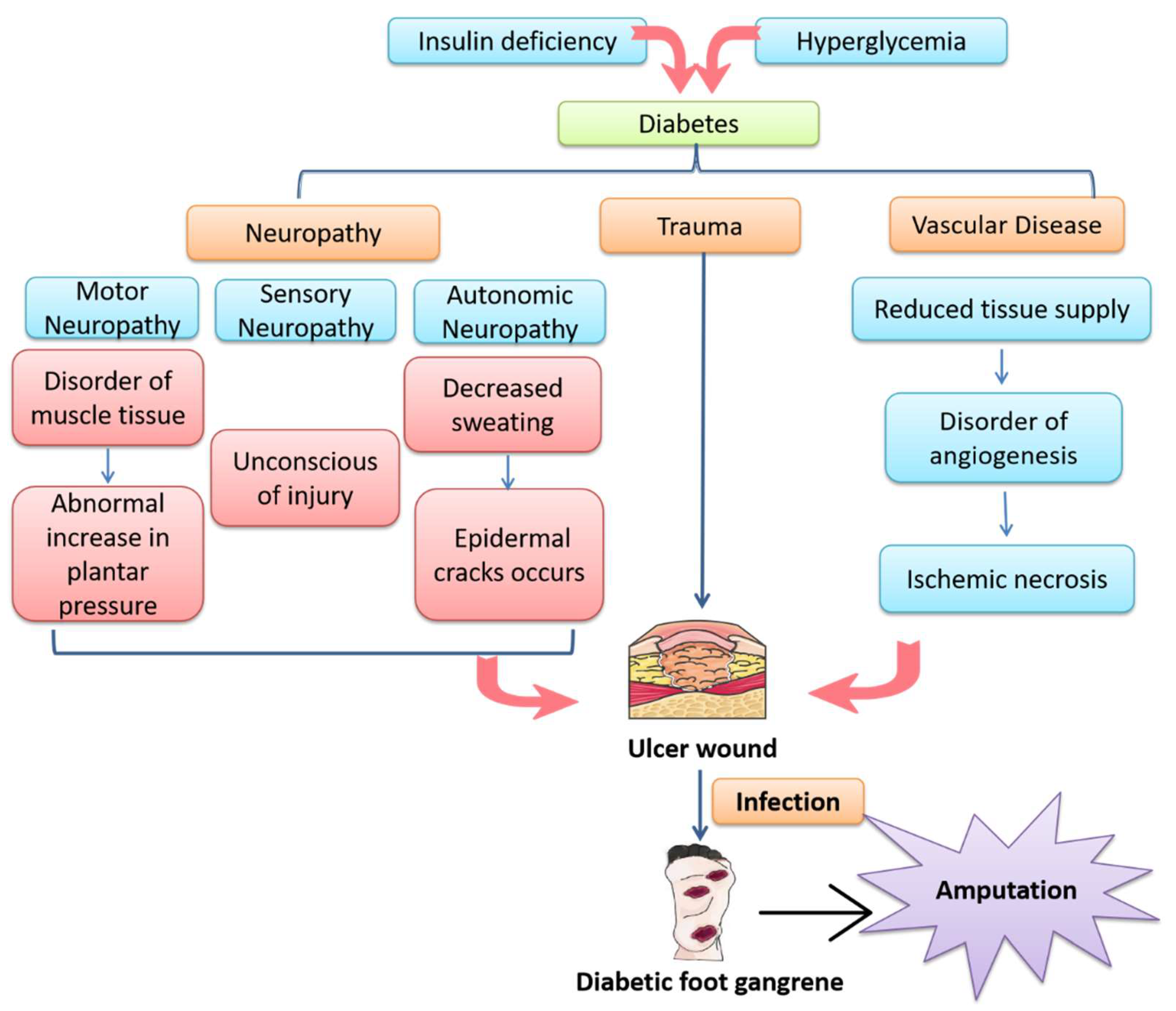
1. Introduction
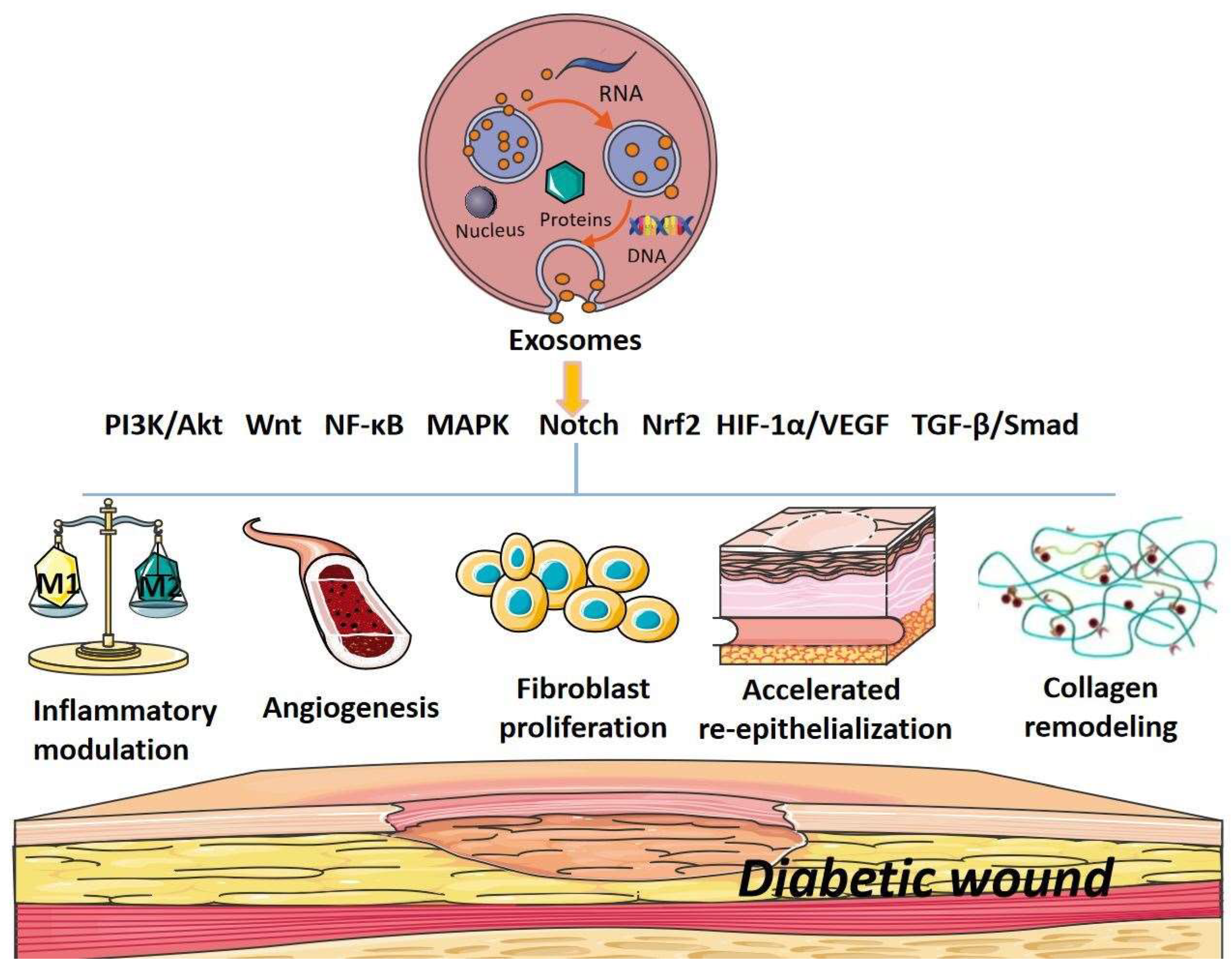
2. The Signaling Pathways of Exosomes in Promoting Diabetic Wound Healing
2.1. PI3K/Akt Signaling Pathway
2.2. Wnt Signaling Pathway
2.3. NF-κB Signaling Pathway
2.4. MAPK Signaling Pathway
2.5. Notch Signaling Pathway
2.6. Nrf2 Signaling Pathway
2.7. HIF-1α/VEGF Signaling Pathway
2.8. TGF-β/Smad Signaling Pathway
2.9. Cross-Talk between Different Signaling Pathways
3. Conclusion and Future Aspects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADSCs | Adipose-tissue-derived stem cells |
| Akt | Serine/threonine kinase or protein kinase B (PKB) |
| APCs | Antigen-presenting cells |
| ATV | Atorvastatin |
| CBF | C-repeat binding transcription factor |
| CK | Cytokines |
| CMC | Carboxymethyl chitosan |
| DFU | Diabetic foot ulcer |
| DM | Diabetes mellitus |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| eNOS | Endothelial nitric oxide synthase |
| EPC | Endothelial progenitor cell |
| ER | Endoplasmic reticulum |
| ESC | Embryonic stem cell |
| EV | Extracellular vesicles |
| ERK | Extracellular signal-regulated kinase |
| FDMSCs | Fetal dermal mesenchymal stem cells |
| GMSC | Gingival mesenchymal stem cell |
| GSK-3 | Glycogen synthase kinase-3 |
| HaCaT | Human immortalized keratinocytes |
| HAECs | Human amniotic epithelial cells |
| HBMMSCs | Human bone marrow mesenchymal stem cells |
| HCC | Hepatocellular carcinoma |
| HCs | Hypertrophic cardiomyocytes |
| HDFs | Human dermal fibroblasts cells |
| HFBs | Human dermal fibroblasts |
| HIF-1α | Hypoxia-inducible factor-1α |
| HUCMSCs | Human umbilical cord mesenchymal stem cells |
| HUVECs | Human umbilical vein endothelial cells |
| HypadSCs | Hypoxic adipose stem cells |
| IDF | International Diabetes Federation |
| JNK | c-Jun N-terminal kinase |
| Keap1 | Kelch-like ECH-associated protein-1 |
| LEF | Lymph enhancer factor |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MenSCs | Menstrual-blood-derived mesenchymal stem cells |
| MMP-9 | Matrix metalloproteinase-9 |
| MSC | Mesenchymal stem cell |
| MT | Melatonin |
| MTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear factor-kappa B |
| NGF | Nerve growth factor |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OSCC | Oral squamous cell carcinoma |
| PCNA | Proliferative cell nuclear antigen |
| PCP | Planner cell polarity |
| P-exos | Plasma exosomes |
| PF-127 | PluronicF-127 |
| PGZ | Pioglitazone |
| PI3K | Phosphatidylinositol 3-hydroxy kinase |
| PUM2 | Pumilio 2 |
| ROS | Reactive oxygen species |
| SERS | Surface-enhanced Raman spectroscopy |
| SMSCs | Synovial mesenchymal stem cells |
| TGF-β | Transforming growth factor-β |
| UC-Mscs | Umbilical cord mesenchymal stem cells |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless/Integrated |
References
- Thewjitcharoen, Y.; Sripatpong, J.; Krittiyawong, S.; Porramatikul, S.; Srikummoon, T.; Mahaudomporn, S.; Butadej, S.; Nakasatien, S.; Himathongkam, T. Changing the patterns of hospitalized diabetic foot ulcer (DFU) over a 5-year period in a multi-disciplinary setting in Thailand. BMC Endocr. Disord. 2020, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula, W.V.; Bus, S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 2020, 13, 16. [Google Scholar] [CrossRef]
- Spanos, K.; Saleptsis, V.; Athanasoulas, A.; Karathanos, C.; Bargiota, A.; Chan, P.; Giannoukas, A.D. Factors Associated With Ulcer Healing and Quality of Life in Patients With Diabetic Foot Ulcer. Angiology 2016, 68, 242–250. [Google Scholar] [CrossRef]
- Alavi, A.; Sibbald, R.G.; Mayer, D.; Goodman, L.; Botros, M.; Armstrong, D.G.; Woo, K.; Boeni, T.; Ayello, E.A.; Kirsner, R.S. Diabetic foot ulcers: Part I. Pathophysiology and prevention. J. Am. Acad. Dermatol. 2014, 70, 1.e1–1.e18; ; quiz 19–20. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. J. Lab. Clin. Med. 2021, 236, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.-J.; Choi, H.J.; Kang, J.S.; Tak, M.S.; Park, E.S. Comparison of five systems of classification of diabetic foot ulcers and predictive factors for amputation. Int. Wound J. 2016, 14, 537–545. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Reardon, R.; Simring, D.; Kim, B.; Mortensen, J.; Williams, D.; Leslie, A. The diabetic foot ulcer. Aust. J. Gen. Pract. 2020, 49, 250–255. [Google Scholar] [CrossRef]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and Treatment of Impaired Wound Healing in Diabetes Mellitus: New Insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Theocharidis, G.; Baltzis, D.; Roustit, M.; Tellechea, A.; Dangwal, S.; Khetani, R.S.; Shu, B.; Zhao, W.; Fu, J.; Bhasin, S.; et al. Integrated Skin Transcriptomics and Serum Multiplex Assays Reveal Novel Mechanisms of Wound Healing in Diabetic Foot Ulcers. Diabetes 2020, 69, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Gao, M.; Yang, P.; Liu, D.; Wang, D.; Song, F.; Zhang, X.; Liu, Y. Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-γ signaling during diabetic wound healing. J. Cell. Physiol. 2018, 234, 4217–4231. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Guo, J.; Li, J.; Shi, X.; Xu, N.; Jiang, Y.; Chen, W.; Hu, Q. lncRNA-H19 in Fibroblasts Promotes Wound Healing in Diabetes. Diabetes 2022, 71, 1562–1578. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.; Zhang, Y.; Long, Q.; Lu, T. Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing. Int. J. Nanomed. 2020, 15, 9717–9743. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liang, P.; Jiang, B.; Zhang, P.; Yu, W.; Duan, M.; Guo, L.; Cui, X.; Huang, M.; Huang, X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020, 259, 118246. [Google Scholar] [CrossRef] [PubMed]
- Buchberger, B.; Follmann, M.; Freyer, D.; Huppertz, H.; Ehm, A.; Wasem, J. The Evidence for the Use of Growth Factors and Active Skin Substitutes for the Treatment of Non-Infected Diabetic Foot Ulcers (DFU): A Health Technology Assessment (HTA). Exp. Clin. Endocrinol. Diabetes 2011, 119, 472–479. [Google Scholar] [CrossRef]
- Campitiello, F.; Mancone, M.; Della Corte, A.; Guerniero, R.; Canonico, S. Expanded negative pressure wound therapy in healing diabetic foot ulcers: A prospective randomised study. J. Wound Care 2021, 30, 121–129. [Google Scholar] [CrossRef]
- Shi, R.; Lian, W.; Jin, Y.; Cao, C.; Han, S.; Yang, X.; Zhao, S.; Li, M.; Zhao, H. Role and effect of vein-transplanted human umbilical cord mesenchymal stem cells in the repair of diabetic foot ulcers in rats. Acta Biochim. Biophys. Sin. 2020, 52, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.B.; Nguyen, H.T.; Palumbo, R.; Pellicano, R.; Fagoonee, S.; Pham, P.V. Stem cell-derived exosomes for wound healing: Current status and promising directions. Minerva Med. 2020, 112, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Didiot, M.-C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tan, W.-F.; Yang, M.-Q.; Li, J.-Y.; Geller, D.A. The therapeutic potential of exosomes derived from different cell sources in liver diseases. Am. J. Physiol. Liver Physiol. 2022, 322, G397–G404. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Dutta, S.; Liu, Z.; Yu, X.; Mesgarzadeh, N.; Ji, F.; Bitan, G.; Xie, Y.-H. A Label-Free Platform for Identification of Exosomes from Different Sources. ACS Sensors 2019, 4, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tao, Q.; Wu, X.; Zhang, L.; Liu, Q.; Wang, L. The Utility of Exosomes in Diagnosis and Therapy of Diabetes Mellitus and Associated Complications. Front. Endocrinol. 2021, 12, 756581. [Google Scholar] [CrossRef]
- Rice, G.E.; Scholz-Romero, K.; Sweeney, E.; Peiris, H.; Kobayashi, M.; Duncombe, G.; Mitchell, M.; Salomon, C. The Effect of Glucose on the Release and Bioactivity of Exosomes From First Trimester Trophoblast Cells. J. Clin. Endocrinol. Metab. 2015, 100, E1280–E1288. [Google Scholar] [CrossRef]
- Abe, H.; Sakurai, A.; Ono, H.; Hayashi, S.; Yoshimoto, S.; Ochi, A.; Ueda, S.; Nishimura, K.; Shibata, E.; Tamaki, M.; et al. Urinary Exosomal mRNA of WT1 as Diagnostic and Prognostic Biomarker for Diabetic Nephropathy. J. Med. Investig. 2018, 65, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Raghav, A.; Tripathi, P.; Mishra, B.K.; Jeong, G.-B.; Banday, S.; Gautam, K.A.; Mateen, Q.N.; Singh, P.; Singh, M.; Singla, A.; et al. Mesenchymal Stromal Cell-Derived Tailored Exosomes Treat Bacteria-Associated Diabetes Foot Ulcers: A Customized Approach From Bench to Bed. Front. Microbiol. 2021, 12, 712588. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Kim, H.-K.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res. Ther. 2020, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Luo, L.; Bai, X.; Shen, K.; Liu, K.; Wang, J.; Hu, D. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch. Biochem. Biophys. 2020, 681, 108259. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, H.; Peng, Y.; Zhao, Y.; Qin, Y.; Zhang, Y.; Xiao, Z. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J. Nanobiotechnology 2021, 19, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ouyang, P.; He, G.; Wang, X.; Song, D.; Yang, Y.; He, X. Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. J. Cell. Mol. Med. 2020, 25, 2148–2162. [Google Scholar] [CrossRef]
- Li, B.; Luan, S.; Chen, J.; Zhou, Y.; Wang, T.; Li, Z.; Fu, Y.; Zhai, A.; Bi, C. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol. Ther. Nucleic Acids 2019, 19, 814–826. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zhou, X.; Luo, X.; Liu, K.; Jiang, E.; Chen, Y.; Shao, Z.; Shang, Z. OSCC Exosomes Regulate miR-210-3p Targeting EFNA3 to Promote Oral Cancer Angiogenesis through the PI3K/AKT Pathway. BioMed. Res. Int. 2020, 2020, 2125656. [Google Scholar] [CrossRef]
- Wei, P.; Zhong, C.; Yang, X.; Shu, F.; Xiao, S.; Gong, T.; Luo, P.; Li, L.; Chen, Z.; Zheng, Y.; et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burn. Trauma 2020, 8, tkaa020. [Google Scholar] [CrossRef]
- Wei, F.; Wang, A.; Wang, Q.; Han, W.; Rong, R.; Wang, L.; Liu, S.; Zhang, Y.; Dong, C.; Li, Y. Plasma endothelial cells-derived extracellular vesicles promote wound healing in diabetes through YAP and the PI3K/Akt/mTOR pathway. Aging 2020, 12, 12002–12018. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, L.; Yan, C.; Zhou, W.; Endo, Y.; Liu, J.; Hu, L.; Hu, Y.; Mi, B.; Liu, G. Circulating Exosomal miR-20b-5p Inhibition Restores Wnt9b Signaling and Reverses Diabetes-Associated Impaired Wound Healing. Small 2019, 16, e1904044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, F.; Yuan, Y.; Jin, C.; Chang, C.; Zhu, Y.; Zhang, X.; Tian, C.; He, F.; Wang, J. Inflammasome-Derived Exosomes Activate NF-κB Signaling in Macrophages. J. Proteome Res. 2017, 16, 170–178. [Google Scholar] [CrossRef]
- Dalirfardouei, R.; Jamialahmadi, K.; Jafarian, A.H.; Mahdipour, E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue Eng. Regen. Med. 2019, 13, 555–568. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Xiao, B.; Liu, H.; Su, Y. Circ_0075932 in adipocyte-derived exosomes induces inflammation and apoptosis in human dermal keratinocytes by directly binding with PUM2 and promoting PUM2-mediated activation of AuroraA/NF-κB pathway. Biochem. Biophys. Res. Commun. 2019, 511, 551–558. [Google Scholar] [CrossRef]
- Li, H.; Rong, P.; Ma, X.; Nie, W.; Chen, Y.; Zhang, J.; Dong, Q.; Yang, M.; Wang, W. Mouse Umbilical Cord Mesenchymal Stem Cell Paracrine Alleviates Renal Fibrosis in Diabetic Nephropathy by Reducing Myofibroblast Transdifferentiation and Cell Proliferation and Upregulating MMPs in Mesangial Cells. J. Diabetes Res. 2020, 2020, 3847171. [Google Scholar] [CrossRef]
- Yu, H.; Qin, L.; Peng, Y.; Bai, W.; Wang, Z. Exosomes Derived From Hypertrophic Cardiomyocytes Induce Inflammation in Macrophages via miR-155 Mediated MAPK Pathway. Front. Immunol. 2021, 11, 606045. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chenguang, Z. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef]
- Li, M.; Ke, Q.-F.; Tao, S.-C.; Guo, S.-C.; Rui, B.-Y.; Guo, Y.-P. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J. Mater. Chem. B 2016, 4, 6830–6841. [Google Scholar] [CrossRef]
- Gonzalez-King, H.; García, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepúlveda, P. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, S.; Bi, J.; Huo, R. Exosomes from human adipose-derived mesenchymal stem cells inhibit production of extracellular matrix in keloid fibroblasts via downregulating transforming growth factor-β2 and Notch-1 expression. Bioengineered 2022, 13, 8515–8525. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiao, Y.; Pan, Y.; Zhang, L.; Gong, H.; Qi, Y.; Wang, M.; Gong, H.; Shao, M.; Wang, X.; et al. Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int. 2019, 2019, 2402916. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Y.; Zhang, Q.; Zhang, Y. Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells. Int. J. Stem Cells 2022, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shi, Y.; Li, M.; Wang, T.; Zhao, L. Plasma Exosomes Loaded pH-Responsive Carboxymethylcellulose Hydrogel Promotes Wound Repair by Activating the Vascular Endothelial Growth Factor Signaling Pathway in Type 1 Diabetic Mice. J. Biomed. Nanotechnol. 2021, 17, 2021–2033. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhao, J.; Nie, F.; Qin, Z.; Xue, H.; Wang, G.; Li, D. Exosomes from Adipose-Derived Stem Cells (ADSCs) Overexpressing miR-21 Promote Vascularization of Endothelial Cells. Sci. Rep. 2019, 9, 12861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, F.; Gu, L.; Ji, P.; Yang, X.; Liu, M.; Tao, K.; Hu, D. Adipose mesenchymal stem cell exosomes promote wound healing through accelerated keratinocyte migration and proliferation by activating the AKT/HIF-1α axis. Histochem. J. 2020, 51, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, W.; Li, J.; Lu, J.; Lu, H.; Jia, W.; Liu, F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res. Ther. 2020, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol. Med. Rep. 2018, 19, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Jere, S.W.; Houreld, N.N.; Abrahamse, H. Role of the PI3K/AKT (mTOR and GSK3β) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019, 50, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.Q.; Zhang, H.; Shi, X.J.; Zhao, J.F.; Chen, Y.; Wu, F.M.; Yang, J.W.; Li, X.H. Asiaticoside nitric oxide gel accelerates diabetic cutaneous ulcers healing by activating Wnt/beta-catenin signaling pathway. Int. Immunopharmacol. 2020, 79, 106109. [Google Scholar] [CrossRef] [PubMed]
- De, A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. 2011, 43, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nie, X.; Shi, X.; Zhao, J.; Chen, Y.; Yao, Q.; Sun, C.; Yang, J. Regulatory Mechanisms of the Wnt/β-Catenin Pathway in Diabetic Cutaneous Ulcers. Front. Pharmacol. 2018, 9, 1114. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Thomas, A.A.; Chen, S.; Aref-Eshghi, E.; Feng, B.; Gonder, J.; Sadikovic, B.; Chakrabarti, S. MALAT1: An Epigenetic Regulator of Inflammation in Diabetic Retinopathy. Sci. Rep. 2018, 8, 6526. [Google Scholar] [CrossRef]
- Ma, T.; Fu, B.; Yang, X.; Xiao, Y.; Pan, M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J. Cell. Biochem. 2019, 120, 10847–10854. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-κB: Its role in health and disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Bourebaba, N.; Kornicka-Garbowska, K.; Turlej, E.; Marycz, K.; Bourebaba, L. Hyoscyamus albus nortropane alkaloids reduce hyperglycemia and hyperinsulinemia induced in HepG2 cells through the regulation of SIRT1/NF-kB/JNK pathway. Cell Commun Signal 2021, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Dong, J.; He, X.; Zhang, C.; Zhang, T. Bone marrow mesenchymal stem cells-derived exosomes reduce apoptosis and inflammatory response during spinal cord injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway. Hum. Exp. Toxicol. 2021, 40, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Kuo, P.-J.; Rau, C.-S.; Huang, L.-H.; Lin, C.-W.; Wu, Y.-C.; Wu, C.-J.; Tsai, C.-W.; Hsieh, T.-M.; Liu, H.-T.; et al. Increased Angiogenesis by Exosomes Secreted by Adipose-Derived Stem Cells upon Lipopolysaccharide Stimulation. Int. J. Mol. Sci. 2021, 22, 8877. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yan, W.; He, J.; Liu, X.; Zhang, Q.; Wang, X. Identification, evolution and expression analyses of mapk gene family in Japanese flounder (Paralichthys olivaceus) provide insight into its divergent functions on biotic and abiotic stresses response. Aquat. Toxicol. 2021, 241, 106005. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; Zhang, Q.; Zhang, D.; Xiang, F.; Jia, J.; Wei, P.; Zhang, J.; Hu, J.; Huang, Y. High Glucose Suppresses Keratinocyte Migration Through the Inhibition of p38 MAPK/Autophagy Pathway. Front. Physiol. 2019, 10, 24. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Fan, L.; Chen, B.; Liu, J.; Tao, Y.; Wang, X. Negative pressure wound therapy promoted wound healing by suppressing inflammation via down-regulating MAPK-JNK signaling pathway in diabetic foot patients. Diabetes Res. Clin. Pr. 2019, 150, 81–89. [Google Scholar] [CrossRef]
- Meurette, O.; Mehlen, P. Notch Signaling in the Tumor Microenvironment. Cancer Cell 2018, 34, 536–548. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Chen, J.; Ma, Y.; Song, Y.; Cen, Y.; You, M.; Yang, G. The Notch signaling pathway regulates macrophage polarization in liver diseases. Int. Immunopharmacol. 2021, 99, 107938. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.S.; Joshi, A.D.; Boniakowski, A.E.; Schaller, M.; Chung, J.; Allen, R.; Bermick, J.; Carson, W.F., IV; Henke, P.K.; Maillard, I.; et al. Notch Regulates Macrophage-Mediated Inflammation in Diabetic Wound Healing. Front. Immunol. 2017, 8, 635. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Mo, Z. Keap1-Nrf2 signaling pathway in angiogenesis and vascular diseases. J. Tissue Eng. Regen. Med. 2020, 14, 869–883. [Google Scholar] [CrossRef]
- Kahroba, H.; Taghipour, Y.D. Exosomal Nrf2: From anti-oxidant and anti-inflammation response to wound healing and tissue regeneration in aged-related diseases. Biochimie 2020, 171, 103–109. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Zhang, J.; Zhu, Q.; Yang, Y.; Niu, X.; Deng, Z.; Li, Q.; Wang, Y. Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Res. Ther. 2019, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Duscher, D.; Maan, Z.; Whittam, A.J.; Sorkin, M.; Hu, M.S.; Walmsley, G.G.; Baker, H.; Fischer, L.H.; Januszyk, M.; Wong, V.W.; et al. Fibroblast-Specific Deletion of Hypoxia Inducible Factor-1 Critically Impairs Murine Cutaneous Neovascularization and Wound Healing. Plast. Reconstr. Surg. 2015, 136, 1004–1013. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Jia, Y.; Xu, J.; Chai, Y. Roxadustat promotes angiogenesis through HIF-1α/VEGF/VEGFR2 signaling and accelerates cutaneous wound healing in diabetic rats. Wound Repair Regen. 2019, 27, 324–334. [Google Scholar] [CrossRef]
- Schiller, M.; Javelaud, D.; Mauviel, A. TGF-beta-induced SMAD signaling and gene regulation: Consequences for extracellular matrix remodeling and wound healing. J. Dermatol. Sci. 2004, 35, 83–92. [Google Scholar] [CrossRef]
- Hu, H.-H.; Chen, D.-Q.; Wang, Y.-N.; Feng, Y.-L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Interactions 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, Z.; Sun, J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res. Ther. 2020, 11, 198. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Liu, Y.; Li, X.; Tang, L.; Duan, M.; Li, J.; Zhang, G. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Res. Ther. 2021, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A.C.; et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J. Nanobiotechnology 2021, 19, 150. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abujamra, B.A.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Wang, S.; Xu, M.; Li, X.; Su, X.; Xiao, X.; Keating, A.; Zhao, R.C. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J. Hematol. Oncol. 2018, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity, Biochimica et biophysica acta. Rev. Cancer 2019, 1871, 455–468. [Google Scholar]
- Chen, K.; Yu, T.; Wang, X. Inhibition of Circulating Exosomal miRNA-20b-5p Accelerates Diabetic Wound Repair. Int. J. Nanomed. 2021, 16, 371–381. [Google Scholar] [CrossRef]
| Exosome Species | Experimental Models | Mechanism/Results | Signaling Pathway | Ref. | |
|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||
| Adipose-tissue-derived stem cells | STZ-induced diabetic rats | Fibroblasts and ADSCs in patients | Promote fibroblast proliferation and migration | PI3K/Akt | [32] |
| Adipose-tissue-derived stem cells | STZ-induced diabetic rats | HaCaT cells | Up-regulate MMP-9 expression; enhance migration and proliferation of HaCaT cells | PI3K/Akt | [35] |
| Hypoxia adipose stem cells | STZ-induced diabetic rats | - | Down-regulate genes miRNA-99b and miRNA-146-a; promote collagen remodeling | PI3K/Akt | [36] |
| Human bone marrow mesenchymal stem cells | STZ-induced diabetic rats | HUVECs | Combine with miRNA– 126; promote the proliferation and migration of HUVECs | PI3K/Akt | [37] |
| Mesenchymal stem cells | STZ-induced diabetic rats | Fibroblast | Promote the proliferation and migration of fibroblasts; inhibit cell apoptosis and inflammation | PI3K/Akt | [38] |
| Oral squamous cell carcinoma | - | HUVECs | Up-regulate the expression of miRNA-210-3p and down-regulate the expression of adrenaline A3; promote angiogenesis | PI3K/Akt | [39] |
| Human amniotic epithelial cells | STZ-induced diabetic rats | HFBs and HUVECs | Promote angiogenesis and activate fibroblasts | PI3K/Akt | [40] |
| Plasma | STZ-induced diabetic rats | - | Reduce YAP phosphorylation; inhibit fibroblast senescence | PI3K/Akt | [41] |
| Circulating exosomes | STZ-induced diabetic rats | - | Knock out miRNA-20b-5p; promote angiogenesis | Wnt | [42] |
| Inflammasome | - | Macrophages | Act on macrophages; reduce inflammation and increase immune response | NF-κB | [43] |
| Menstrual-blood-derived mesenchymal stem cells | STZ-induced diabetic rats | - | Induce polarization of M1-M2 macrophages; up-regulation of VEGF-A; reduce inflammation and promote angiogenesis | NF-κB | [44] |
| Human dermal keratinocytes | - | Keratinocytes | Combine with PUM2; activate inflammation and induce apoptosis of keratinocytes | NF-κB | [45] |
| Mesenchymal stem cells | - | Mouse beta cell line βTC-6 | Carry miRNA-21 and alleviate endoplasmic reticulum (ER) stress; inhibition of β cell apoptosis | MAPK | [34] |
| Umbilical cord mesenchymal stem cells | STZ-induced diabetic rats | Mesangial cells | Reduce the deposition of fibronectin and collagen I; increase the level of matrix metalloproteinase | MAPK | [46] |
| Hypertrophic cardiomyocytes | - | Mouse macrophage line RAW264.7 | Inhibit miRNA-155 expression and weaken the effect of p38, JNK and ERK; regulate inflammatory response | MAPK | [47] |
| Adipose-tissue-derived stem cells | - | HDFs | Adjust the proportion of type Ⅲ collagen; increase MMP-3 expression and reduce scar formation | MAPK | [48] |
| Synovial mesenchymal stem cells | STZ-induced diabetic rats | HDFs | Promote re-epithelialization; accelerate angiogenesis and collagen maturation | MAPK | [49] |
| Mesenchymal stem cells | - | Tissue explant model | Over-express HIF-1α; induce endothelial angiogenesis | Notch | [50,51] |
| Fetal dermal mesenchymal stem cells | STZ-induced diabetic rats | HDFs | Enhance ADF cell proliferation; promote extracellular matrix (ECM) deposition | Notch | [52] |
| Adipose-tissue-derived stem cells | STZ-induced diabetic rats | EPCs | Overexpress Nrf2; promote the expression of growth factor and decrease inflammation | Nrf2 | [8] |
| Human bone marrow mesenchymal stem cells | STZ-induced diabetic rats | EPCs | Binding to small molecule Nrf2 activator; accelerate epithelial remodeling, collagen deposition and angiogenesis | Nrf2 | [53] |
| Plasma | STZ-induced diabetic rats | - | Loaded on CMC hydrogel; enhance angiogenesis | HIF-1α/VEGF | [54] |
| Adipose tissue-derived stem cells | - | HUVECs | Over-express miRNA-21; enhance angiogenesis | HIF-1α/VEGF | [55] |
| Adipose-tissue-derived stem cells | STZ-induced diabetic rats | HaCaTs | Up-regulate the phosphorylation of AKT; promote the proliferation and migration of keratinocytes | HIF-1α/VEGF | [56] |
| Human umbilical cord mesenchymal stem cells | STZ-induced diabetic rats | Myofibroblasts | Inhibit the formation of myofibroblasts and reduce scar formation | TGF-β/Smad | [57] |
| Mesenchymal stem cells | STZ-induced diabetic rats | HUVECs | Pretreatment with ATV; up-regulate miRNA-221-3p; promote angiogenesis | Akt/eNOS | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, J.; Chen, J.; Xu, R.; Groth, T.; Wan, H.; Zhou, G. The Signaling Pathways Induced by Exosomes in Promoting Diabetic Wound Healing: A Mini-Review. Curr. Issues Mol. Biol. 2022, 44, 4960-4976. https://doi.org/10.3390/cimb44100337
Wang Y, Zhu J, Chen J, Xu R, Groth T, Wan H, Zhou G. The Signaling Pathways Induced by Exosomes in Promoting Diabetic Wound Healing: A Mini-Review. Current Issues in Molecular Biology. 2022; 44(10):4960-4976. https://doi.org/10.3390/cimb44100337
Chicago/Turabian StyleWang, Yanying, Jiayan Zhu, Jing Chen, Ruojiao Xu, Thomas Groth, Haitong Wan, and Guoying Zhou. 2022. "The Signaling Pathways Induced by Exosomes in Promoting Diabetic Wound Healing: A Mini-Review" Current Issues in Molecular Biology 44, no. 10: 4960-4976. https://doi.org/10.3390/cimb44100337
APA StyleWang, Y., Zhu, J., Chen, J., Xu, R., Groth, T., Wan, H., & Zhou, G. (2022). The Signaling Pathways Induced by Exosomes in Promoting Diabetic Wound Healing: A Mini-Review. Current Issues in Molecular Biology, 44(10), 4960-4976. https://doi.org/10.3390/cimb44100337






