Tissue Levels of CD80, CD163 and CD206 and Their Ratios in Periodontal and Peri-Implant Health and Disease
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berglundh, T.; Zitzmann, N.U.; Donati, M. Are Peri-Implantitis Lesions Different from Periodontitis Lesions? J. Clin. Periodontol. 2011, 38, 188–202. [Google Scholar] [CrossRef]
- Carcuac, O.; Berglundh, T. Composition of Human Peri-Implantitis and Periodontitis Lesions. J. Dent. Res. 2014, 93, 1083–1088. [Google Scholar] [CrossRef]
- Wilson, T.G.; Valderrama, P.; Burbano, M.; Blansett, J.; Levine, R.; Kessler, H.; Rodrigues, D.C. Foreign Bodies Associated with Peri-Implantitis Human Biopsies. J. Periodontol. 2015, 86, 9–15. [Google Scholar] [CrossRef]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef]
- Ross, E.A.; Devitt, A.; Johnson, J.R. Macrophages: The good, the bad, and the gluttony. Front. Immunol. 2021, 12, 708186. [Google Scholar] [CrossRef] [PubMed]
- Tardito, S.; Martinelli, G.; Soldano, S.; Paolino, S.; Pacini, G.; Patane, M.; Alessandri, E.; Smith, V.; Cutolo, M. Macrophage M1/M2 polarization and rheumatoid arthritis: A systematic review. Autoimmun. Rev. 2019, 18, 102397. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef] [PubMed]
- de Gaetano, M.; Crean, D.; Barry, M.; Belton, O. M1- and M2-type macrophage responses are predictive of adverse outcomes in human atherosclerosis. Front. Immunol. 2016, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.N.; Bi, C.S.; Gao, L.N.; An, Y.; Chen, F.; Chen, F.M. Macrophage Polarization in Human Gingival Tissue in Response to Periodontal Disease. Oral Dis. 2019, 25, 265–273. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, Y.; Duan, D.; Wang, P.; Xin, Y.; Bai, L.; Liu, Y.; Xu, Y. Enhanced Activity of Macrophage M1/M2 Phenotypes in Periodontitis. Arch. Oral Biol. 2018, 96, 234–242. [Google Scholar] [CrossRef]
- Yu, T.; Zhao, L.; Huang, X.; Ma, C.; Wang, Y.; Zhang, J.; Xuan, D. Enhanced Activity of the Macrophage M1/M2 Phenotypes and Phenotypic Switch to M1 in Periodontal Infection. J. Periodontol. 2016, 87, 1092–1102. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yoshizawa-Smith, S.; Glowacki, A.; Maltos, K.; Pacheco, C.; Shehabeldin, M.; Mulkeen, M.; Myers, N.; Chong, R.; Verdelis, K.; et al. Induction of M2 Macrophages Prevents Bone Loss in Murine Periodontitis Models. J. Dent. Res. 2019, 98, 200–208. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.E.; Obreja, K.; Ramanauskaite, A.; Magini, R.; Begic, A.; Sader, R.; Schwarz, F. Macrophage Polarization in Peri-Implantitis Lesions. Clin. Oral Investig. 2021, 25, 2335–2344. [Google Scholar] [CrossRef]
- Fretwurst, T.; Garaicoa-Pazmino, C.; Nelson, K.; Giannobile, W.V.; Squarize, C.H.; Larsson, L.; Castilho, R.M. Characterization of Macrophages Infiltrating Peri-Implantitis Lesions. Clin. Oral Implant. Res. 2020, 31, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Soldano, S.; Trombetta, A.C.; Contini, P.; Tomatis, V.; Ruaro, B.; Brizzolara, R.; Montagna, P.; Sulli, A.; Paolino, S.; Pizzorni, C.; et al. Increase in Circulating Cells Coexpressing M1 and M2 Macrophage Surface Markers in Patients with Systemic Sclerosis. Ann. Rheum. Dis. 2018, 77, 1842–1845. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Trombetta, A.C.; Soldano, S.; Contini, P.; Tomatis, V.; Ruaro, B.; Paolino, S.; Brizzolara, R.; Montagna, P.; Sulli, A.; Pizzorni, C.; et al. A Circulating Cell Population Showing Both M1 and M2 Monocyte/Macrophage Surface Markers Characterizes Systemic Sclerosis Patients with Lung Involvement. Respir. Res. 2018, 19, 186. [Google Scholar] [CrossRef]
- Pinto, M.L.; Rios, E.; Durães, C.; Ribeiro, R.; Machado, J.C.; Mantovani, A.; Barbosa, M.A.; Carneiro, F.; Oliveira, M.J. The Two Faces of Tumor-Associated Macrophages and Their Clinical Significance in Colorectal Cancer. Front. Immunol. 2019, 10, 1875. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Murakami, K.; Saito, R.; Ito, H.; Murata, K.; Nishitani, K.; Hashimoto, M.; Tanaka, M.; Kitagori, K.; Akizuki, S.; et al. Increased Ratio of CD14++ CD80+ Cells/CD14++ CD163+ Cells in the Infrapatellar Fat Pad of End-Stage Arthropathy Patients. Front. Immunol. 2021, 12, 774177. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Viola, N.; Berretta, A.; Galeazzi, R.; Matacchione, G.; Sabbatinelli, J.; Storci, G.; de Matteis, S.; Butini, L.; Rippo, M.R.; et al. Age-Related M1/M2 Phenotype Changes in Circulating Monocytes from Healthy/Unhealthy Individuals. Aging 2018, 10, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Safavian, D.; Leung, C.H.; Kapus, A.; Ailenberg, M.; Szaszi, K.; Shani, R.; di Ciano-Oliveira, C.; Ghazarian, M.; Rotstein, O. Hemorrhagic Shock/Resuscitation Reduces the M2 Phenotype of Alveolar Macrophages: A Potential Mechanism Contributing to Increased LPS-Induced Lung Injury. Shock 2019, 51, 213–220. [Google Scholar] [CrossRef]
- Subauste, C.S.; de Waal Malefyt, R.; Fuh, F. Role of CD80 (B7.1) and CD86 (B7.2) in the Immune Response to an Intracellular Pathogen. J. Immunol. 1998, 160, 1831–1840. [Google Scholar]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.A.; Moestrup, S.K. Identification of the Haemoglobin Scavenger Receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Moestrup, S.; Møller, H. CD163: A Regulated Hemoglobin Scavenger Receptor with a Role in the Anti-inflammatory Response. Ann. Med. 2004, 36, 347–354. [Google Scholar] [CrossRef]
- Taylor, P.R.; Martinez-Pomares, L.; Stacey, M.; Lin, H.-H.; Brown, G.D.; Gordon, S. Macrophage Receptors and Immune Recognition. Annu. Rev. Immunol. 2004, 23, 901–944. [Google Scholar] [CrossRef]
- Thorbert-Mros, S.; Larsson, L.; Berglundh, T. Cellular Composition of Long-Standing Gingivitis and Periodontitis Lesions. J. Periodontal Res. 2015, 50, 535–543. [Google Scholar] [CrossRef]
- Fretwurst, T.; Müller, J.; Larsson, L.; Bronsert, P.; Hazard, D.; Castilho, R.M.; Kohal, R.; Nelson, K.; Iglhaut, G. Immunohistological Composition of Peri-Implantitis Affected Tissue around Ceramic Implants—A Pilot Study. J. Periodontol. 2021, 92, 571–579. [Google Scholar] [CrossRef]
- Almubarak, A.; Tanagala, K.K.K.; Papapanou, P.N.; Lalla, E.; Momen-Heravi, F. Disruption of Monocyte and Macrophage Homeostasis in Periodontitis. Front. Immunol. 2020, 11, 330. [Google Scholar] [CrossRef]
- Albrektsson, T.; Jemt, T.; Mölne, J.; Tengvall, P.; Wennerberg, A. On Inflammation-Immunological Balance Theory—A Critical Apprehension of Disease Concepts around Implants: Mucositis and Marginal Bone Loss May Represent Normal Conditions and Not Necessarily a State of Disease. Clin. Implant Dent. Relat. Res. 2019, 21, 183–189. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-Implant Health, Peri-Implant Mucositis, and Peri-Implantitis: Case Definitions and Diagnostic Considerations. J. Clin. Periodontol. 2018, 45, S278–S285. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Demir, E.; Gürsoy, M.; Firatli, E.; Gürsoy, U.K. Baseline Interleukin-10, CD163 and Tumor Necrosis Factor-like Weak Inducer of Apoptosis Gingival Tissue Levels in Relation to Clinical Periodontal Treatment Outcomes: A 12-Week Follow-up Study. J. Periodontol. 2022. ahead of print. [Google Scholar] [CrossRef]
- Veloso, P.; Fernández, A.; Terraza-Aguirre, C.; Álvarez, C.; Vernal, R.; Escobar, A.; Hernández, M. Macrophages Skew towards M1 Profile through Reduced CD163 Expression in Symptomatic Apical Periodontitis. Clin. Oral Investig. 2020, 24, 4571–4581. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Andersen, M.N.; Rittig, N.; Rødgaard-Hansen, S.; Grønbæk, H.; Moestrup, S.K.; Møller, H.J.; Etzerodt, A. The Macrophage-Related Biomarkers SCD163 and SCD206 Are Released by Different Shedding Mechanisms. J. Leukoc. Biol. 2019, 106, 1129–1138. [Google Scholar] [CrossRef]
- Alves-Januzzi, A.B.; Brunialti, M.K.C.; Salomao, R. CD163 and CD206 Expression Does Not Correlate with Tolerance and Cytokine Production in LPS-Tolerant Human Monocytes. Cytom. Part B Clin. Cytom. 2017, 92, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.C.; Gantzel, R.H.; Clària, J.; Trebicka, J.; Møller, H.J.; Grønbæk, H. Macrophage Activation Markers, CD163 and CD206, in Acute-on-Chronic Liver Failure. Cells 2020, 9, 1175. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.N.; Hønge, B.L.; Jespersen, S.; Medina, C.; da Silva Té, D.; Laursen, A.; Wejse, C.; Erikstrup, C.; Møller, H.J. Soluble Macrophage Mannose Receptor (SCD206/SMR) as a Biomarker in Human Immunodeficiency Virus Infection. J. Infect. Dis. 2018, 218, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Du, R.; Tan, N.S.; Ho, B.; Ding, J.L. CD163 and IgG Codefend against Cytotoxic Hemoglobin via Autocrine and Paracrine Mechanisms. J. Immonol. 2013, 190, 5267–5278. [Google Scholar] [CrossRef] [PubMed]
- Bover, L.C.; Cardó-Vila, M.; Kuniyasu, A.; Sun, J.; Rangel, R.; Takeya, M.; Aggarwal, B.B.; Arap, W.; Pasqualini, R. A Previously Unrecognized Protein-Protein Interaction between TWEAK and CD163: Potential Biological Implications. J. Immonol. 2007, 178, 8183–8194. [Google Scholar] [CrossRef] [PubMed]
- Fabriek, B.O.; van Bruggen, R.; Deng, D.M.; Ligtenberg, A.J.M.; Nazmi, K.; Schornagel, K.; Vloet, R.P.M.; Dijkstra, C.D.; van den Berg, T.K. The Macrophage Scavenger Receptor CD163 Functions as an Innate Immune Sensor for Bacteria. Blood 2009, 113, 887–892. [Google Scholar] [CrossRef]
- Martinez-Pomares, L. The Mannose Receptor. J. Leukoc. Biol. 2012, 92, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Detzen, L.; Chen, S.C.Y.; Cheng, B.; Papapanou, P.N.; Lalla, E. Increased Levels of Soluble CD163 in Periodontitis Patients. J. Clin. Periodontol. 2017, 44, 585–590. [Google Scholar] [CrossRef]
- Bos, I.; Johannisson, R. Foreign Body Reactions in Lymph Nodes ff Oncology Patients with Joint Prostheses—Light-, Electron Microscopic and Immunohistological Investigations. Pathol. Res. Pract. 2004, 200, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Soskic, B.; Jeffery, L.E.; Kennedy, A.; Gardner, D.H.; Hou, T.Z.; Halliday, N.; Williams, C.; Janman, D.; Rowshanravan, B.; Hirschfield, G.M.; et al. CD80 on Human T Cells Is Associated with FoxP3 Expression and Supports Treg Homeostasis. Front. Immunol. 2021, 11, 577655. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, E.; McHugh, G.B.; Grieco, D.A.; Seymour, G.J. Costimulatory Molecules in Human Periodontal Disease Tissues. J. Periodontal Res. 2001, 36, 92–100. [Google Scholar] [CrossRef]
- Nibali, L.; Novoa, L.; Donos, N.; Henderson, B.; Blanco, J.; Tomas, I. Leukocyte Receptor Expression in Chronic Periodontitis. Clin. Oral Investig. 2016, 20, 2559–2564. [Google Scholar] [CrossRef]
- Mahanonda, R.; Sa-Ard-Iam, N.; Yongvanitchit, K.; Wisetchang, M.; Ishikawa, I.; Nagasawa, T.; Walsh, D.S.; Pichyangkul, S. Upregulation of Co-Stimulatory Molecule Expression and Dendritic Cell Marker (CD83) on B Cells in Periodontal Disease. J. Periodontal Res. 2002, 37, 177–183. [Google Scholar] [CrossRef]
- Han, D.C.; Huang, G.T.J.; Lin, L.M.; Warner, N.A.; Gim, J.S.; Jewett, A. Expression of MHC Class II, CD70, CD80, CD86 and pro-Inflammatory Cytokines Is Differentially Regulated in Oral Epithelial Cells Following Bacterial Challenge. Oral Microbiol. Immunol. 2003, 18, 350–358. [Google Scholar] [CrossRef]
- Papadopoulos, G.; Shaik-Dasthagirisaheb, Y.B.; Huang, N.; Viglianti, G.A.; Henderson, A.J.; Kantarci, A.; Gibson, F.C. Immunologic Environment Influences Macrophage Response to Porphyromonas gingivalis. Mol. Oral Microbiol. 2017, 32, 250–261. [Google Scholar] [CrossRef]
- Page, R.C.; Schroeder, H.E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab. Investig. 1976, 34, 235–249. [Google Scholar]
- Hajishengallis, G.; Korostoff, J.M. Revisiting the Page & Schroeder Model: The Good, the Bad and the Unknowns in the Periodontal Host Response 40 Years Later. Periodontology 2000 2017, 75, 116–151. [Google Scholar] [CrossRef]
- Bystrom, J.; Evans, I.; Newson, J.; Stables, M.; Toor, I.; van Rooijen, N.; Crawford, M.; Colville-Nash, P.; Farrow, S.; Gilroy, D.W. Resolution-Phase Macrophages Possess a Unique Inflammatory Phenotype That Is Controlled By cAMP. Blood 2008, 112, 4117–4127. [Google Scholar] [CrossRef]
- Oshi, M.; Tokumaru, Y.; Asaoka, M.; Yan, L.; Satyananda, V.; Matsuyama, R.; Matsuhashi, N.; Futamura, M.; Ishikawa, T.; Yoshida, K.; et al. M1 Macrophage and M1/M2 Ratio Defined by Transcriptomic Signatures Resemble Only Part of Their Conventional Clinical Characteristics in Breast Cancer. Sci. Rep. 2020, 10, 16554. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.; Li, C.; Zhang, X.; Cheng, L.; Dai, L.; Wang, Y.; Wang, F.; Shi, G.; Li, Y.; et al. IL-35 Decelerates the Inflammatory Process by Regulating Inflammatory Cytokine Secretion and M1/M2 Macrophage Ratio in Psoriasis. J. Immonol. 2016, 197, 2131–2144. [Google Scholar] [CrossRef]
- Gürsoy, U.K.; Kantarci, A. Molecular Biomarker Research in Periodontology: A Roadmap for Translation of Science to Clinical Assay Validation. J. Clin. Periodontol. 2022, 49, 556–561. [Google Scholar] [CrossRef]
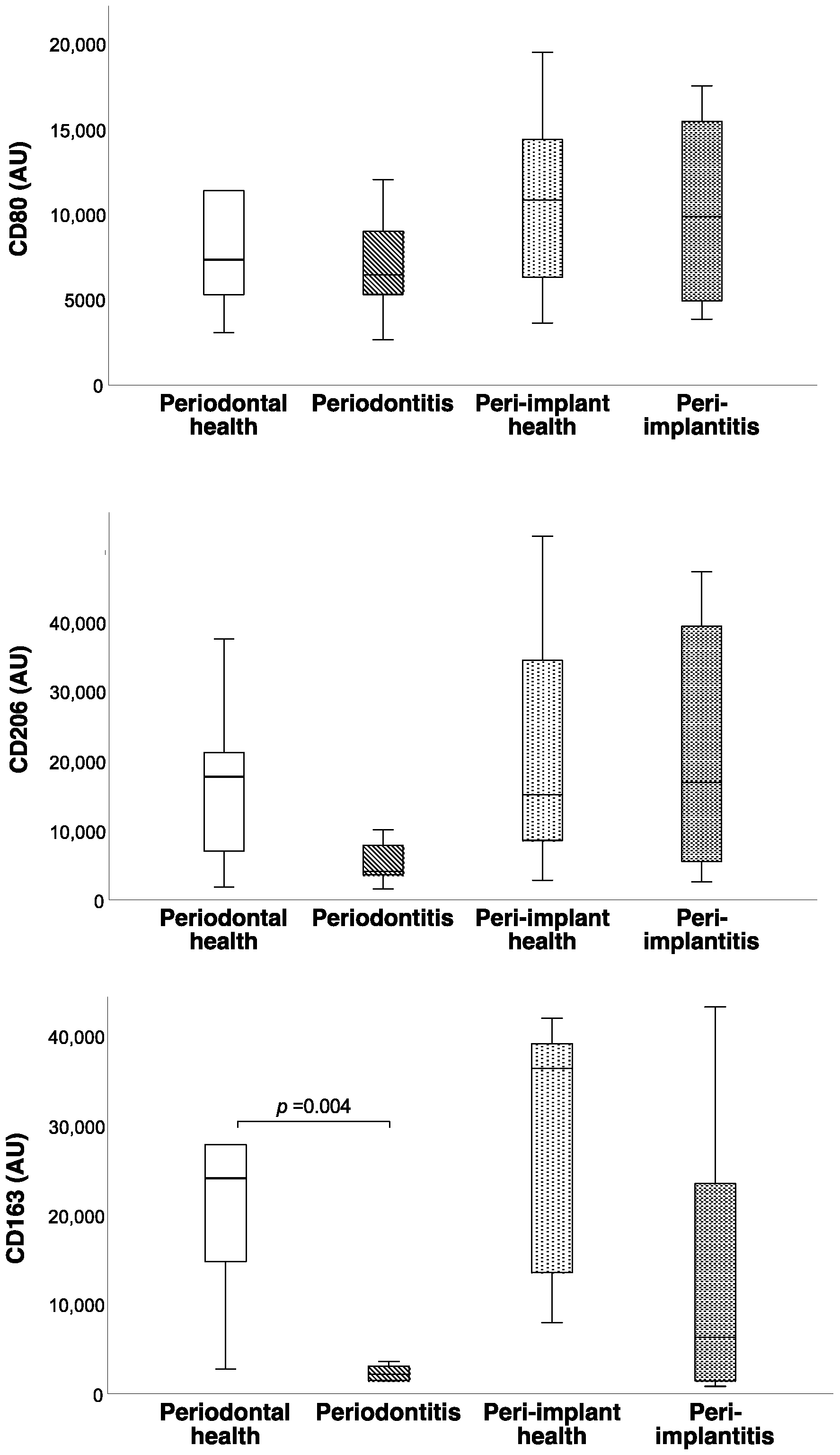
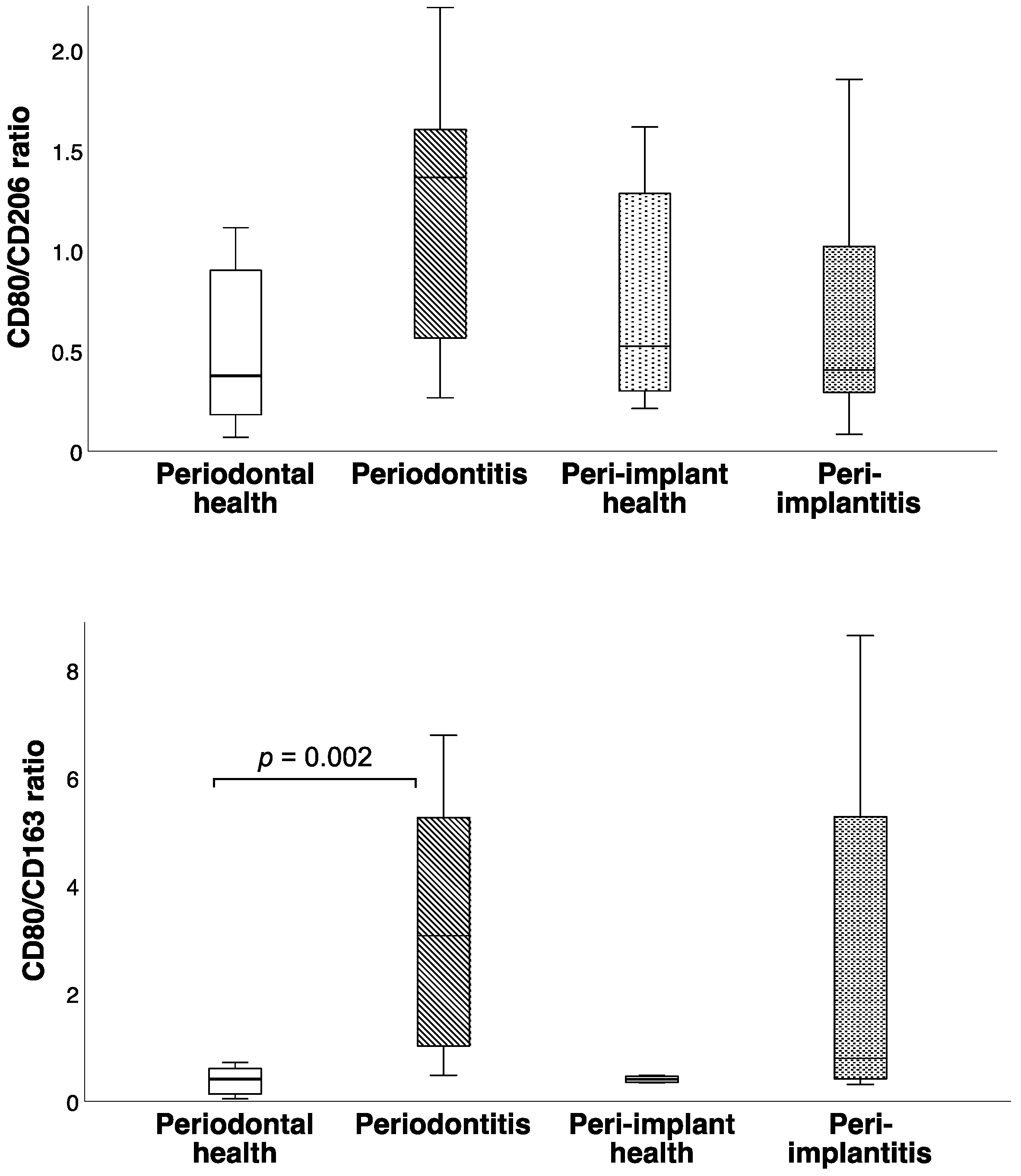
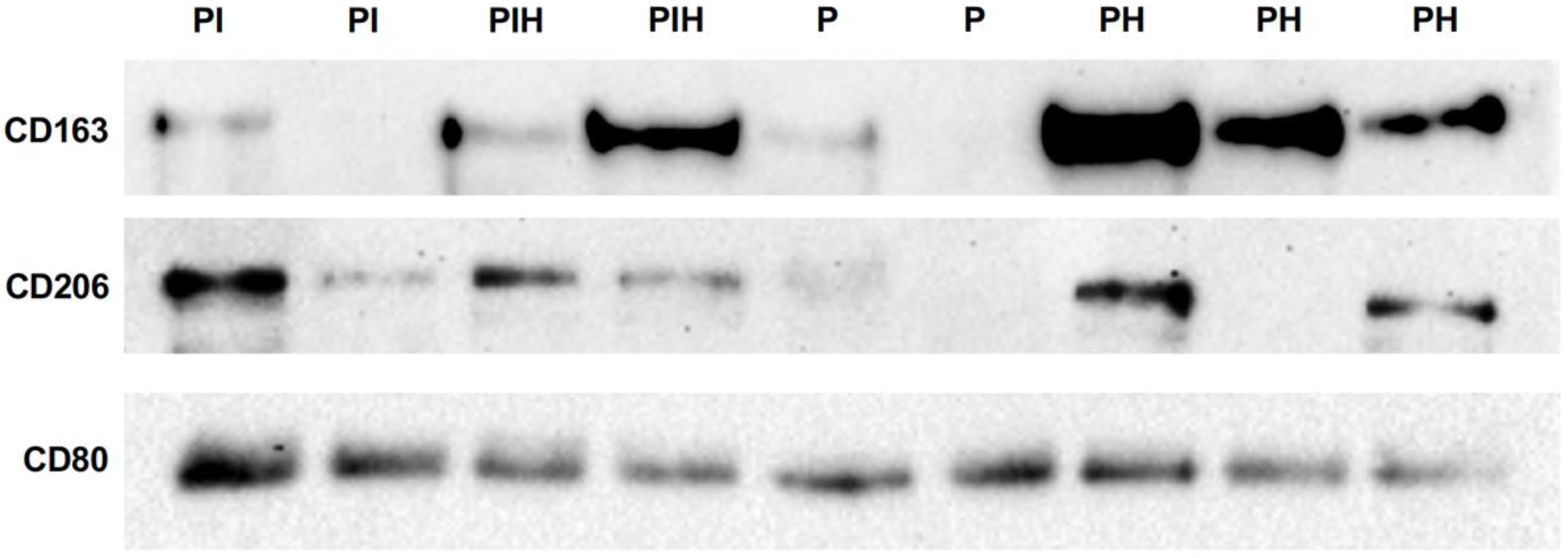
| Periodontal Health (n = 10) | Periodontitis (n = 9) | Peri-Implant Health (n = 8) | Peri-Implantitis (n = 9) | p | |
|---|---|---|---|---|---|
| Age (mean ± SD) | 44.0 ± 11.7 | 44.4 ± 11.4 | 43.0 ± 18.8 | 48.7 ± 10.3 | 0.822 |
| Male % | 50% | 33.3% | 62.5% | 33.3% | 0.556 |
| PPD (mean ± SD) | 1.5 ± 0.71 | 7.78 ± 0.83 | - | 7.78 ± 1.09 | <0.001 |
| CAL (mean ± SD) | - | 8.1 ± 0.88 | - | - | - |
| BoP % | 0 | 100 | - | 100 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, M.; Demir, E.; Firatli, Y.; Firatli, E.; Gürsoy, U.K. Tissue Levels of CD80, CD163 and CD206 and Their Ratios in Periodontal and Peri-Implant Health and Disease. Curr. Issues Mol. Biol. 2022, 44, 4704-4713. https://doi.org/10.3390/cimb44100321
Yilmaz M, Demir E, Firatli Y, Firatli E, Gürsoy UK. Tissue Levels of CD80, CD163 and CD206 and Their Ratios in Periodontal and Peri-Implant Health and Disease. Current Issues in Molecular Biology. 2022; 44(10):4704-4713. https://doi.org/10.3390/cimb44100321
Chicago/Turabian StyleYilmaz, Mustafa, Esra Demir, Yigit Firatli, Erhan Firatli, and Ulvi Kahraman Gürsoy. 2022. "Tissue Levels of CD80, CD163 and CD206 and Their Ratios in Periodontal and Peri-Implant Health and Disease" Current Issues in Molecular Biology 44, no. 10: 4704-4713. https://doi.org/10.3390/cimb44100321
APA StyleYilmaz, M., Demir, E., Firatli, Y., Firatli, E., & Gürsoy, U. K. (2022). Tissue Levels of CD80, CD163 and CD206 and Their Ratios in Periodontal and Peri-Implant Health and Disease. Current Issues in Molecular Biology, 44(10), 4704-4713. https://doi.org/10.3390/cimb44100321







