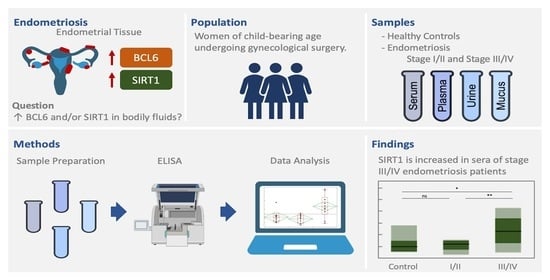
Evaluation of BCL6 and SIRT1 as Non-Invasive Diagnostic Markers of Endometriosis
Abstract
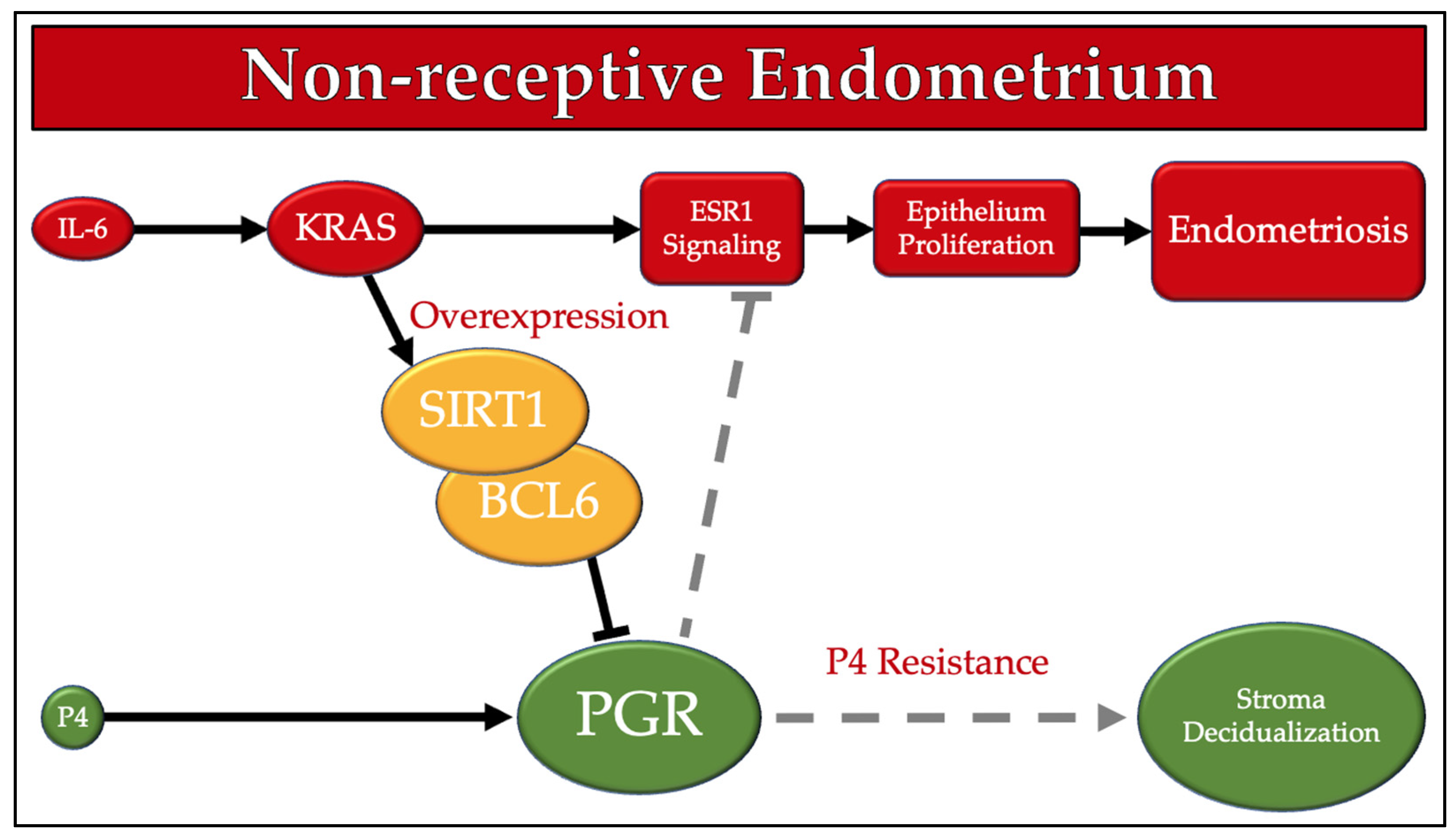
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Sample Processing and Storage
2.3. ELISA Procedure
2.4. Data Analysis
3. Results
3.1. BCL6 ELISA
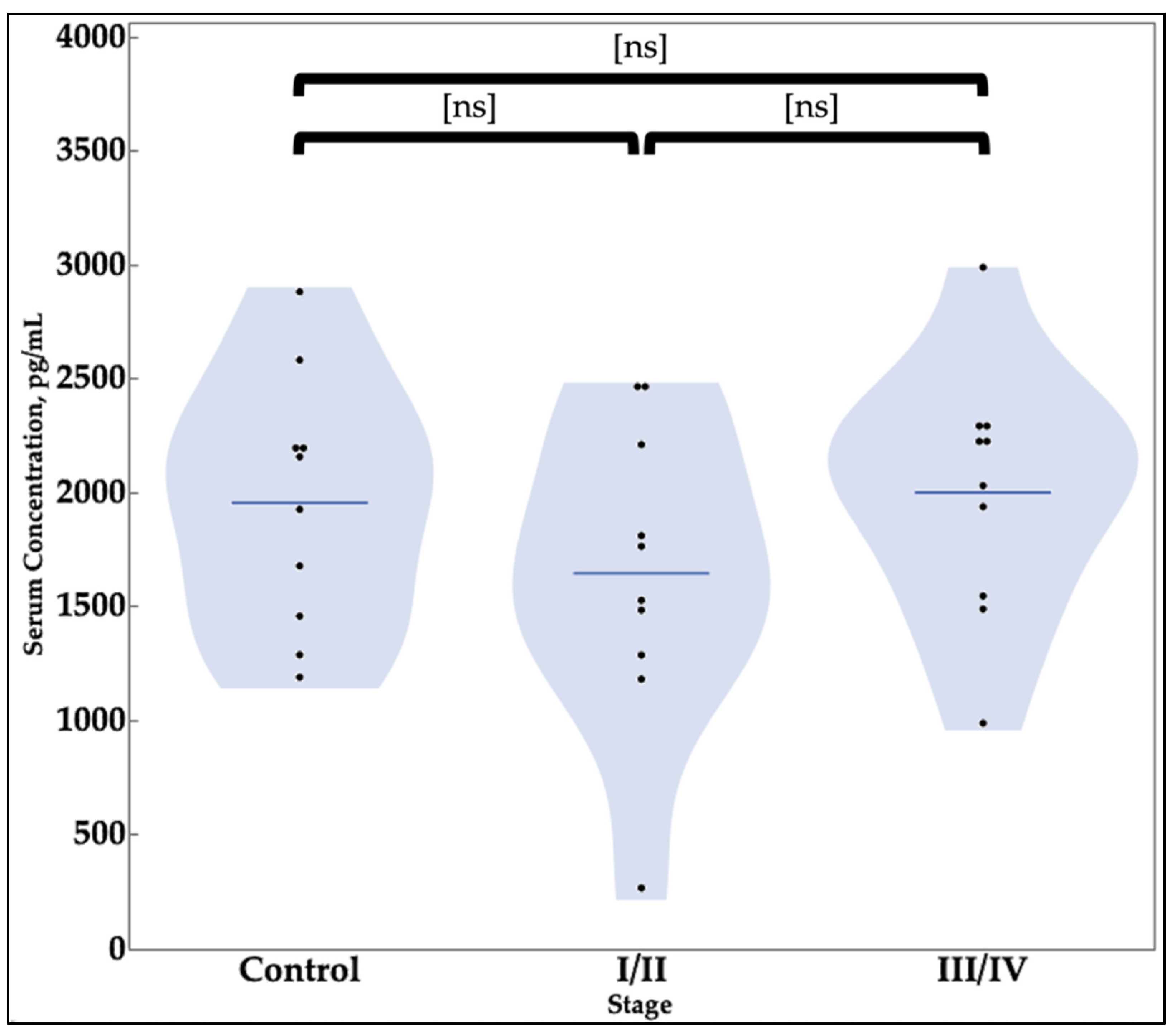
3.2. SIRT1 ELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullen, T.S. Adenomyoma of the uterus. J. Am. Med. Assoc. 1908, L, 107–115. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet (Lond. UK) 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Carter, J.E. Combined hysteroscopic and laparoscopic findings in patients with chronic pelvic pain. J. Am. Assoc. Gynecol. Laparosc. 1994, 2, 43–47. [Google Scholar] [CrossRef]
- Stratton, P.; Winkel, C.; Premkumar, A.; Chow, C.; Wilson, J.; Hearns-Stokes, R.; Heo, S.; Merino, M.; Nieman, L.K. Diagnostic accuracy of laparoscopy, magnetic resonance imaging, and histopathologic examination for the detection of endometriosis. Fertil. Steril. 2003, 79, 1078–1085. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; D’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. World Endometriosis Research Foundation Global Study of Women’s Health consortium Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299. [Google Scholar] [CrossRef]
- Simoens, S.; Hummelshoj, L.; D’Hooghe, T. Endometriosis: Cost estimates and methodological perspective. Hum. Reprod. Update 2007, 13, 395–404. [Google Scholar] [CrossRef]
- Soliman, A.M.; Surrey, E.; Bonafede, M.; Nelson, J.K.; Castelli-Haley, J. Real-World Evaluation of Direct and Indirect Economic Burden Among Endometriosis Patients in the United States. Adv. Ther. 2018, 35, 408–423. [Google Scholar] [CrossRef]
- Marjoribanks, J.; Ayeleke, R.O.; Farquhar, C.; Proctor, M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst. Rev. 2015, 2015, CD001751. [Google Scholar] [CrossRef]
- Rafique, S.; Decherney, A.H. Medical Management of Endometriosis. Clin. Obstet. Gynecol. 2017, 60, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Sourial, S.; Tempest, N.; Hapangama, D.K. Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 1984, 64, 151–154. [Google Scholar]
- Chantalat, E.; Valera, M.-C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef]
- Lessey, B.A.; Young, S.L. Homeostasis imbalance in the endometrium of women with implantation defects: The role of estrogen and progesterone. Semin. Reprod. Med. 2014, 32, 365–375. [Google Scholar] [CrossRef]
- Bulun, S.E.; Monsivais, D.; Kakinuma, T.; Furukawa, Y.; Bernardi, L.; Pavone, M.E.; Dyson, M. Molecular biology of endometriosis: From aromatase to genomic abnormalities. Semin. Reprod. Med. 2015, 33, 220–224. [Google Scholar] [CrossRef]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef]
- Patel, B.G.; Rudnicki, M.; Yu, J.; Shu, Y.; Taylor, R.N. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 2017, 96, 623–632. [Google Scholar] [CrossRef]
- Yoo, J.-Y.; Kim, T.H.; Fazleabas, A.T.; Palomino, W.A.; Ahn, S.H.; Tayade, C.; Schammel, D.P.; Young, S.L.; Jeong, J.-W.; Lessey, B.A. KRAS Activation and over-expression of SIRT1/BCL6 Contributes to the Pathogenesis of Endometriosis and Progesterone Resistance. Sci. Rep. 2017, 7, 6765. [Google Scholar] [CrossRef]
- Gaben, A.-M.; Saucier, C.; Bedin, M.; Redeuilh, G.; Mester, J. Mitogenic Activity of Estrogens in Human Breast Cancer Cells Does Not Rely on Direct Induction of Mitogen-Activated Protein Kinase/Extracellularly Regulated Kinase or Phosphatidylinositol 3-Kinase. Mol. Endocrinol. 2004, 18, 2700–2713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marquardt, R.M.; Kim, T.H.; Shin, J.-H.; Jeong, J.-W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Baron, B.W.; Nucifora, G.; McCabe, N.; Espinosa, R.; Le Beau, M.M.; McKeithan, T.W. Identification of the gene associated with the recurring chromosomal translocations t(3;14)(q27;q32) and t(3;22)(q27;q11) in B-cell lymphomas. Proc. Natl. Acad. Sci. USA 1993, 90, 5262–5266. [Google Scholar] [CrossRef]
- Chang, C.C.; Ye, B.H.; Chaganti, R.S.; Dalla-Favera, R. BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc. Natl. Acad. Sci. USA 1996, 93, 6947–6952. [Google Scholar] [CrossRef] [PubMed]
- Arima, M.; Fukuda, T.; Tokuhisa, T. Role of the Transcriptional Repressor BCL6 in Allergic Response and Inflammation. World Allergy Organ. J. 2008, 1, 115–122. [Google Scholar] [CrossRef]
- Iqbal, J.; Greiner, T.C.; Patel, K.; Dave, B.J.; Smith, L.; Ji, J.; Wright, G.; Sanger, W.G.; Pickering, D.L.; Jain, S.; et al. Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B-cell lymphoma. Leukemia 2007, 21, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Allman, D.; Jain, A.; Dent, A.; Maile, R.R.; Selvaggi, T.; Kehry, M.R.; Staudt, L.M. BCL-6 expression during B-cell activation. Blood 1996, 87, 5257–5268. [Google Scholar] [CrossRef] [PubMed]
- Pasqualucci, L.; Migliazza, A.; Basso, K.; Houldsworth, J.; Chaganti, R.S.K.; Dalla-Favera, R. Mutations of the BCL6 proto-oncogene disrupt its negative autoregulation in diffuse large B-cell lymphoma. Blood 2003, 101, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Basso, K.; Dalla-Favera, R. BCL6: Master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv. Immunol. 2010, 105, 193–210. [Google Scholar] [CrossRef]
- Evans-Hoeker, E.; Lessey, B.A.; Jeong, J.W.; Savaris, R.F.; Palomino, W.A.; Yuan, L.; Schammel, D.P.; Young, S.L. Endometrial BCL6 Overexpression in Eutopic Endometrium of Women With Endometriosis. Reprod. Sci. 2016, 23, 1234–1241. [Google Scholar] [CrossRef]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef]
- SIRT1 Sirtuin 1 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/23411 (accessed on 6 August 2021).
- Frye, R.A. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem. Biophys. Res. Commun. 1999, 260, 273–279. [Google Scholar] [CrossRef]
- Sinclair, D.A.; Guarente, L. Unlocking the secrets of longevity genes. Sci. Am. 2006, 294, 48–51, 54–57. [Google Scholar] [CrossRef]
- Langley, E.; Pearson, M.; Faretta, M.; Bauer, U.-M.; Frye, R.A.; Minucci, S.; Pelicci, P.G.; Kouzarides, T. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002, 21, 2383–2396. [Google Scholar] [CrossRef]
- Luo, J.; Nikolaev, A.Y.; Imai, S.; Chen, D.; Su, F.; Shiloh, A.; Guarente, L.; Gu, W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 2001, 107, 137–148. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Ng Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on its biological functions. Cell Commun. Signal. 2011, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K. The Enigmatic Role of Sir2 in Aging. Cell 2005, 123, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fang, D. The Roles of SIRT1 in Cancer. Genes Cancer 2013, 4, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Rezk, N.A.; Lashin, M.B.; Sabbah, N.A. MiRNA 34-a regulate SIRT-1 and Foxo-1 expression in endometriosis. Non-Coding RNA Res. 2021, 6, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Buyalos, R.P.; Funari, V.A.; Azziz, R.; Watson, J.M.; Martinez-Maza, O. Elevated interleukin-6 levels in peritoneal fluid of patients with pelvic pathology*†. Fertil. Steril. 1992, 58, 302–306. [Google Scholar] [CrossRef]
- Pellicer, A.; Albert, C.; Mercader, A.; Bonilla-Musoles, F.; Remohí, J.; Simón, C. The follicular and endocrine environment in women with endometriosis: Local and systemic cytokine production. Fertil. Steril. 1998, 70, 425–431. [Google Scholar] [CrossRef]
- Kim, B.G.; Yoo, J.-Y.; Kim, T.H.; Shin, J.-H.; Langenheim, J.F.; Ferguson, S.D.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Jeong, J.-W. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum. Reprod. 2015, 30, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Arguni, E.; Arima, M.; Tsuruoka, N.; Sakamoto, A.; Hatano, M.; Tokuhisa, T. JunD/AP-1 and STAT3 are the major enhancer molecules for high Bcl6 expression in germinal center B cells. Int. Immunol. 2006, 18, 1079–1089. [Google Scholar] [CrossRef]
| Categories | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Age, years | 18–42 | >42 |
| Menopausal Status | Premenopausal | Postmenopausal |
| Menstruation Status | Cycle frequency 21–35 days | Cycle < 21 days or >35 days |
| Past Medical History * | NA | PCOS, psychiatric history, diabetes, known communicable infections |
| Medications | NA | Hormonal therapy in the past 3 months |
| Pregnancy Status | None | Pregnant |
| Surgery Type | Gynecological ** | Oncologic |
| Surgery, Count | Control Group | Stage I/II | Stage III/IV |
|---|---|---|---|
| Adhesion Lysis | 0 | 0 | 2 |
| Adnexal Removal | 1 | 0 | 0 |
| Chromotubation | 0 | 1 | 1 |
| Dilation and Curettage | 0 | 0 | 1 |
| Endometriosis Resection | 2 | 6 | 3 |
| Hysterectomy | 1 | 2 | 1 |
| Myomectomy | 0 | 3 | 0 |
| Ovarian Cystectomy | 1 | 0 | 2 |
| Oviduct Fulguration | 2 | 0 | 0 |
| Salpingectomy | 2 | 0 | 0 |
| Characteristic | Control | I/II | III/IV |
|---|---|---|---|
| Age, mean (SD) | 29.7 (4.9) | 34.5 (5.8) | 31.6 (3.5) |
| Race, % | |||
| African American | 40 | 20 | 0 |
| Asian | 0 | 0 | 10 |
| White | 50 | 60 | 90 |
| White/Hispanic | 0 | 10 | 0 |
| Hispanic | 10 | 0 | 0 |
| Unknown | 0 | 10 | 0 |
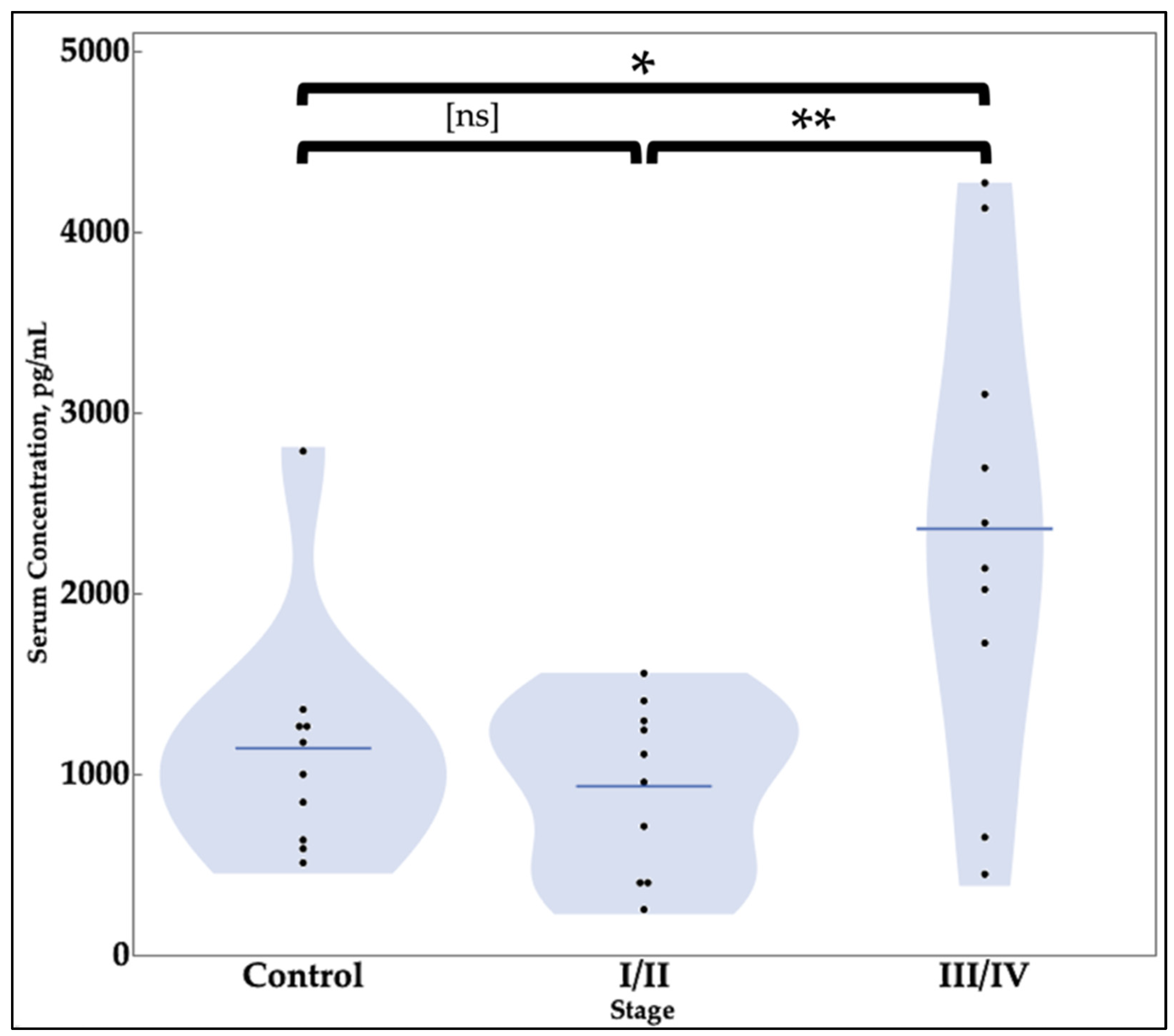
| Value Category | SIRT1 | BCL6 |
|---|---|---|
| Concentration—pg/mL, mean (SD) * | ||
| Negative Controls | 1141.7 (655.4) | 1953.8 (554.0) |
| Stage I/II Endometriosis | 932.3 (466.3) | 1645.7 (665.9) |
| Stages III/IV Endometriosis | 2357.5 (1274.5) | 2000.5 (552.4) |
| t-test Results | ||
| Negative Controls vs. Stages I/II | 0.4213 | 0.2754 |
| Negative Controls vs. Stages III/IV | 0.0152 ** | 0.8525 |
| Stages I/II vs. Stages III/IV | 0.0038 ** | 0.2111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansone, A.M.; Hisrich, B.V.; Young, R.B.; Abel, W.F.; Bowens, Z.; Blair, B.B.; Funkhouser, A.T.; Schammel, D.P.; Green, L.J.; Lessey, B.A.; et al. Evaluation of BCL6 and SIRT1 as Non-Invasive Diagnostic Markers of Endometriosis. Curr. Issues Mol. Biol. 2021, 43, 1350-1360. https://doi.org/10.3390/cimb43030096
Sansone AM, Hisrich BV, Young RB, Abel WF, Bowens Z, Blair BB, Funkhouser AT, Schammel DP, Green LJ, Lessey BA, et al. Evaluation of BCL6 and SIRT1 as Non-Invasive Diagnostic Markers of Endometriosis. Current Issues in Molecular Biology. 2021; 43(3):1350-1360. https://doi.org/10.3390/cimb43030096
Chicago/Turabian StyleSansone, Alison M., Brooke V. Hisrich, R. Brandt Young, William F. Abel, Zachary Bowens, Bailey B. Blair, Avery T. Funkhouser, David P. Schammel, Lisa J. Green, Bruce A. Lessey, and et al. 2021. "Evaluation of BCL6 and SIRT1 as Non-Invasive Diagnostic Markers of Endometriosis" Current Issues in Molecular Biology 43, no. 3: 1350-1360. https://doi.org/10.3390/cimb43030096
APA StyleSansone, A. M., Hisrich, B. V., Young, R. B., Abel, W. F., Bowens, Z., Blair, B. B., Funkhouser, A. T., Schammel, D. P., Green, L. J., Lessey, B. A., & Blenda, A. V. (2021). Evaluation of BCL6 and SIRT1 as Non-Invasive Diagnostic Markers of Endometriosis. Current Issues in Molecular Biology, 43(3), 1350-1360. https://doi.org/10.3390/cimb43030096