Amelioration of Diabetic Nephropathy by Targeting Autophagy via Rapamycin or Fasting: Relation to Cell Apoptosis/Survival
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Drugs and Chemicals
2.3. Animals
2.4. Experimental Design
2.5. Preliminary Pilot Studies for Rapamycin Dosage Optimization
2.6. Experimental Induction of Diabetes Mellitus
2.7. Urine Collection
2.8. Blood Sampling
2.9. Blood Chemistry
2.10. Tissue Sampling
2.11. Western Blot (WB) Analysis
2.12. Transmission Electron Microscope (TEM)
2.13. Statistical Analysis
3. Results
3.1. DM Markers
3.2. Kidney and Liver Function Tests
3.3. Nucleic Acid Integrity
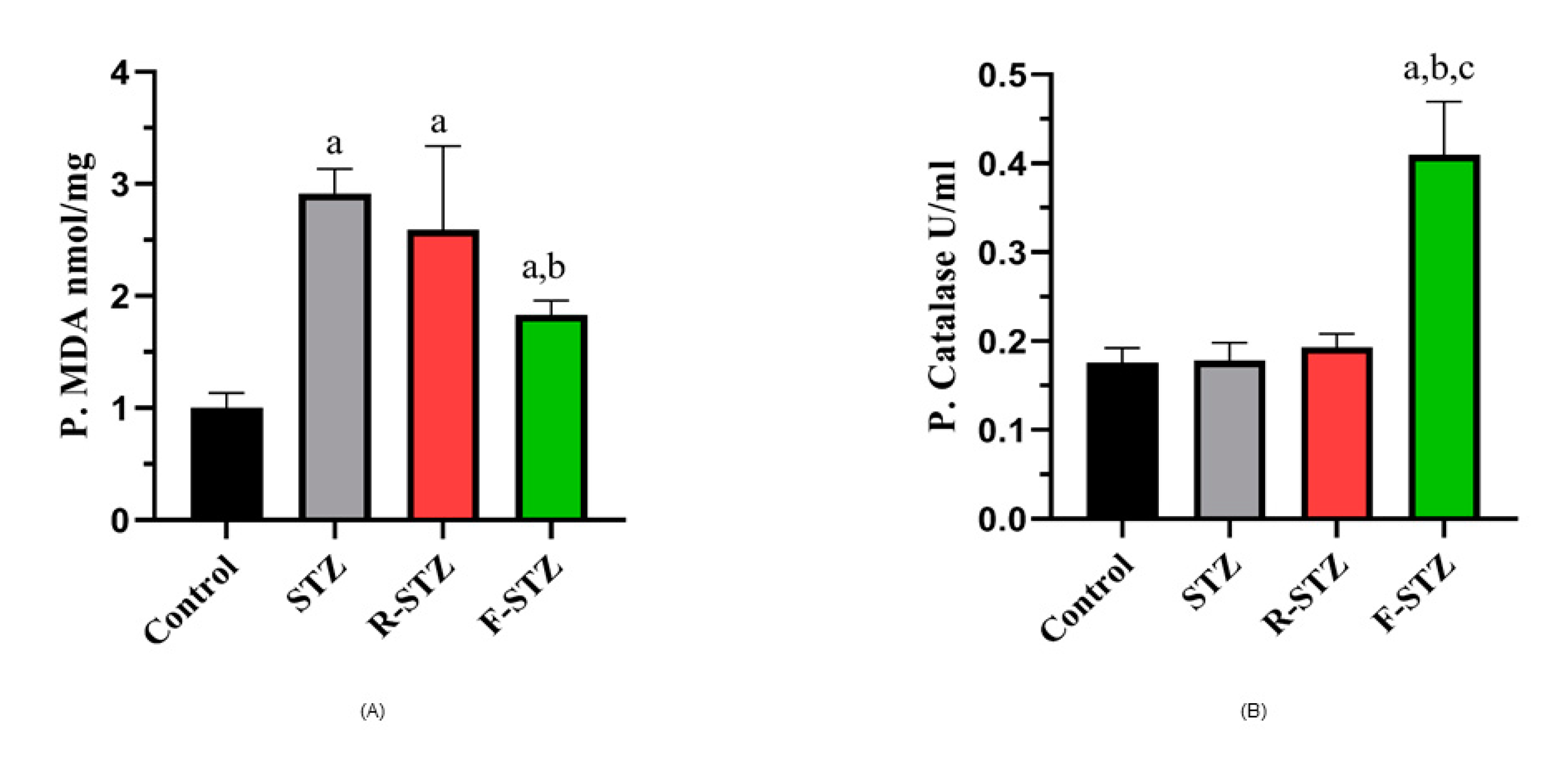
3.4. Tissue Oxidative Stress Estimation
3.5. Cellular Autophagy
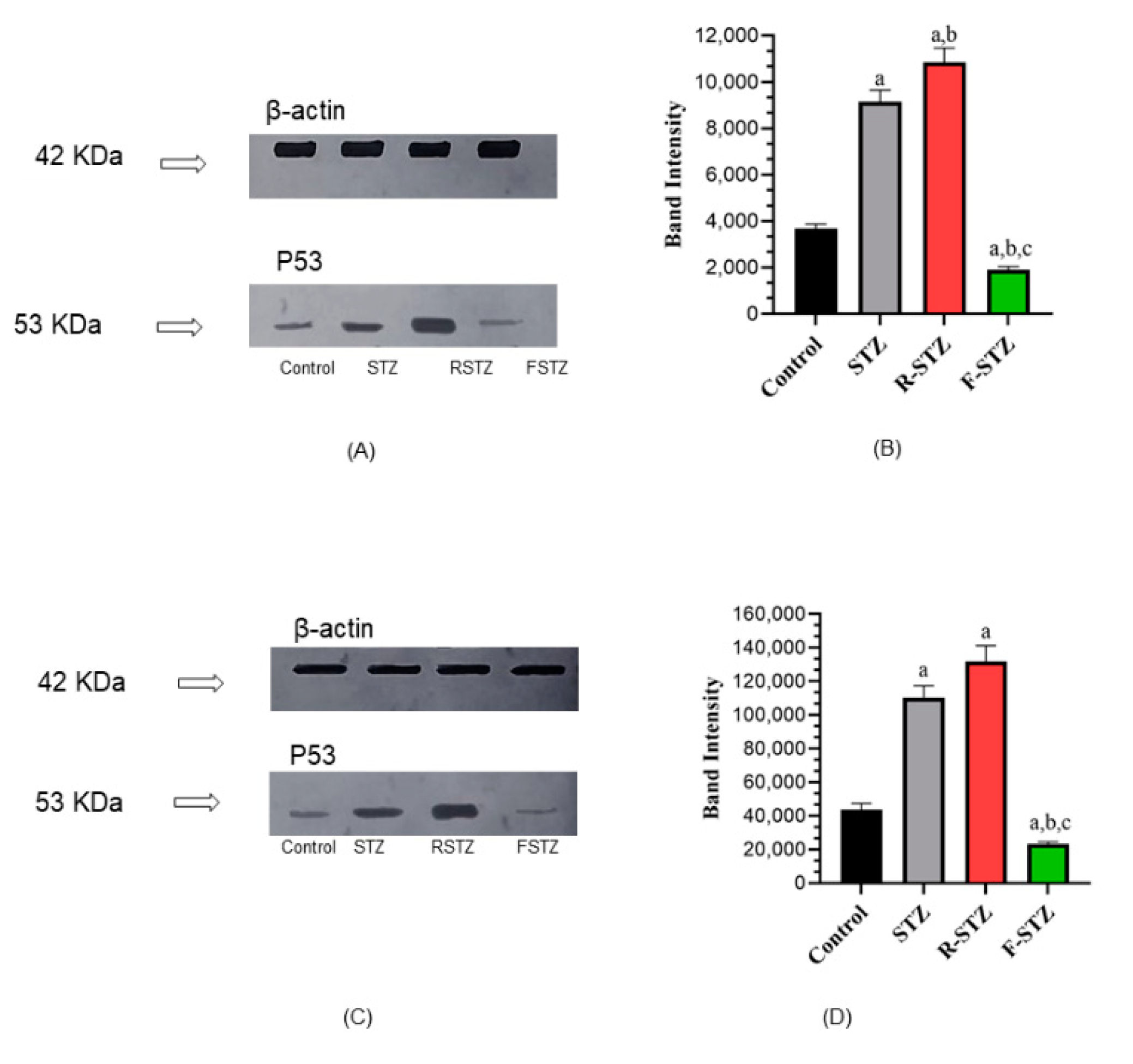
3.6. Cellular Apoptosis
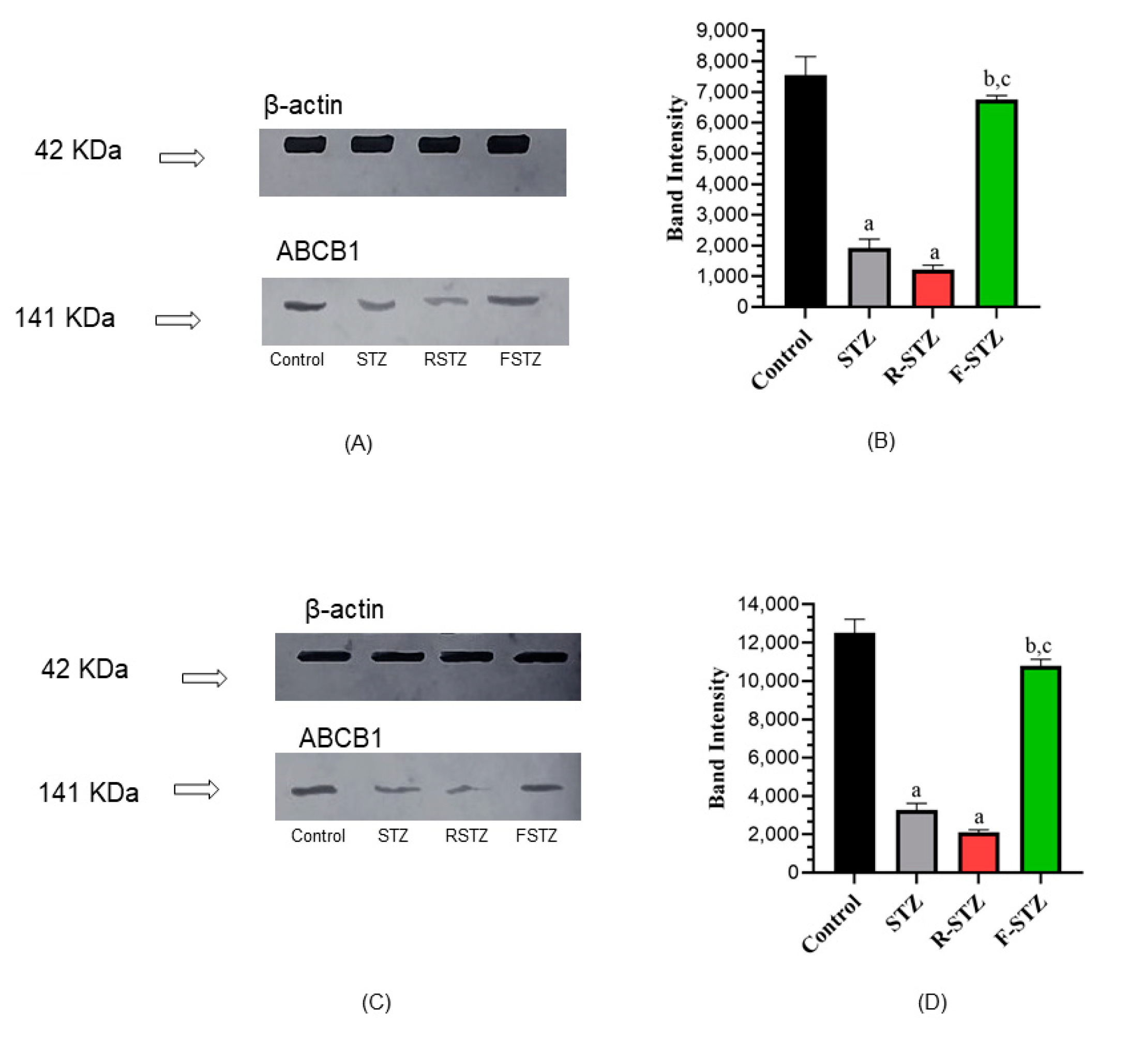
3.7. Cellular Permeability-Glycoprotein; ABCB1 Estimation
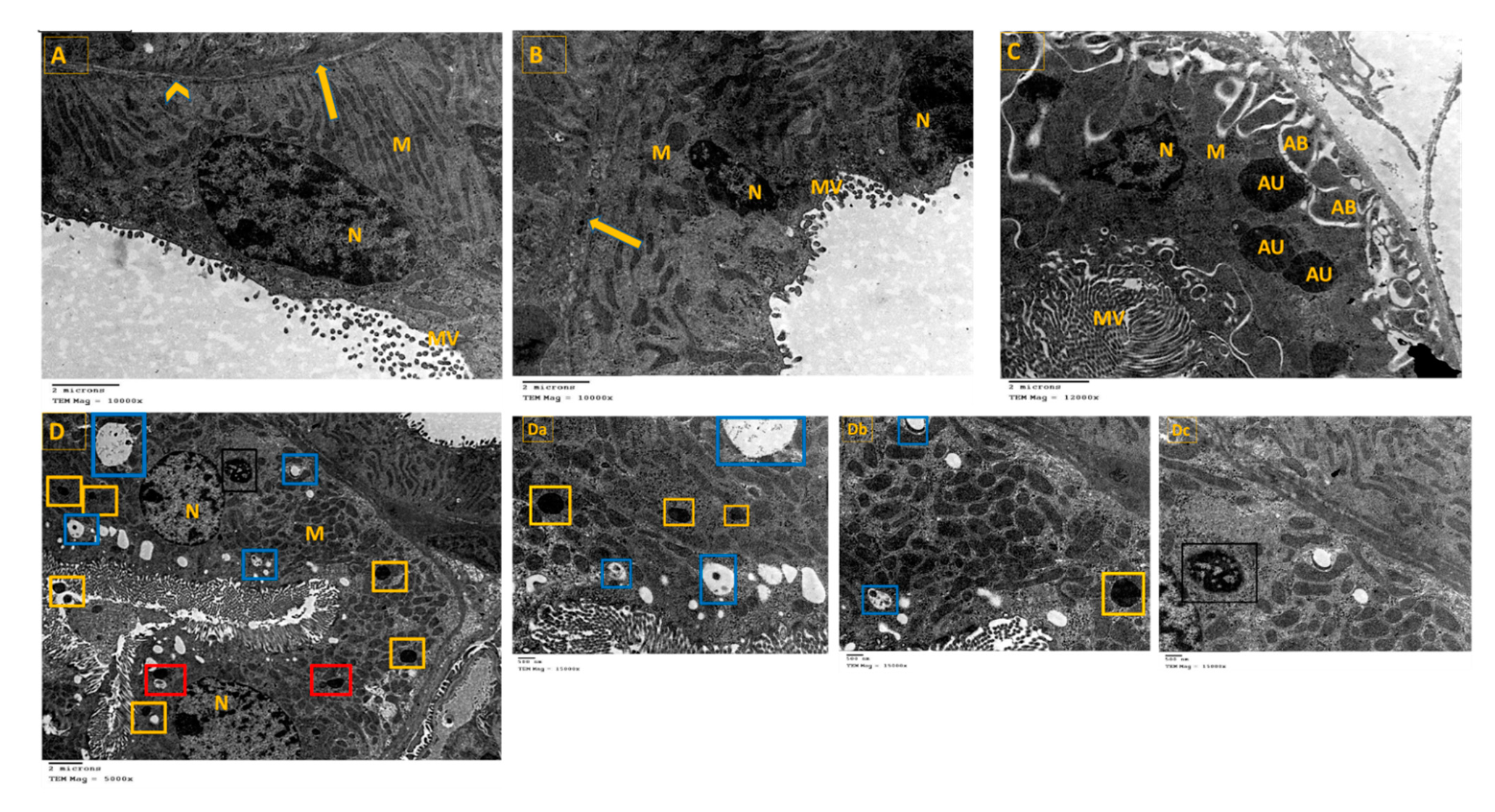
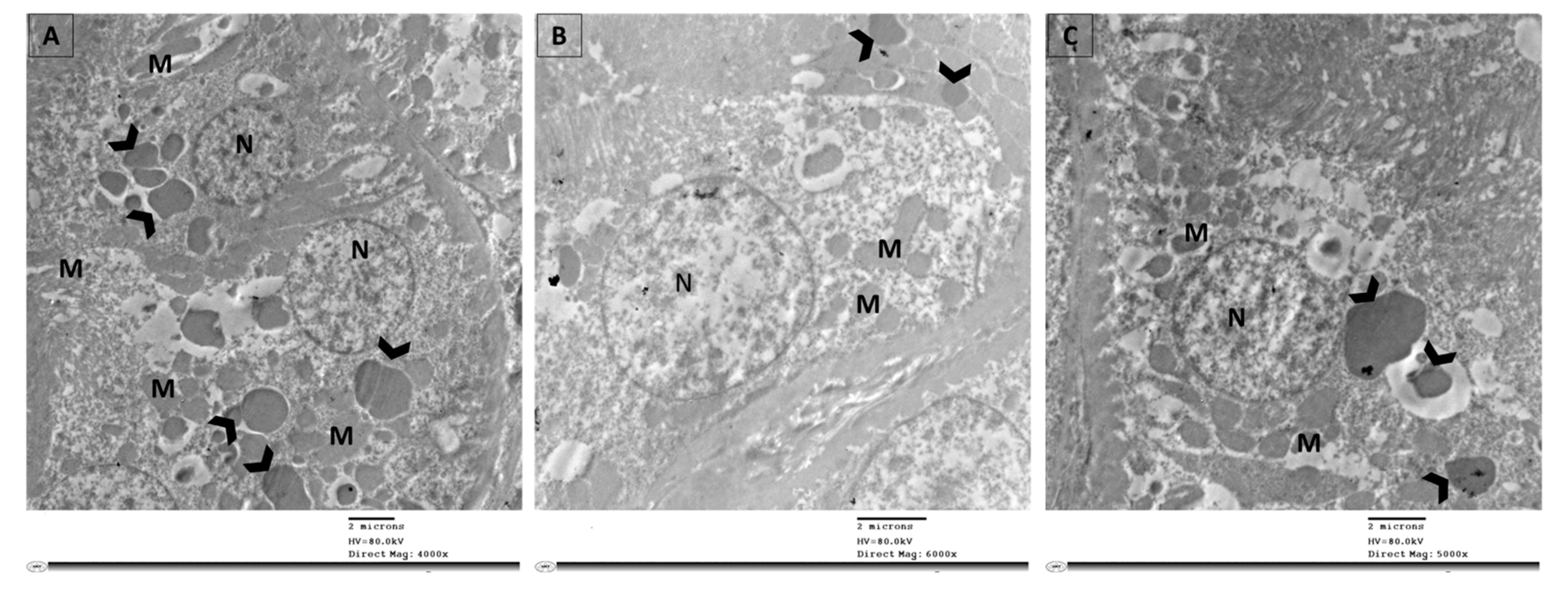
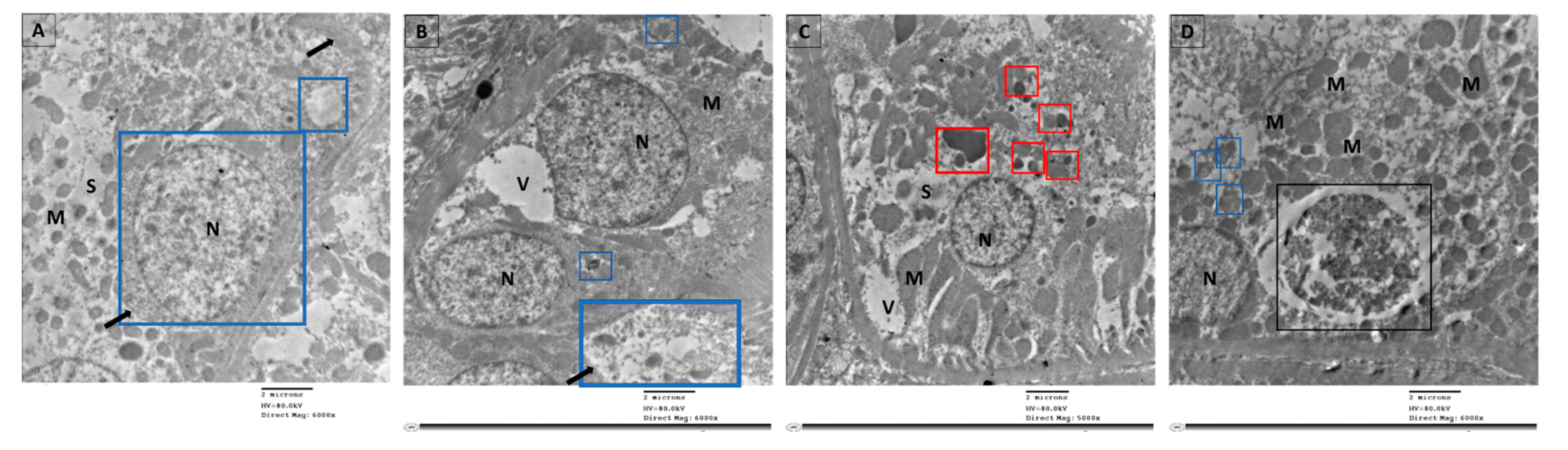
3.8. TEM Was Employed to Visualize the Occurrence of Autophagy as Evaluated by Autophagosome Formation
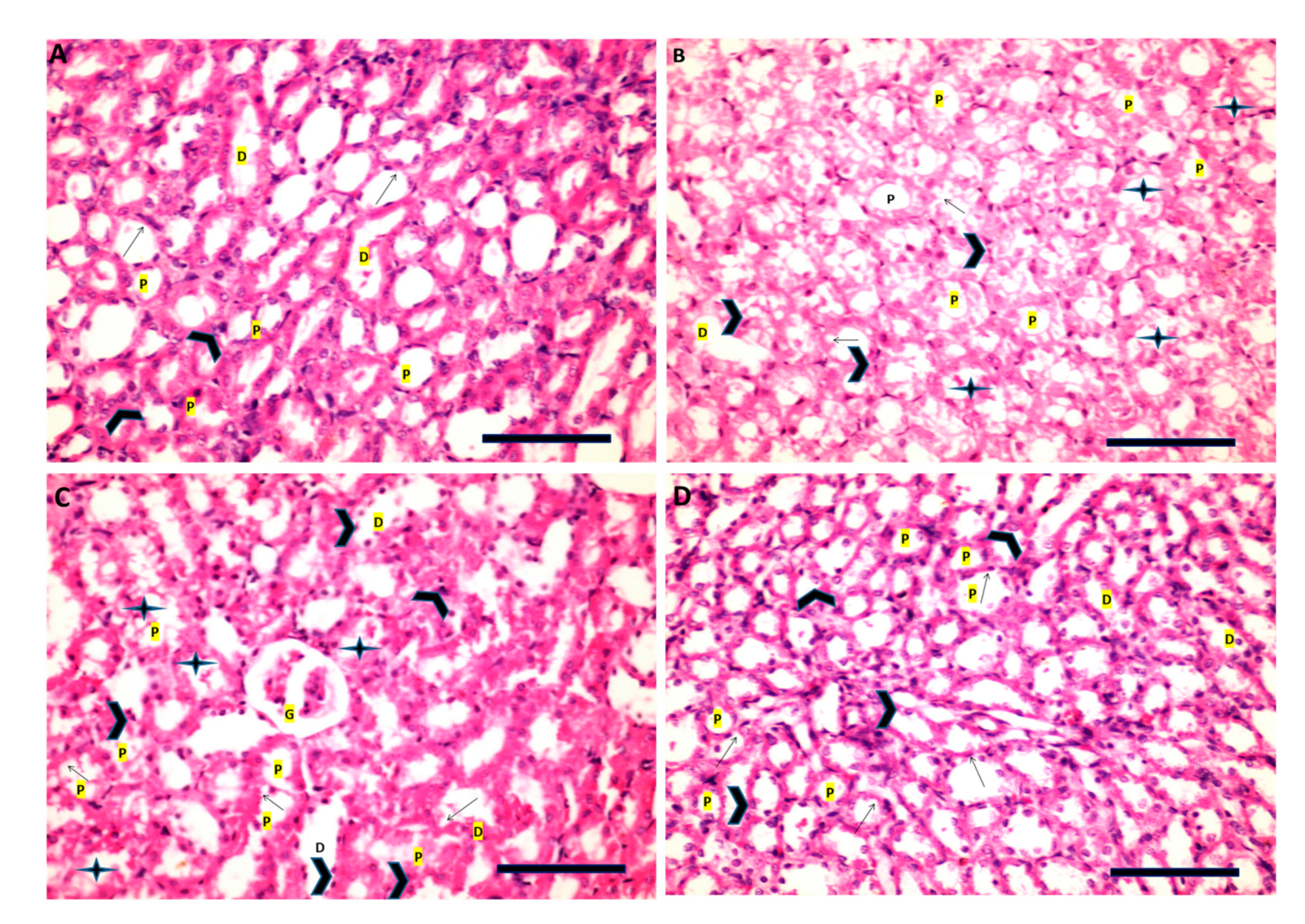
3.9. Histopathological Examination (H&E)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.H.; Kim, J.; Park, K.; Lee, M.S. β-cell autophagy: Mechanism and role in β-cell dysfunction. Mol. Metab. 2019, 27, S92–S103. [Google Scholar] [CrossRef]
- Hur, K.Y.; Jung, H.S.; Lee, M.S. Role of autophagy in β-cell function and mass. Diabetes Obes. Metab. 2010, 12 (Suppl. 2), 20–26. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Turner, N. Mitochondrial dysfunction and insulin resistance: An update. Endocr. Connect. 2015, 4, R1–R15. [Google Scholar] [CrossRef] [Green Version]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Sadraie, B.; Steckhan, N.; Kessler, C.; Stange, R.; Jeitler, M.; Michalsen, A. Effects of A One-week Fasting Therapy in Patients with Type-2 Diabetes Mellitus and Metabolic Syndrome—A Randomized Controlled Explorative Study. Exp. Clin. Endocrinol. Diabetes 2017, 125, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; De Cabo, R. A time to fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall, K.B.; Færgeman, N.J. Metabolic regulation of lifespan from a C. Elegans perspective. Genes Nutr. 2019, 14, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Mukhopadhyay, M.; Bhattacharyya, M.; Karmakar, P. Is autophagy associated with diabetes mellitus and its complications? A review. EXCLI J. 2018, 17, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, M.; Ohmura, Y.; Deguchi, T.; Machida, T.; Tsukamoto, R.; Wada, H.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; Ito, T.; et al. Rapamycin Causes Upregulation of Autophagy and Impairs Islets Function Both In Vitro and In Vivo. Arab. Archaeol. Epigr. 2012, 12, 102–114. [Google Scholar] [CrossRef]
- Renna, M.; Jimenez-Sanchez, M.; Sarkar, S.; Rubinsztein, D.C. Chemical Inducers of Autophagy That Enhance the Clearance of Mutant Proteins in Neurodegenerative Diseases. J. Biol. Chem. 2010, 285, 11061–11067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Klionsky, D.J. Regulation Mechanisms and Signaling Pathways of Autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [Green Version]
- Ballou, L.M.; Lin, R.Z. Rapamycin and mTOR kinase inhibitors. J. Chem. Biol. 2008, 1, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, S.; Dancourt, J.; Shteyn, V.; Puente, G.; Fong, W.M.; Nag, S.; Bewersdorf, J.; Yamamoto, A.; Antonny, B.; Melia, T.J. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat. Cell Biol. 2014, 16, 415–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livesey, K.M.; Kang, R.; Vernon, P.; Buchser, W.; Loughran, P.; Watkins, S.; Zhang, L.; Manfredi, J.J.; Zeh, H.J.; Li, L.; et al. p53/HMGB1 Complexes Regulate Autophagy and Apoptosis. Cancer Res. 2012, 72, 1996–2005. [Google Scholar] [CrossRef] [Green Version]
- Maiuri, M.C.; Galluzzi, L.; Morselli, E.; Kepp, O.; Malik, S.A.; Kroemer, G. Autophagy regulation by p53. Curr. Opin. Cell Biol. 2010, 22, 181–185. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kume, S.; Kitada, M.; Kanasaki, K.; Uzu, T.; Maegawa, H.; Koya, D. Autophagy as a Therapeutic Target in Diabetic Nephropathy. Exp. Diabetes Res. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Choi, M.E. Autophagy in diabetic nephropathy. J. Endocrinol. 2014, 224, R15–R30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maric, C.; Hall, J.E. Obesity, Metabolic Syndrome and Diabetic Nephropathy. Contrib. Nephrol. 2011, 170, 28–35. [Google Scholar]
- USRDS: The United States Renal Data System. Am. J. Kidney Dis. 2003, 42 (Suppl. 5), 1–230. [CrossRef]
- Masereeuw, R.; Russel, F.G.M. Regulatory Pathways for ATP-binding Cassette Transport Proteins in Kidney Proximal Tubules. AAPS J. 2012, 14, 883–894. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornilova, E.S. Receptor-mediated endocytosis and cytoskeleton. Biochemistry 2014, 79, 865–878. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Jonker, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Liu, H.; Xu, X.; Yang, Z.; Deng, Y.; Liu, X.; Xie, L. Impaired function and expression of P-glycoprotein in blood–brain barrier of streptozotocin-induced diabetic rats. Brain Res. 2006, 1123, 245–252. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. MTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Kume, S.; Thomas, M.C.; Koya, D. Nutrient sensing, autophagy, and diabetic nephropathy. Diabetes 2012, 61, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Lopez, N.; Tarabra, E.; Toledo, M.; Garcia-Macia, M.; Sahu, S.; Coletto, L.; Batista-Gonzalez, A.; Barzilai, N.; Pessin, J.E.; Schwartz, G.J.; et al. System-wide Benefits of Intermeal Fasting by Autophagy. Cell Metab. 2017, 26, 856–871.e5. [Google Scholar] [CrossRef] [Green Version]
- Benevenga, N.J.; Calvert, C.; Eckhert, C.D.; Fahey, G.C.; Greger, J.L.; Keen, C.L.; Knapka, J.J.; Magalhaes, H.; Oftedal, O.T.; Reeves, P.G.; et al. Nutrient Requirements of the Laboratory Rat; The National Academic Press: Washington, DC, USA, 1995. [Google Scholar]
- Lu, Z.; Liu, F.; Chen, L.; Zhang, H.; Ding, Y.; Liu, J.; Wong, M.; Zeng, L.-H. Effect of Chronic Administration of Low Dose Rapamycin on Development and Immunity in Young Rats. PLoS ONE 2015, 10, e0135256. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Erustes, A.; Costa, A.J.; Nascimento, A.C.; Bincoletto, C.; Ureshino, R.P.; Pereira, G.J.S.; Smaili, S.S. Autophagy and intermittent fasting: The connection for cancer therapy? Clinics 2018, 73, e814s. [Google Scholar] [CrossRef] [PubMed]
- Norecopa. Fasting in Rodents. Norecopa Vet. Inst. 2009, 1–11. Available online: https://norecopa.no/media/6351/food-deprivation.pdf (accessed on 7 September 2021).
- Food Regulation and Restriction in Rodents | Research Support. [Online]. Available online: https://www.bu.edu/researchsupport/compliance/animal-care/working-with-animals/food-regulation-and-restriction-in-rodents/ (accessed on 29 September 2021).
- Guidelines on Use of Streptozotocin in Rodents|Research A to Z. 2018. [Online]. Available online: https://az.research.umich.edu/animalcare/guidelines/guidelines-use-streptozotocin-rodents (accessed on 21 March 2020).
- King, A.J.F. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [Green Version]
- Ruehl-Fehlert, C.; Kittel, B.; Morawietz, G.; Deslex, P.; Keenan, C.; Mahrt, C.R.; Nolte, T.; Robinson, M.; Stuart, B.P.; Deschl, U. Revised guides for organ sampling and trimming in rats and Mice—Part 1: A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 2003, 55, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Ruehl-Fehlert, C.; Kittel, B.; Morawietz, G.; Deslex, P.; Keenan, C.; Mahrt, C.R.; Nolte, T.; Robinson, M.; Stuart, B.P.; Deschl, U. Revised guides for organ sampling and trimming in rats and Mice—Part 3. Exp. Toxicol. Pathol. 2003, 55, 433–449. [Google Scholar] [CrossRef] [Green Version]
- Ylä-Anttila, P.; Vihinen, H.; Jokitalo, E.; Eskelinen, E.L. Monitoring autophagy by electron microscopy in Mammalian cells. Methods Enzym. 2009, 452, 143–164. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Mahmood, T.; Yang, P.C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar] [PubMed]
- Ayache, J.; Beaunier, L.; Boumendil, J.; Ehret, G.; Laub, D. Sample Preparation Handbook for Transmission Electron Microscopy; Springer: New York, NY, USA, 2010. [Google Scholar]
- Lin, X.; Han, L.; Weng, J.; Wang, K.; Chen, T. Rapamycin inhibits proliferation and induces autophagy in human neuroblastoma cells. Biosci. Rep. 2018, 38, BSR20181822. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Livingston, M.J.; Liu, Z.; Dong, G.; Zhang, M.; Chen, J.K.; Dong, Z. Autophagy in diabetic kidney disease: Regulation, pathological role and therapeutic potential. Cell. Mol. Life Sci. 2018, 75, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Goginashvili, A.; Zhang, Z.; Erbs, E.; Spiegelhalter, C.; Kessler, P.; Mihlan, M.; Pasquier, A.; Krupina, K.; Schieber, N.; Cinque, L. Insulin secretory granules control autophagy in Pancreatic β cells. Science 2015, 347, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Javaheri, A.; Godar, R.J.; Murphy, J.; Ma, X.; Rohatgi, N.; Mahadevan, J.; Hyrc, K.; Saftig, P.; Marshall, C. Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy 2017, 13, 1952–1968. [Google Scholar] [CrossRef] [PubMed]
- Cernea, S.; Dobreanu, M. Diabetes and beta cell function: From mechanisms to evaluation and clinical implications. Biochem. Med. 2013, 23, 266–280. [Google Scholar] [CrossRef]
- Vetere, A.; Choudhary, A.; Burns, S.M.; Wagner, B.K. Targeting the pancreatic β-cell to treat diabetes. Nat. Rev. Drug Discov. 2014, 13, 278–289. [Google Scholar] [CrossRef]
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free. Radic. Biol. Med. 1996, 20, 463–466. [Google Scholar] [CrossRef]
- Lin, T.-A.; Wu, V.C.-C.; Wang, C.-Y. Autophagy in Chronic Kidney Diseases. Cells 2019, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Kume, S.; Koya, D.; Uzu, T.; Maegawa, H. Role of Nutrient-Sensing Signals in the Pathogenesis of Diabetic Nephropathy. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Yeh, S.-Y.; Pan, H.-J.; Lin, C.-C.; Kao, Y.-H.; Chen, Y.-H.; Lin, C.-J. Hyperglycemia induced down-regulation of renal P-glycoprotein expression. Eur. J. Pharmacol. 2012, 690, 42–50. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Su, Y.-J.; Li, J.-Y.; Yao, X.-C.; Liang, G.-J. Rapamycin, an mTOR inhibitor, induced apoptosis via independent mitochondrial and death receptor pathway in retinoblastoma Y79 cell. Int. J. Clin. Exp. Med. 2015, 8, 10723–10730. [Google Scholar]
- Zheng, Y.; Jiang, Y. mTOR Inhibitors at a Glance. Mol. Cell. Pharmacol. 2016, 7, 15–20. [Google Scholar]
- Wessler, J.D.; Grip, L.T.; Mendell, J.; Giugliano, R.P. The P-glycoprotein transport system and cardiovascular drugs. J. Am. Coll. Cardiol. 2013, 61, 2495–2502. [Google Scholar] [CrossRef] [Green Version]
- Smyth, M.J.; Krasovskis, E.; Sutton, V.R.; Johnstone, R. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc. Natl. Acad. Sci. USA 1998, 95, 7024–7029. [Google Scholar] [CrossRef] [Green Version]
- Tanemura, M.; Saga, A.; Kawamoto, K.; Machida, T.; Deguchi, T.; Nishida, T.; Sawa, Y.; Doki, Y.; Mori, M.; Ito, T. Rapamycin Induces Autophagy in Islets: Relevance in Islet Transplantation. Transplant. Proc. 2009, 41, 334–338. [Google Scholar] [CrossRef]
- PReifsnyder, P.C.; Flurkey, K.; Te, A.; Harrison, D.E. Rapamycin treatment benefits glucose metabolism in mouse models of type 2 diabetes. Aging 2016, 8, 3120–3130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlow, A.D.; Nicholson, M.L.; Herbert, T.P. Evidence for Rapamycin Toxicity in Pancreatic β-Cells and a Review of the Underlying Molecular Mechanisms. Diabetes 2013, 62, 2674–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Fu, R.; Duan, Z.; Lu, J.; Gao, J.; Tian, L.; Lv, Z.; Chen, Z.; Han, J.; Jia, L.; et al. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol. Res. Pract. 2016, 212, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, N.; Richardson, D.; Jansson, P.J. Glucose modulation induces reactive oxygen species and increases P-glycoprotein-mediated multidrug resistance to chemotherapeutics. Br. J. Pharmacol. 2015, 172, 2557–2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seebacher, N.; Lane, D.; Jansson, P.J.; Richardson, D.R. Glucose Modulation Induces Lysosome Formation and Increases Lysosomotropic Drug Sequestration via the P-Glycoprotein Drug Transporter. J. Biol. Chem. 2016, 291, 3796–3820. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Morales, P.; Tapia, E.; León-Contreras, J.C.; González-Reyes, S.; Jiménez-Osorio, A.S.; Trujillo, J.; Pavón, N.; Granados-Pineda, J.; Hernández-Pando, R.; Sánchez-Lozada, L.G.; et al. Mechanisms of Fasting-Mediated Protection against Renal Injury and Fibrosis Development after Ischemic Acute Kidney Injury. Biomolecules 2019, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- Riedl, R.A.; Atkinson, S.N.; Burnett, C.M.L.; Grobe, J.L.; Kirby, J.R. The Gut Microbiome, Energy Homeostasis, and Implications for Hypertension. Curr. Hypertens. Rep. 2017, 19, 27. [Google Scholar] [CrossRef]
- De Toledo, F.W.; Grundler, F.; Sirtori, C.R.; Ruscica, M. Unravelling the health effects of fasting: A long road from obesity treatment to healthy life span increase and improved cognition. Ann. Med. 2020, 52, 147–161. [Google Scholar] [CrossRef] [PubMed]



| Serum Parameters (mg/dl)\ Group (n) | Control (7) | STZ (6) | R-STZ (6) | F-STZ (6) |
|---|---|---|---|---|
| T.Cholesterol | 119.6 ± 9.843 | 194.9 ± 4.889 a | 240.8 ± 50.09 a | 144.9 ± 4.02 a,b,c |
| HDL-C | 38.53 ± 3.327 | 18.06 ± 1.346 a | 16.46 ± 1.16 a | 26.73 ± 1.47 a,b,c |
| LDL-C | 52.57 ± 11.89 | 117.7 ± 5.38 a | 182.2 ± 51.68 a,b | 79.69 ± 4.52 a,b,c |
| VLDL-C | 28.54 ± 0.5757 | 59.06 ± 0.96 a | 42.07 ± 2.71 a,b | 38.46 ± 0.81 a,b,c |
| TAG | 142.7 ± 2.878 | 295.3 ± 4.82 a | 210.3 ± 13.54 a,b | 192.3 ± 4.06 a,b,c |
| Parameters\Group (n) | Control (7) | STZ (6) | R-STZ (6) | F-STZ (6) |
|---|---|---|---|---|
| S. Urea (mg/dl) | 36.57 ± 1.25 | 117.9 ± 10.57 a | 147.3 ± 4.78 a,b | 65.05 ± 7.02 a,b,c |
| S. Creatinine (mg/dl) | 0.68 ± 0.08 | 1.947 ± 0.22 a | 2.3 ± 0.26 a,b | 1.008 ± 0.08 a,b,c |
| Micro albuminuria (mg/24 h) | 1.49 ± 0.04 | 2.493 ± 0.49 a | 2.962 ± 0.31 a | 1.580 ± 0.09 b,c |
| S. Albumin (g/dl) | 4.38 ± 0.36 | 4.160 ± 0.72 | 2.943 ± 0.299 a | 4.073 ± 0.279 c |
| S. T.Bilirubin (mg/dl) | 0.363 ± 0.042 | 0.787 ± 0.214 a | 1.132 ± 0.23 a,b | 0.365 ± 0.041 b,c |
| S. D.Bilirubin (mg/dl) | 0.064 ± 0.042 | 0.381 ± 0.033 a | 0.0225 ± 0.015 a,b | 0.104 ± 0.022 b,c |
| S. Uric acid (mg/dl) | 2.06 ± 0.12 | 5.492 ± 0.57 a | 5.09 ± 0.87 a | 3.05 ± 0.11 a,b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouda, K.; AbdelHamid, S.; Mansour, A.; Omar, N.; El-Mesallamy, H. Amelioration of Diabetic Nephropathy by Targeting Autophagy via Rapamycin or Fasting: Relation to Cell Apoptosis/Survival. Curr. Issues Mol. Biol. 2021, 43, 1698-1714. https://doi.org/10.3390/cimb43030120
Gouda K, AbdelHamid S, Mansour A, Omar N, El-Mesallamy H. Amelioration of Diabetic Nephropathy by Targeting Autophagy via Rapamycin or Fasting: Relation to Cell Apoptosis/Survival. Current Issues in Molecular Biology. 2021; 43(3):1698-1714. https://doi.org/10.3390/cimb43030120
Chicago/Turabian StyleGouda, Khaled, Sherihan AbdelHamid, Ahmed Mansour, Nesreen Omar, and Hala El-Mesallamy. 2021. "Amelioration of Diabetic Nephropathy by Targeting Autophagy via Rapamycin or Fasting: Relation to Cell Apoptosis/Survival" Current Issues in Molecular Biology 43, no. 3: 1698-1714. https://doi.org/10.3390/cimb43030120
APA StyleGouda, K., AbdelHamid, S., Mansour, A., Omar, N., & El-Mesallamy, H. (2021). Amelioration of Diabetic Nephropathy by Targeting Autophagy via Rapamycin or Fasting: Relation to Cell Apoptosis/Survival. Current Issues in Molecular Biology, 43(3), 1698-1714. https://doi.org/10.3390/cimb43030120






