Regulation of Immunity in Clear Cell Renal Carcinoma: Role of PD-1, PD-L1, and PD-L2
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. PD-1, PD-L1, PD-L2 Expression in ccRCC Oncogenesis
3.2. VHL Expression and p-VHL Content in ccRCC Oncogenesis
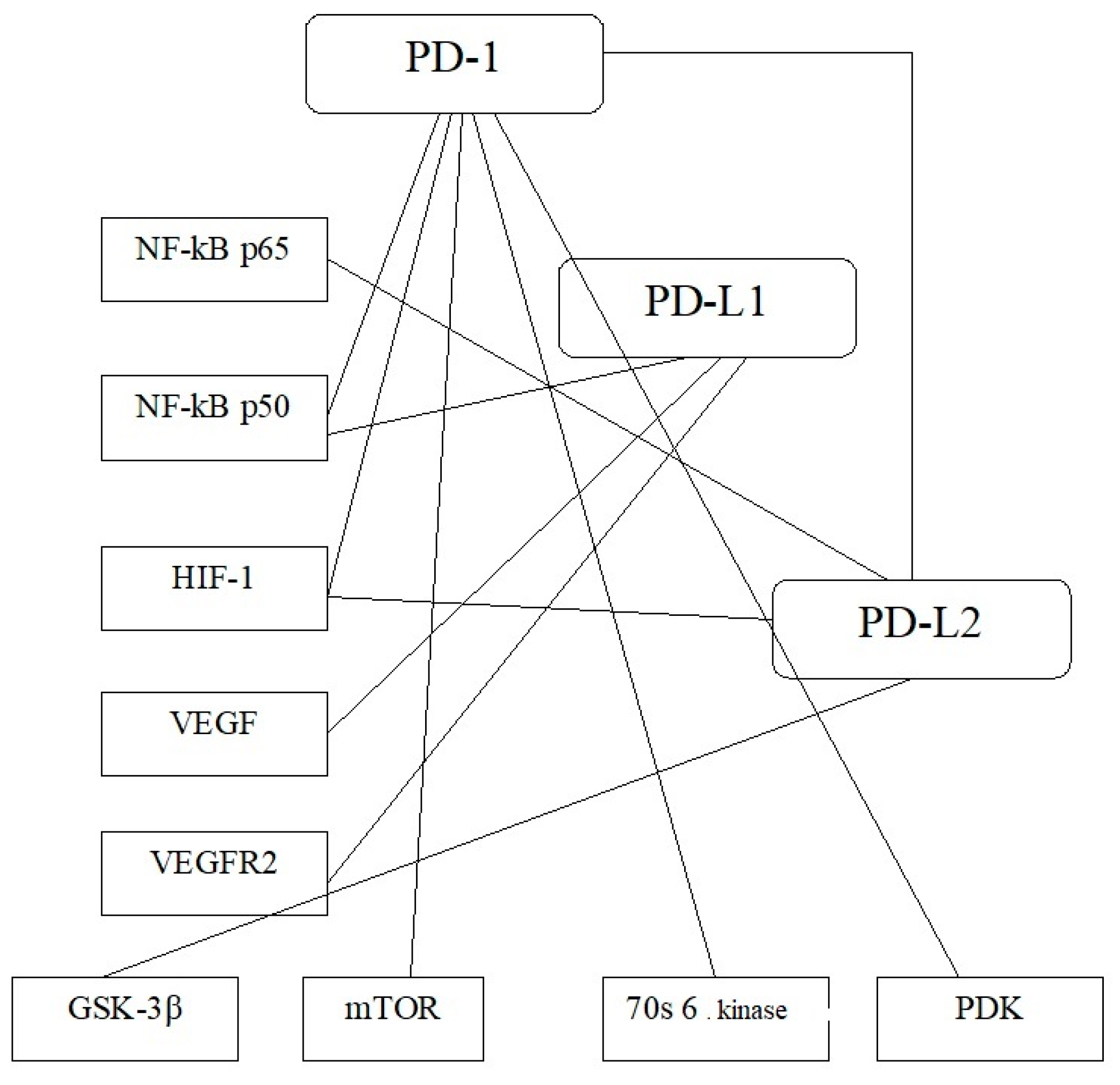
3.3. AKT/mTOR Activation and Implementation of Transcriptional and Growth Factors in ccRCC Oncogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kushlinskii, N.E.; Fridman, M.V.; Morozov, A.A.; Gershtei, E.S.; Kadagidze, Z.G.; Matveev, V.B. Modern approaches to kidney cancer immunotherapy. Cancer Urol. 2018, 14, 54–67. [Google Scholar] [CrossRef]
- Yearley, J.H.; Gibson, C.; Yu, N.; Moon, C.; Murphy, E.; Juco, J.; Lunceford, J.; Cheng, J.; Chow, L.Q.M.; Seiwert, T.Y.; et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin. Cancer Res. 2017, 23, 3158–3167. [Google Scholar] [CrossRef]
- Zhu, S.; Ding, W.; Chen, Y.; Wang, W.; Xu, R.; Liu, C.; Liu, X.; Deng, H. High VHL Expression Reverses Warburg Phenotype and Enhances Immunogenicity in Kidney Tumor Cells. Genom. Proteom. Bioinform. 2021. [Google Scholar] [CrossRef]
- Messai, Y.; Gad, S.; Noman, M.Z.; Le Teuff, G.; Couve, S.; Janji, B.; Kammerer, S.F.; Rioux-Leclerc, N.; Hasmim, M.; Ferlicot, S.; et al. Renal Cell Carcinoma Programmed Death-ligand 1, a New Direct Target of Hypoxia-inducible Factor-2 Alpha, is Regulated by von Hippel-Lindau Gene Mutation Status. Eur. Urol. 2016, 70, 623–632. [Google Scholar] [CrossRef]
- Hong, B.; Cai, L.; Wang, J.; Liu, S.; Zhou, J.; Ma, K.; Zhang, J.; Zhou, B.; Peng, X.; Zhang, N.; et al. Differential Expression of PD-L1 Between Sporadic and VHL-Associated Hereditary Clear-Cell Renal Cell Carcinoma and Its Correlation With Clinicopathological Features. Clin. Genitourin. Cancer 2019, 17, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kammerer-Jacquet, S.F.; Crouzet, L.; Brunot, A.; Dagher, J.; Pladys, A.; Edeline, J.; Laguerre, B.; Peyronnet, B.; Mathieu, R.; Verhoest, G.; et al. Independent association of PD-L1 expression with noninactivated VHL clear cell renal cell carcinoma-A finding with therapeutic potential. Int. J. Cancer 2017, 140, 142–148. [Google Scholar] [CrossRef]
- Lu, Y.; Song, Y.; Xu, Y.; Ou, N.; Liang, Z.; Hu, R.; Zhang, W.; Kang, J.; Wang, X.; Liu, L.; et al. The prevalence and prognostic and clinicopathological value of PD-L1 and PD-L2 in renal cell carcinoma patients: A systematic review and meta-analysis involving 3389 patients. Transl. Androl. Urol. 2020, 9, 367–381. [Google Scholar] [CrossRef]
- Ueda, K.; Suekane, S.; Kurose, H.; Chikui, K.; Nakiri, M.; Nishihara, K.; Matsuo, M.; Kawahara, A.; Yano, H.; Igawa, T. Prognostic value of PD-1 and PD-L1 expression in patients with metastatic clear cell renal cell carcinoma. Urol. Oncol. 2018, 36, 499.e9–499.e16. [Google Scholar] [CrossRef] [PubMed]
- Tanegashima, T.; Togashi, Y.; Azuma, K.; Kawahara, A.; Ideguchi, K.; Sugiyama, D.; Kinoshita, F.; Akiba, J.; Kashiwagi, E.; Takeuchi, A.; et al. Immune Suppression by PD-L2 against Spontaneous and Treatment-Related Antitumor Immunity. Clin. Cancer Res. 2019, 25, 4808–4819. [Google Scholar] [CrossRef]
- Philips, E.A.; Garcia-España, A.; Tocheva, A.S.; Ahearn, I.M.; Adam, K.R.; Pan, R.; Mor, A.; Kong, X.P. The structural features that distinguish PD-L2 from PD-L1 emerged in placental mammals. J. Biol. Chem. 2020, 295, 4372–4380. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, X.; Zhang, H.; Sun, G.; Yang, Y.; Chen, J.; Zhu, X.; Zhao, P.; Zhao, J.; Liu, J.; et al. Differential expressions of PD-1, PD-L1 and PD-L2 between primary and metastatic sites in renal cell carcinoma. BMC Cancer 2019, 19, 360. [Google Scholar] [CrossRef]
- Lastwika, K.J.; Wilson, W., III; Li, Q.K.; Norris, J.; Xu, H.; Ghazarian, S.R.; Kitagawa, H.; Kawabata, S.; Taube, J.M.; Yao, S.; et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 2016, 76, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F.; Lin, J.F.; Lin, Y.C.; Chou, K.Y.; Chen, H.E.; Ho, C.Y.; Chen, P.C.; Hwang, T.I. Cisplatin contributes to programmed death-ligand 1 expression in bladder cancer through ERK1/2-AP-1 signaling pathway. Biosci. Rep. 2019, 39, BSR20190362. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Lv, H.; Li, W.; Song, Z.; Li, L.; Zhou, S.; Qiu, L.; Qian, Z.; Liu, X.; Feng, L.; et al. Co-expression of PD-L1 and p-AKT is associated with poor prognosis in diffuse large B-cell lymphoma via PD-1/PD-L1 axis activating intracellular AKT/mTOR pathway in tumor cells. Oncotarget 2016, 7, 33350–33362. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef]
- Ruf, M.; Moch, H.; Schraml, P. PD-L1 expression is regulated by hypoxia-inducible factor in clear cell renal cell carcinoma. Int. J. Cancer 2016, 139, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Tatli Dogan, H.; Kiran, M.; Bilgin, B.; Kiliçarslan, A.; Sender, M.A.N.; Yalçin, B.; Ardiçoglu, A.; Atmaca, A.F.; Gumuskaya, B. Prognostic significance of the programmed death-ligand 1 expression in clear cell renal cell carcinoma and correlation with the tumor microenvironment and hypoxia-inducible factor expression. Diagn. Pathol. 2018, 13, 60. [Google Scholar] [CrossRef]
- Wu, X.-F.; Cheng, T.; Li, J.; Ma, D.-Y.; Zhu, Q.-F.; Lian, X. Correlation analysis of VHL and Jade-1 gene expression in human renal cell carcinoma. Open Med. 2016, 11, 226–230. [Google Scholar] [CrossRef]
- Spirina, L.V.; Yurmazov, Z.A.; Gorbunov, A.K.; Usynin, E.A.; Lushnikova, N.A.; Kovaleva, I.V. Molecular Protein and Expression Profile in the Primary Tumors of Clear Cell Renal Carcinoma and Metastases. Cells 2020, 9, 1680. [Google Scholar] [CrossRef]
- Spirina, L.V.; Kondakova, I.V.; Yurmazov, Z.A.; Usynin, E.A.; Slonimskaya, E.M.; Lushnikova, N.A.; Podnebesnova, D.V. VHL Expression in Kidney Cancer: Relation to Metastasis Development, Transcription and Growth Factors and Component of Akt/m-TOR Signaling Pathway. Bull. Exp. Biol. Med. 2019, 167, 671–675. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, J.H.; Kim, H.S.; Zang, D.Y. Prognostic and predictive value of VHL gene alteration in renal cell carcinoma: A meta-analysis and review. Oncotarget 2017, 8, 13979–13985. [Google Scholar] [CrossRef] [PubMed]
- Less, F.; Mazzanti, C.M.; Tomei, S.; Di Cristofano, C.; Minervini, A.; Menicagli, M.; Apollo, A.; Masieri, L.; Collecchi, P.; Minervini, R.; et al. VHL and HIF-1α: Gene variations and prognosis in early-stage clear cell renal cell carcinoma. Med. Oncol. 2014, 31, 840. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Y.; Jiang, H.; Zhu, H.; Liu, L.; Sun, B.; Pan, S.; Kristiansen, G.W.; Sun, X. Overexpression of von Hippel-Lindau protein synergizes with doxorubicin to suppress hepatocellular carcinoma in mice. J. Hepatol. 2011, 55, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, S.R.; McGovern, N.N.; Whyte, M.K.; Chilvers, E.R. The HIF/VHL pathway: From oxygen sensing to innate immunity. Am. J. Respir. Cell Mol. Biol. 2008, 38, 251–255. [Google Scholar] [CrossRef]
- Diez-Calzadilla, N.A.; Noguera Salvá, R.; Soriano Sarrió, P.; Martínez-Jabaloyas, J.M. Genetic profile and immunohistochemical study of clear cell renal carcinoma: Pathological-anatomical correlation and prognosis. Cancer Treat. Res. Commun. 2021, 27, 100374. [Google Scholar] [CrossRef]
- Yang, H.; Minamishima, Y.A.; Yan, Q.; Schlisio, S.; Ebert, B.L.; Zhang, X.; Zhang, L.; Kim, W.Y.; Olumi, A.F.; Kaelin, W.G., Jr. pVHL acts as an adaptor to promote the inhibitory phosphorylation of the NF-kappaB agonist Card9 by CK2. Mol. Cell 2007, 28, 15–27. [Google Scholar] [CrossRef][Green Version]

| Indicator | Tumor Size | Cancer Dissemination | ||||
|---|---|---|---|---|---|---|
| T1N0M0 | T2N0M0-1 | T3N0M1 | Localized Cancers | Disseminated Cancers | Metastases | |
| PD-1 | 4.19 (3.45; 20.40) | 0.81 (0.11; 2.00) | 0.41 (0.20; 1.94) | 2.91 (1.44; 7.63) | 0.61 (0.12; 1.94) * | 0.57 (0.135; 1.82) |
| Kruskal–Wallis Test: H = 11.95785 p = 0.0025 | ||||||
| PD-L1 | 2.85 (0.40; 6.77) | 1.00 (0.19; 1.00) | 0.32 (0.28; 0.50) | 1.00 (0.25; 4.27) | 0.37 (0.24; 1.00) | 0.75 (0.375; 1.50) |
| Kruskal–Wallis Test: H = 3.792281 p = 0.1501 | ||||||
| PD-L2 | 11.8 (1.49; 21.10) | 1.14 (0.64; 2.00) | 1.77 (0.50; 10.54) | 1.28 (0.50; 2.17) | 6.81 (2.16; 17.8) * | 0.605 (0.435; 0.755) ** |
| Kruskal–Wallis Test: H = 4.837951 p = 0.0490 | ||||||
| Indicator | Tumor Size | Cancer Dissemination | |||
|---|---|---|---|---|---|
| T1-2N0M0 | T3-4N0-1M0-1 | Localized Cancer (T1-2N0M0) | Metastatic Cancers (T1-4N0-1M1) | Metastases | |
| VHL expression, Relative Units | 7.00 (2.00; 31.32) | 87.50 (4.22; 128.00) * | 2.00 (0.50;4.00) | 64.00 (8.46; 128.00) * | 1.09 (0.09; 5.44) ** |
| VHL content,% to unaltered tissue | 77.20 (42.25; 99.79) | 15.30 (11.30; 46.07) * | 55.00 (20.10; 93.61) | 147.54 (100.54; 156.78) * | 20.80 (10.60; 58.80) ** |
| Indicator | Tumor Size | Cancer Dissemination | |||
|---|---|---|---|---|---|
| T1-2N0M0 | T3-4N0-1M0-1 | Localized Cancer (T1-2N0M0) | Metastatic Cancers (T1-4N0-1M1) | Metastases | |
| Transcriptional and growth factors | |||||
| HIF-1 | 1.29 (0.11; 2.53) | 0.79 (0.09; 3.50) | 1.20 (0.11; 3.30) | 1.07 (0.01; 6.60) | 20.80 (9.60; 35.30) ** |
| Kruskal–Wallis Test: H = 3.845614; p = 0.0156 | |||||
| HIF-2 | 0.94 (0.13; 2.87) | 2.68 (0.13; 128.00) * | 1.00 (0.13; 4.00) | 1.13 (0.01; 3.50) | 2.95 (1.24; 12.27) |
| Kruskal–Wallis Test: H = 3.792281; p = 0.1501 | |||||
| NF-κB p50 | 1.07 (0.1; 12.72) | 3.00 (0.03; 9.13) * | 1.00 (0.08; 12.72) | 4.08 (0.10; 6.92) ** | 6.00 (2.05; 28.14) ** |
| Kruskal–Wallis Test: H = 5.845614 df = 1 p = 0.0156 | |||||
| NF-κB p65 | 1.39 (0.22; 4.60) | 1.64 (0.66; 12.73) | 1.00 (0.08; 12.72) | 2.83 (0.01; 6.60) ** | 4.11 (0.14; 16.24) |
| Kruskal–Wallis Test: H = 5.792281; p = 0.8501 | |||||
| VEGF | 1.39 (0.13; 25.00) | 1.00 (0.13; 16.00) | 1.35 (0.13; 25.00) | 1.03 (0.00; 3.43) | 21.75 (0.50; 31.31) |
| Kruskal–Wallis Test: H = 5.792281; p = 0.0501 | |||||
| CAIX | 1.97 (0.31; 2.00) | 2.56 (0.85; 5.00) * | 0.81 (0.02; 2.00) | 0.07 (0.01; 25.1) | 1.85 (1.14; 2.28) ** |
| Kruskal–Wallis Test: H = 4.845614; p = 0.0056 | |||||
| VEGFR2 | 0.74 (0.16; 8.57) | 0.75 (0.25; 16.00) | 1,00 (0.33; 16.00) | 0.32 (0.04; 12.5) | 0.91 (0.45; 1.82) |
| Kruskal–Wallis Test: H = 5.792281; p = 0.8501 | |||||
| AKT/mTOR signaling cascade components | |||||
| PTEN | 4.72 (1.00; 8.78) | 1.46 (0.50; 9.07) * | 0.81 (0.50; 7.80) | 2.23 (1.18; 8.11) | 0.50 (0.00; 48.00) |
| Kruskal–Wallis Test: H = 3.612530; p = 0.0490 | |||||
| AKT | 0.93 (0.63; 16.00) | 1.48 (0.34; 3.42) | 1.02 (0.54; 4.00) | 4.57 (0.70; 23.23) | 0.34 (0.28; 8.00) |
| Kruskal–Wallis Test: H = 3.792281; p = 0.1501 | |||||
| GSK-3β | 3.78 (1.24; 17.00) | 8.00 (4.00; 12.64) | 2.59 (0.64; 9.50) | 4.00 (0.54; 16.00) | 0.24 (0.13; 8.00) |
| Kruskal–Wallis Test: H = 3.792281; p = 0.1501 | |||||
| PDK | 3.65 (1.07; 15.60) | 3.00 (0.51; 14.06) | 5.48 (0.70; 17.30) | 0.68 (0.03; 6.35) ** | 0.29 (0.01; 2.00) |
| Kruskal–Wallis Test: H = 3.792281; p = 0.1501 | |||||
| c-RAF | 4.44 (1.96; 16.41) | 2.51 (2.00; 8.00) * | 1.90 (0.22; 4.88) | 2.59 (0.16; 12.20) | 0.25 (0.00; 10.00) |
| Kruskal–Wallis Test: H = 4.371429 df = 1 p = 0.0365 | |||||
| mTOR | 4.28 (2.80; 16.00) | 0.36 (0.16; 1.14) * | 3.39 (0.84; 13.28) | 1.15 (0.42; 7.47) | 0.31 (0.25; 2.00) |
| Kruskal–Wallis Test: H = 5.792281; p = 0.8501 | |||||
| 70S 6 kinase | 2.01 (0.50; 5.58) | 1.00 (0.20; 3.64) | 0.98 (0.49; 4.64) | 0.69 (0.55; 7.91) | 0.13 (0.10; 1.18) |
| Kruskal–Wallis Test: H = 4.792281; p = 0.1501 | |||||
| 4E-BP1 | 1.05 (0.35; 3.66) | 1.00 (0.33; 2.00) | 0.71 (0.28; 3.83) | 0.83 (0.21; 6.22) | 0.25 (0.00; 10.56) |
| Kruskal–Wallis Test: H = 2.792281; p = 0.5501 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spirina, L.; Yurmazov, Z.; Usynin, E.; Kondakova, I.; Ladutko, E.; Choynzonov, E. Regulation of Immunity in Clear Cell Renal Carcinoma: Role of PD-1, PD-L1, and PD-L2. Curr. Issues Mol. Biol. 2021, 43, 1072-1080. https://doi.org/10.3390/cimb43020076
Spirina L, Yurmazov Z, Usynin E, Kondakova I, Ladutko E, Choynzonov E. Regulation of Immunity in Clear Cell Renal Carcinoma: Role of PD-1, PD-L1, and PD-L2. Current Issues in Molecular Biology. 2021; 43(2):1072-1080. https://doi.org/10.3390/cimb43020076
Chicago/Turabian StyleSpirina, Liudmila, Zahar Yurmazov, Evgeny Usynin, Irina Kondakova, Ekaterine Ladutko, and Evgeny Choynzonov. 2021. "Regulation of Immunity in Clear Cell Renal Carcinoma: Role of PD-1, PD-L1, and PD-L2" Current Issues in Molecular Biology 43, no. 2: 1072-1080. https://doi.org/10.3390/cimb43020076
APA StyleSpirina, L., Yurmazov, Z., Usynin, E., Kondakova, I., Ladutko, E., & Choynzonov, E. (2021). Regulation of Immunity in Clear Cell Renal Carcinoma: Role of PD-1, PD-L1, and PD-L2. Current Issues in Molecular Biology, 43(2), 1072-1080. https://doi.org/10.3390/cimb43020076







