Analysis of Dip2B Expression in Adult Mouse Tissues Using the LacZ Reporter Gene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
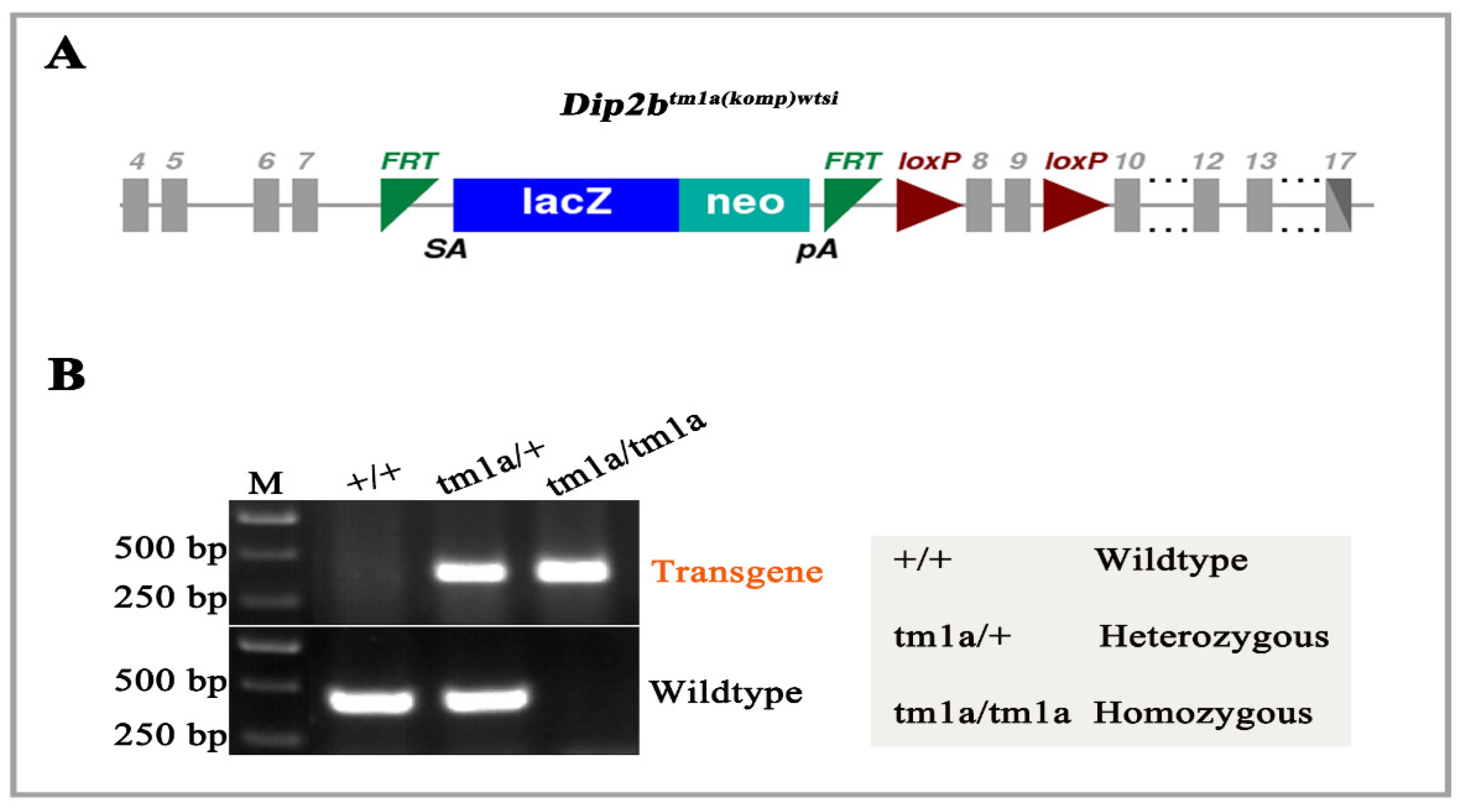
2.2. Genotyping
2.3. LacZ Staining of Whole-Mount and Frozen Sections
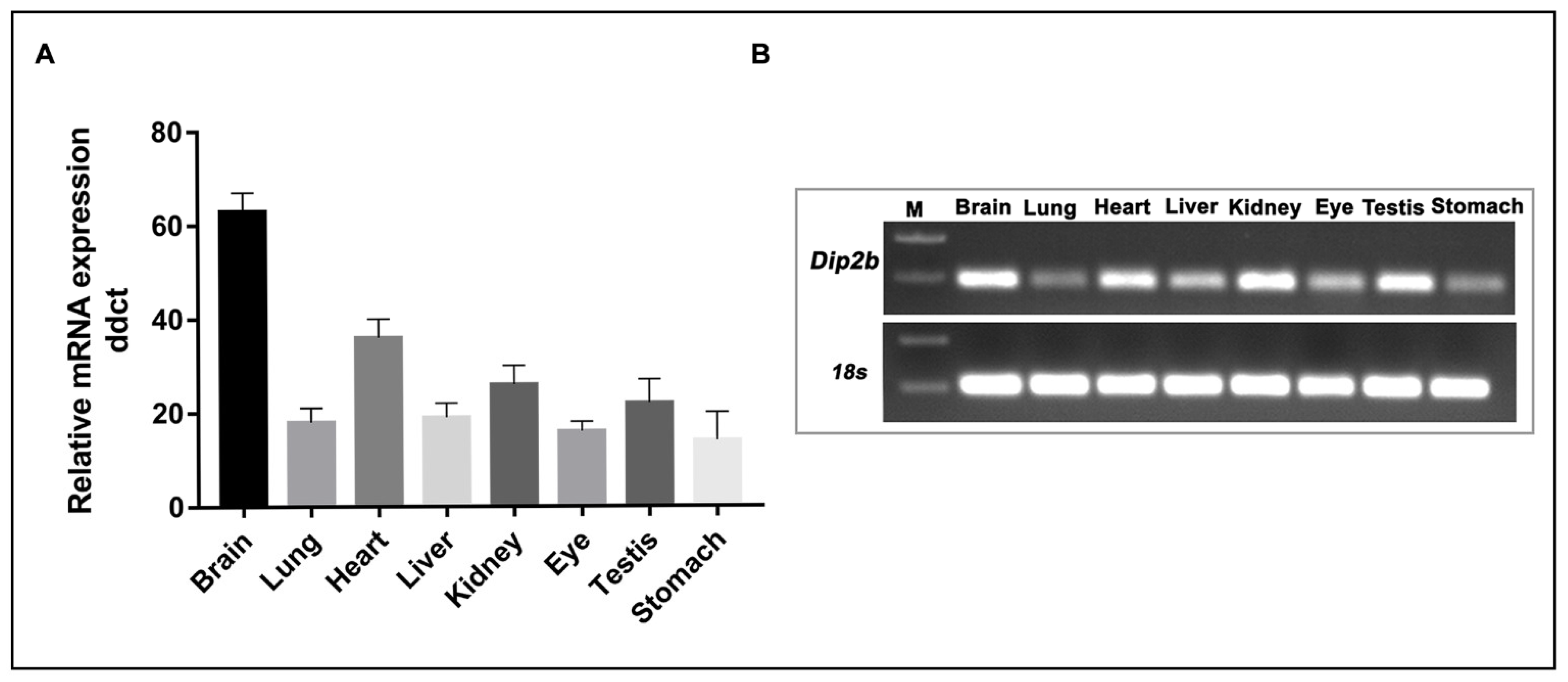
2.4. RNA Isolation and Real-Time PCR
3. Results
3.1. Genotyping of Dip2btm1a Mice
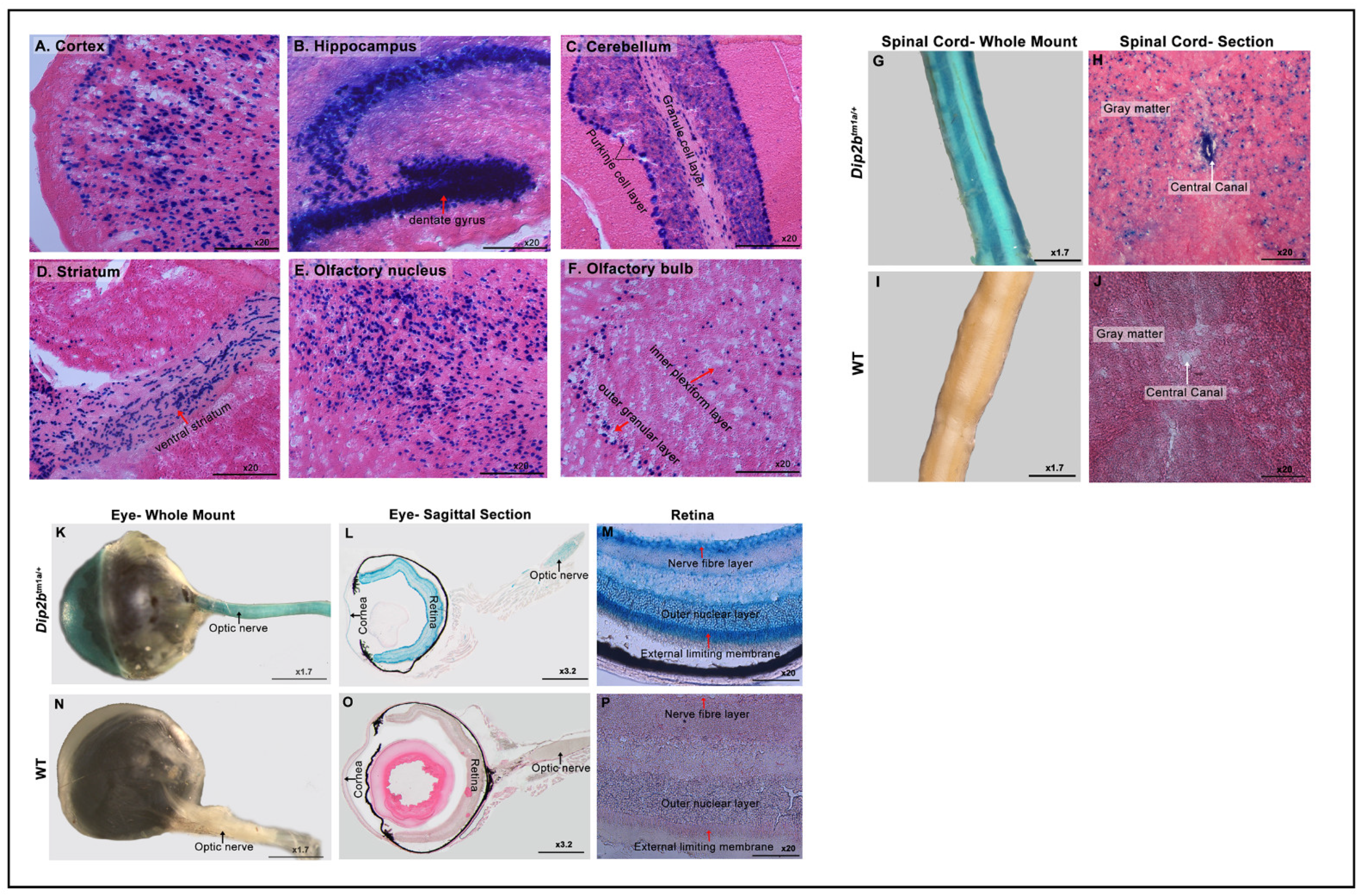
3.2. Expression of LacZ in the Nervous System
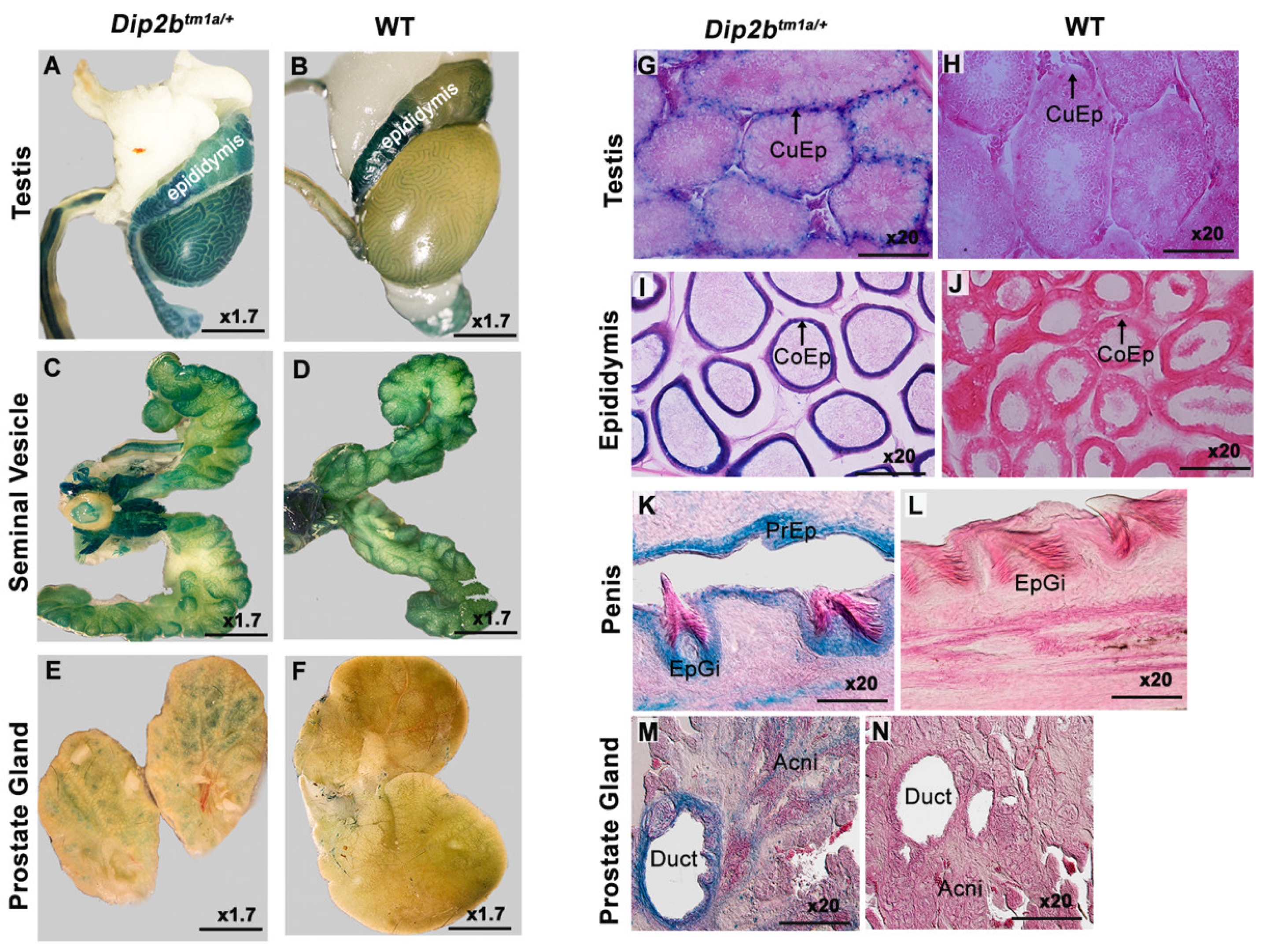
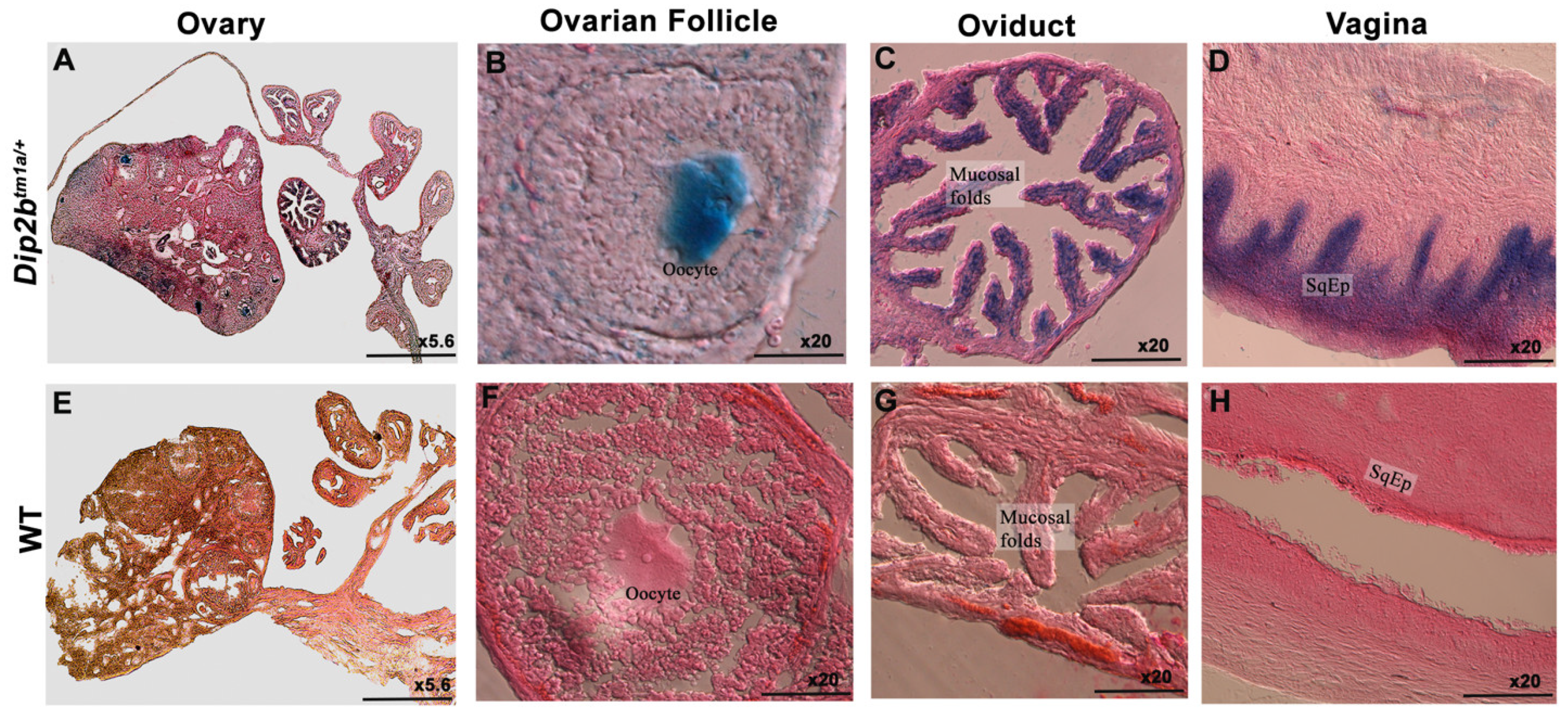
3.3. LacZ Expression in the Reproductive System
3.4. LacZ Expression in the Digestive System
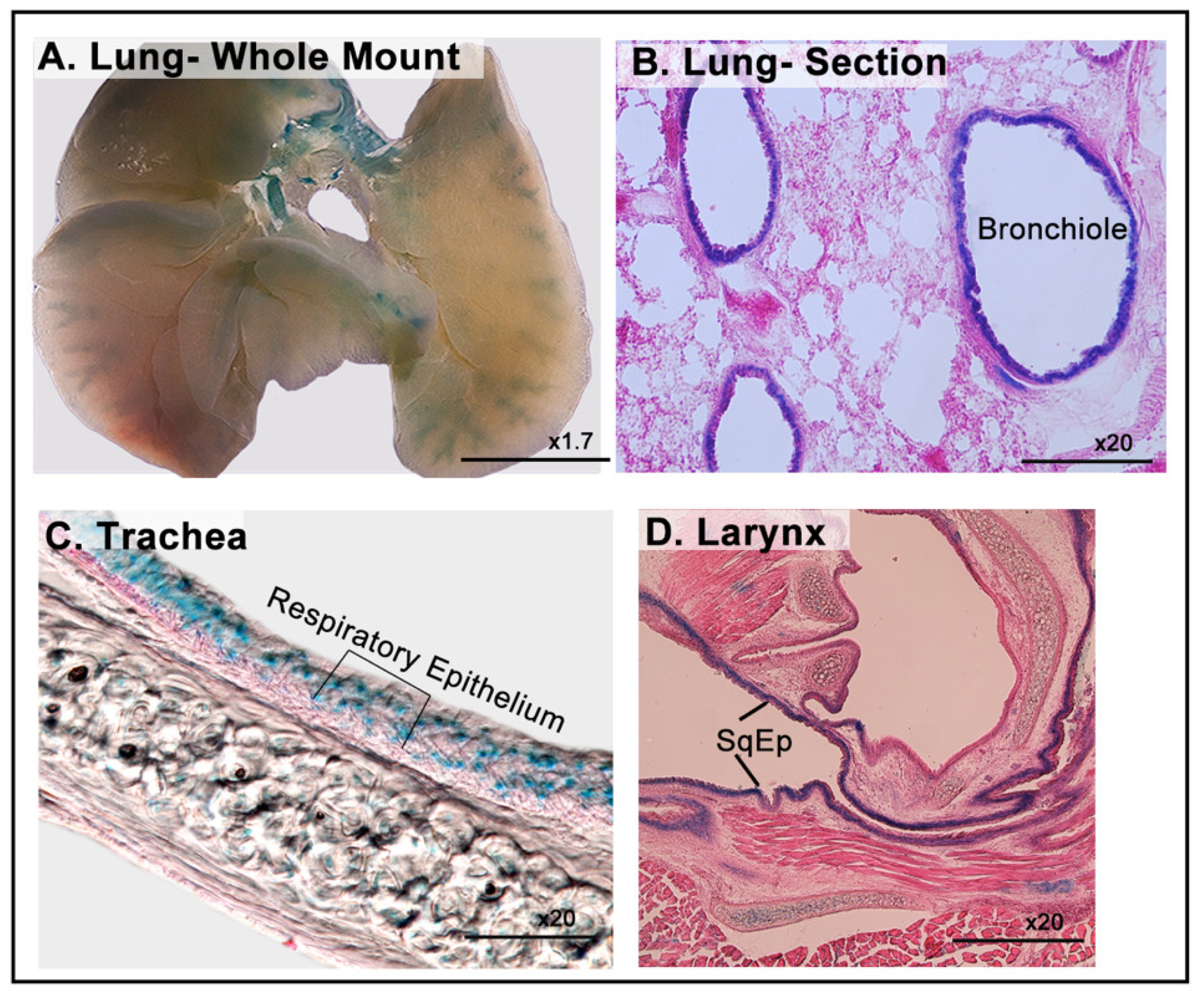
3.5. LacZ Expression of in the Respiratory System
3.6. LacZ Expression in the Genitourinary System
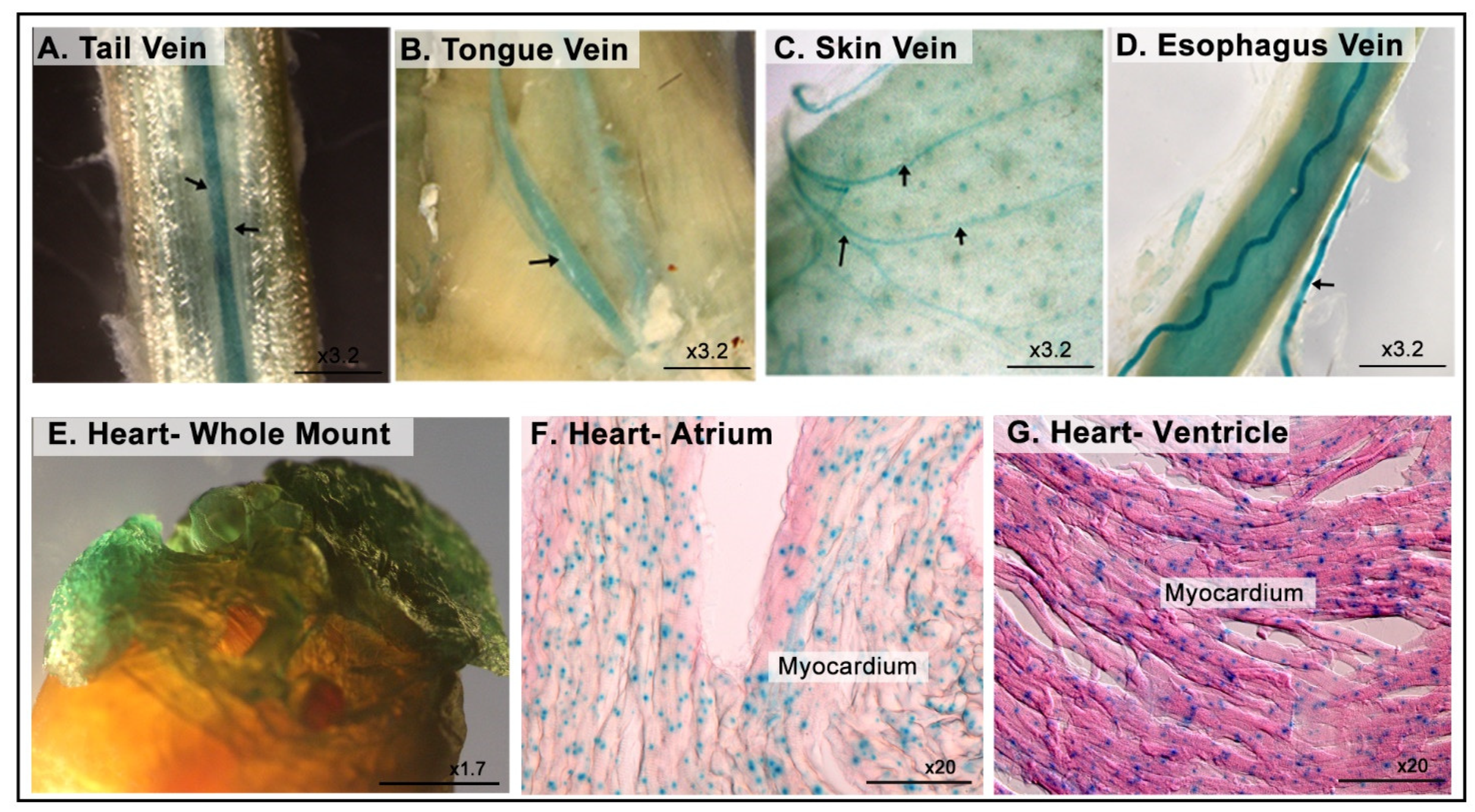
3.7. LacZ Expression in the Cardiovascular System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Mukhopadhyay, M.; Pelka, P.; DeSousa, D.; Kablar, B.; Schindler, A.; Rudnicki, M.A.; Campos, A.R. Cloning, Genomic Organization and Expression Pattern of a Novel Drosophila Gene, the Disco-Interacting Protein 2 (Dip2), and Its Murine Homolog. Gene 2002, 293, 59–65. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.; He, X.-X.; Wang, Y.-J.; Yu, H.-L.; He, Z.-X.; Zhang, L.-Q.; Zheng, Y.-W.; Zhu, X.-J. Functional Prediction and Characterization of Dip2 Gene in Mice. Cell Biol. Int. 2019, 43, 421–428. [Google Scholar] [CrossRef]
- Winnepenninckx, B.; Debacker, K.; Ramsay, J.; Smeets, D.; Smits, A.; FitzPatrick, D.R.; Kooy, R.F. CGG-Repeat Expansion in the DIP2B Gene Is Associated with the Fragile Site FRA12A on Chromosome 12q13.1. Am. J. Hum. Genet. 2007, 80, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Campos, A.R.; Lee, K.J.; Steller, H. Establishment of Neuronal Connectivity during Development of the Drosophila Larval Visual System. J. Neurobiol. 1995, 28, 313–329. [Google Scholar] [CrossRef]
- Heilig, J.S.; Freeman, M.; Laverty, T.; Lee, K.J.; Campos, A.R.; Rubin, G.M.; Steller, H. Isolation and Characterization of the Disconnected Gene of Drosophila Melanogaster. EMBO J. 1991, 10, 809–815. [Google Scholar] [CrossRef]
- Steller, H.; Fischbach, K.-F.; Rubin, G.M. Disconnected: A Locus Required for Neuronal Pathway Formation in the Visual System of Drosophila. Cell 1987, 50, 1139–1153. [Google Scholar] [CrossRef]
- Nitta, Y.; Yamazaki, D.; Sugie, A.; Hiroi, M.; Tabata, T. DISCO Interacting Protein 2 Regulates Axonal Bifurcation and Guidance of Drosophila Mushroom Body Neurons. Dev. Biol. 2017, 421, 233–244. [Google Scholar] [CrossRef]
- Nitta, Y.; Sugie, A. DISCO Interacting Protein 2 Determines Direction of Axon Projection under the Regulation of C-Jun N-Terminal Kinase in the Drosophila Mushroom Body. Biochem. Biophys. Res. Commun. 2017, 487, 116–121. [Google Scholar] [CrossRef]
- Noblett, N.; Wu, Z.; Ding, Z.H.; Park, S.; Roenspies, T.; Flibotte, S.; Chisholm, A.D.; Jin, Y.; Colavita, A. DIP-2 Suppresses Ectopic Neurite Sprouting and Axonal Regeneration in Mature Neurons. J. Cell Biol. 2019, 218, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Closa, A.; Cordero, D.; Sanz-Pamplona, R.; Solé, X.; Crous-Bou, M.; Paré-Brunet, L.; Berenguer, A.; Guino, E.; Lopez-Doriga, A.; Guardiola, J.; et al. Identification of Candidate Susceptibility Genes for Colorectal Cancer through EQTL Analysis. Carcinogenesis 2014, 35, 2039–2046. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Qiu, C.; Huang, D.; Zhang, Y.; Yu, S.; Zeng, C. Integrative Functional Analysis of Super Enhancer SNPs for Coronary Artery Disease. J. Hum. Genet. 2018, 63, 627–638. [Google Scholar] [CrossRef]
- Zhao, Y.; He, A.; Zhu, F.; Ding, M.; Hao, J.; Fan, Q.; Li, P.; Liu, L.; Du, Y.; Liang, X.; et al. Integrating Genome-Wide Association Study and Expression Quantitative Trait Locus Study Identifies Multiple Genes and Gene Sets Associated with Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 50–54. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Wang, C.; Li, C. The Correlation Analysis of MiRNAs and Target Genes in Metastasis of Cervical Squamous Cell Carcinoma. Epigenomics 2018, 10, 259–275. [Google Scholar] [CrossRef]
- Gerdin, A. The Sanger Mouse Genetics Programme: High Throughput Characterisation of Knockout Mice. Acta Ophthalmol. 2010, 88. [Google Scholar] [CrossRef]
- Van der Weyden, L.; White, J.K.; Adams, D.J.; Logan, D.W. The Mouse Genetics Toolkit: Revealing Function and Mechanism. Genome Biol. 2011, 12, 224. [Google Scholar] [CrossRef] [Green Version]
- Sah, R.K.; Ma, J.; Bah, F.B.; Xing, Z.; Adlat, S.; Oo, Z.M.; Wang, Y.; Bahadar, N.; Bohio, A.A.; Nagi, F.H.; et al. Targeted Disruption of Mouse Dip2B Leads to Abnormal Lung Development and Prenatal Lethality. Int. J. Mol. Sci. 2020, 21, 8223. [Google Scholar] [CrossRef]
- Skarnes, W.C.; Rosen, B.; West, A.P.; Koutsourakis, M.; Bushell, W.; Iyer, V.; Mujica, A.O.; Thomas, M.; Harrow, J.; Cox, T.; et al. A Conditional Knockout Resource for the Genome-Wide Study of Mouse Gene Function. Nature 2011, 474, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Casadaban, M.J.; Chou, J.; Cohen, S.N. In Vitro Gene Fusions That Join an Enzymatically Active Beta-Galactosidase Segment to Amino-Terminal Fragments of Exogenous Proteins: Escherichia Coli Plasmid Vectors for the Detection and Cloning of Translational Initiation Signals. J. Bacteriol. 1980, 143, 971–980. [Google Scholar] [CrossRef] [Green Version]
- Cooper, M.A.; Zhou, R. β-Galactosidase Staining of LacZ Fusion Proteins in Whole Tissue Preparations. Methods Mol. Biol. 2013, 1018, 189–197. [Google Scholar] [CrossRef]
- Burn, S.F. Detection of β-Galactosidase Activity: X-Gal Staining. Methods Mol. Biol. 2012, 886, 241–250. [Google Scholar] [CrossRef]
- Seymour, P.A.; Sander, M. Immunohistochemical Detection of Beta-Galactosidase or Green Fluorescent Protein on Tissue Sections. Methods Mol. Biol. 2007, 411, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.A. Whole-Mount Histochemical Detection of β-Galactosidase Activity. Curr. Protoc. Mol. Biol. 2000, 50, 14.14.1–14.14.8. [Google Scholar] [CrossRef] [PubMed]
- Gioglio, L.; De Angelis, M.G.C.; Boratto, R.; Poggi, P. An Improved Method for β-Galactosidase Activity Detection on Muscle Tissue. A Light and Electron Microscopic Study. Ann. Anat. Anat. Anz. 2002, 184, 153–157. [Google Scholar] [CrossRef]
- Aguzzi, A.; Theuring, F. Improved in Situ β-Galactosidase Staining for Histological Analysis of Transgenic Mice. Histochemistry 1994, 102, 477–481. [Google Scholar] [CrossRef]
- Xing, Z.-K.; Zhang, L.-Q.; Zhang, Y.; Sun, X.; Sun, X.-L.; Yu, H.-L.; Zheng, Y.-W.; He, Z.-X.; Zhu, X.-J. DIP2B Interacts With α-Tubulin to Regulate Axon Outgrowth. Front. Cell. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Lombaert, I.M.A.; Hauser, B.R.; Patel, V.N.; Hoffman, M.P. Exosomal MicroRNA Transport from Salivary Mesenchyme Regulates Epithelial Progenitor Expansion during Organogenesis. Dev. Cell 2017, 40, 95–103. [Google Scholar] [CrossRef] [Green Version]


| Dip2btm1a/+ Expressional Pattern | |
|---|---|
| Nervous system | Cortical layers |
| Ammon’s horn (CA1, CA2, CA3) and dentate gyrus of Hippocampus | |
| Granular and purkinje cell layer of cerebellum | |
| Ventral Straitum | |
| Olfactory nucleus | |
| Inner plexiform and outer nuclear layer of olfactory bulb | |
| Spinal cord gray matter and central canal | |
| Optic nerve, cornea, retinal nerve fiber layer, outer nuclear layer, and external limiting membrane | |
| Reproductive system | cuboidal epithelium (CuEp) lining of convoluted seminiferous tubules |
| columnar epithelium (CoEp) lining of epididymal ducts | |
| Glans and prepuce epithelium of penis | |
| Duct wall and acne of prostrate gland | |
| columnar epithelium of oviduct’s mucosal fold | |
| stratified squamous epithelium lining of vagina | |
| oocyte | |
| Digestive system | squamous epithelium lining of fore-stomach |
| non-keratinized stratified squamous epithelium layer of esophagus | |
| Crypts of lieberkuhn of jejunum | |
| Dorsal epithelium lining of stomach | |
| Respiratory system | Bronchial epithelium of lung |
| Respiratory epithelium of trachea | |
| Squamous epithelium of larynx | |
| Genitourinary system | Renal pelvis and glomeruli of kidney |
| Epithelium lining of urinary bladder | |
| Medulla of adrenal gland | |
| Cardiovascular system | Veins |
| Cardiac muscle | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sah, R.K.; Bahadar, N.; Bah, F.B.; Adlat, S.; Oo, Z.M.; Zhang, L.; Ali, F.; Zobaer, M.S.; Feng, X.; Zheng, Y. Analysis of Dip2B Expression in Adult Mouse Tissues Using the LacZ Reporter Gene. Curr. Issues Mol. Biol. 2021, 43, 529-542. https://doi.org/10.3390/cimb43020040
Sah RK, Bahadar N, Bah FB, Adlat S, Oo ZM, Zhang L, Ali F, Zobaer MS, Feng X, Zheng Y. Analysis of Dip2B Expression in Adult Mouse Tissues Using the LacZ Reporter Gene. Current Issues in Molecular Biology. 2021; 43(2):529-542. https://doi.org/10.3390/cimb43020040
Chicago/Turabian StyleSah, Rajiv Kumar, Noor Bahadar, Fatoumata Binta Bah, Salah Adlat, Zin Mar Oo, Luqing Zhang, Fawad Ali, M S Zobaer, Xuechao Feng, and Yaowu Zheng. 2021. "Analysis of Dip2B Expression in Adult Mouse Tissues Using the LacZ Reporter Gene" Current Issues in Molecular Biology 43, no. 2: 529-542. https://doi.org/10.3390/cimb43020040
APA StyleSah, R. K., Bahadar, N., Bah, F. B., Adlat, S., Oo, Z. M., Zhang, L., Ali, F., Zobaer, M. S., Feng, X., & Zheng, Y. (2021). Analysis of Dip2B Expression in Adult Mouse Tissues Using the LacZ Reporter Gene. Current Issues in Molecular Biology, 43(2), 529-542. https://doi.org/10.3390/cimb43020040






