Investigation of NH3 Desorption Kinetics on the LTA and SOD Zeolite Membranes
Abstract
1. Introduction
2. Materials and Methods
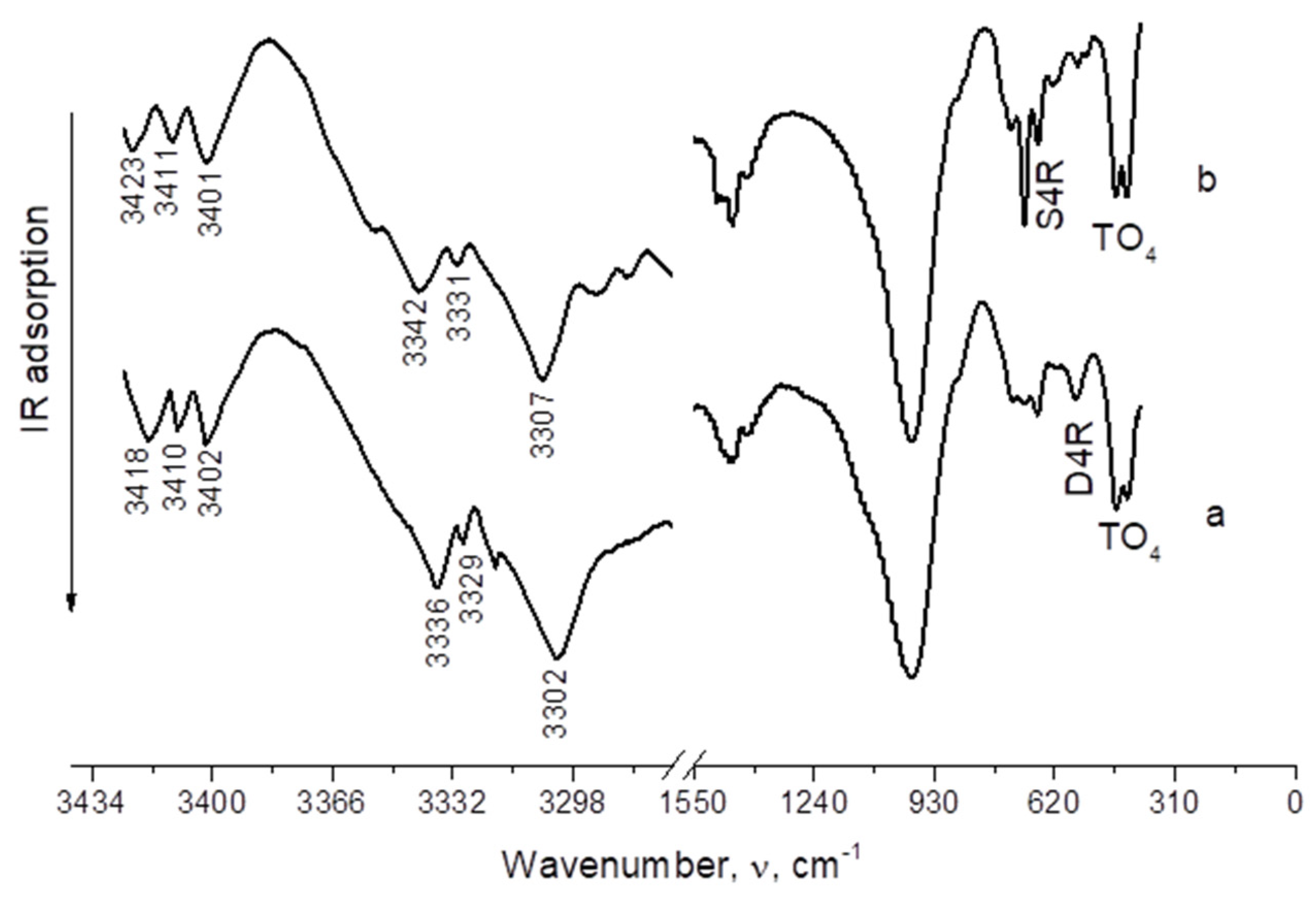
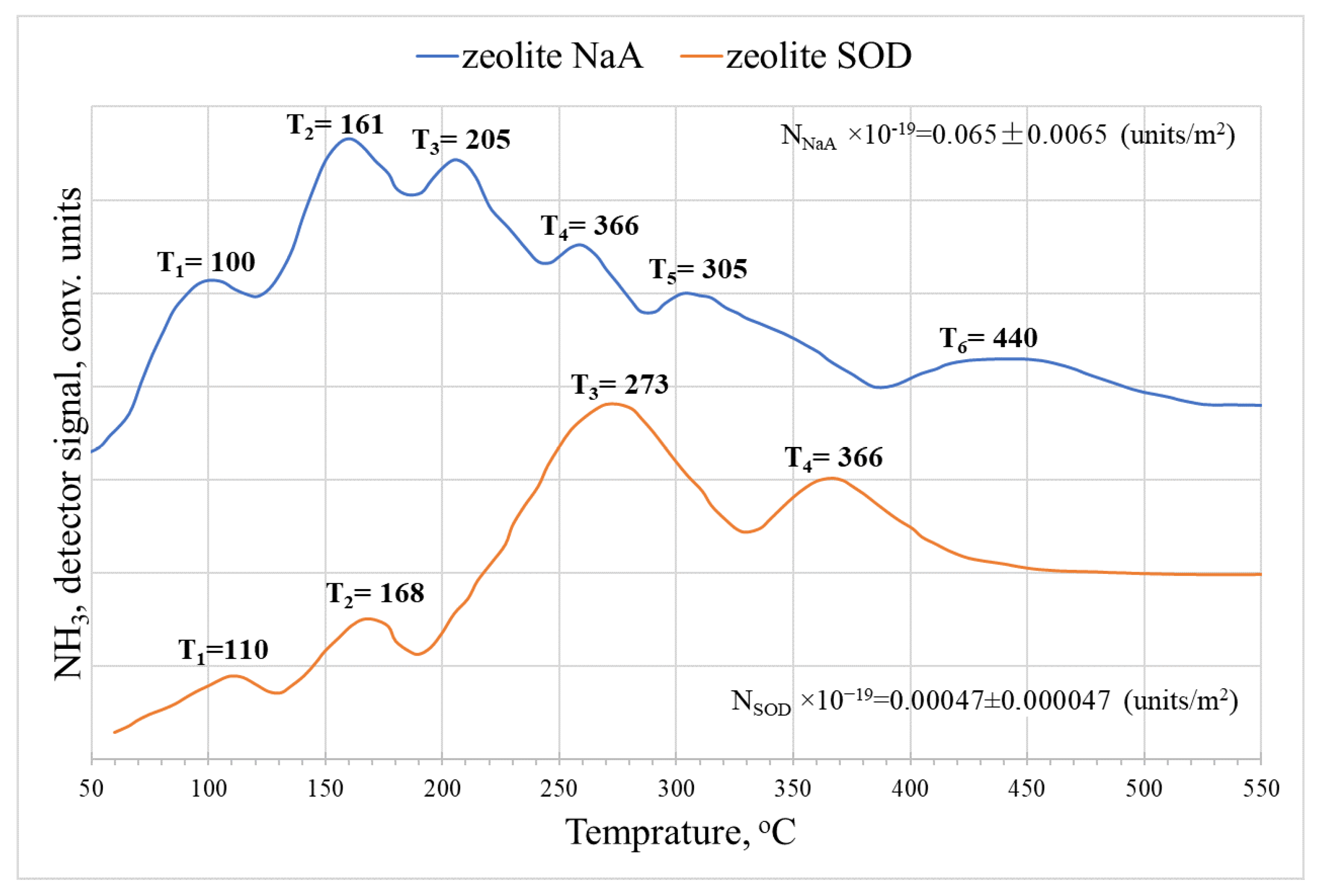
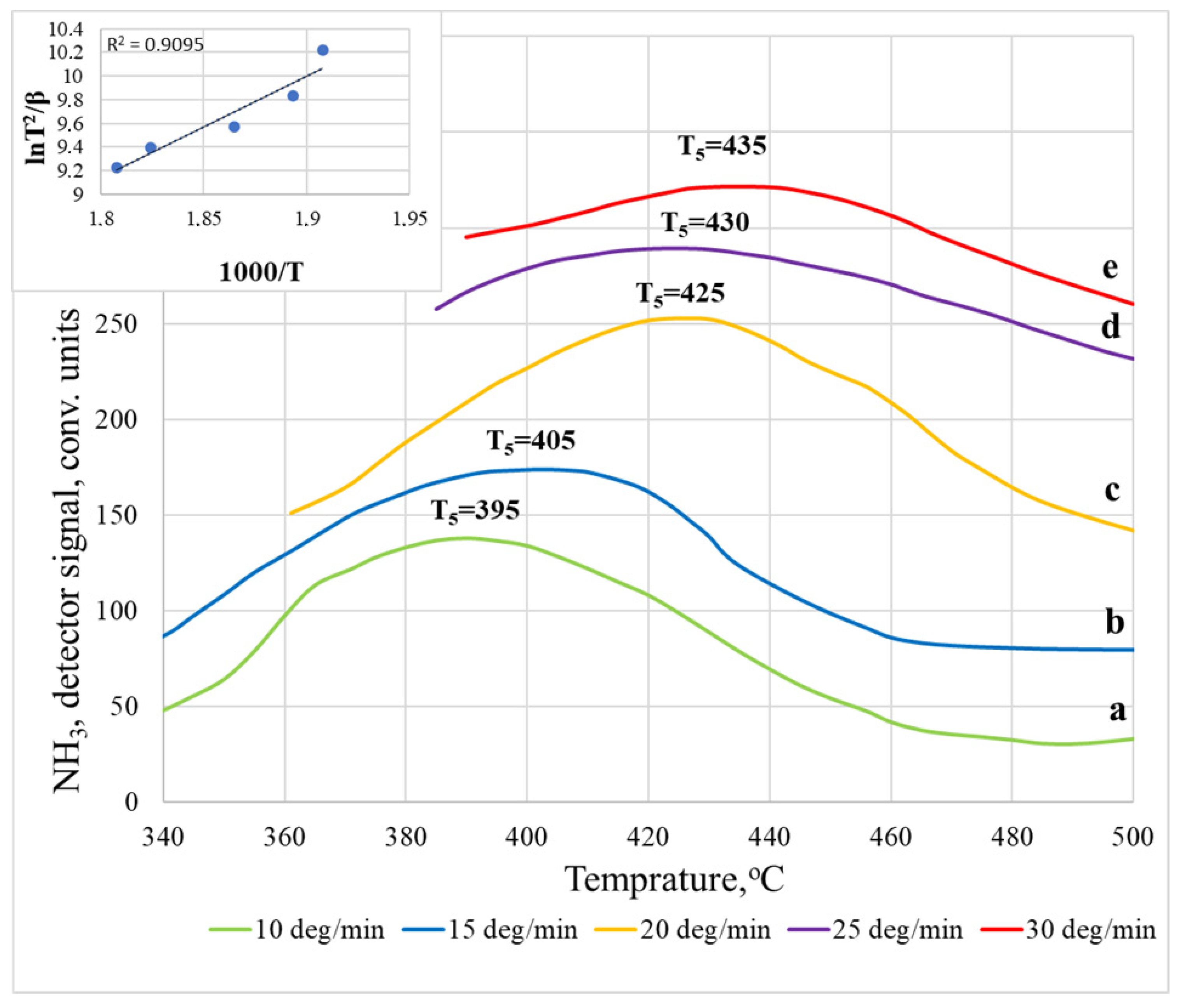
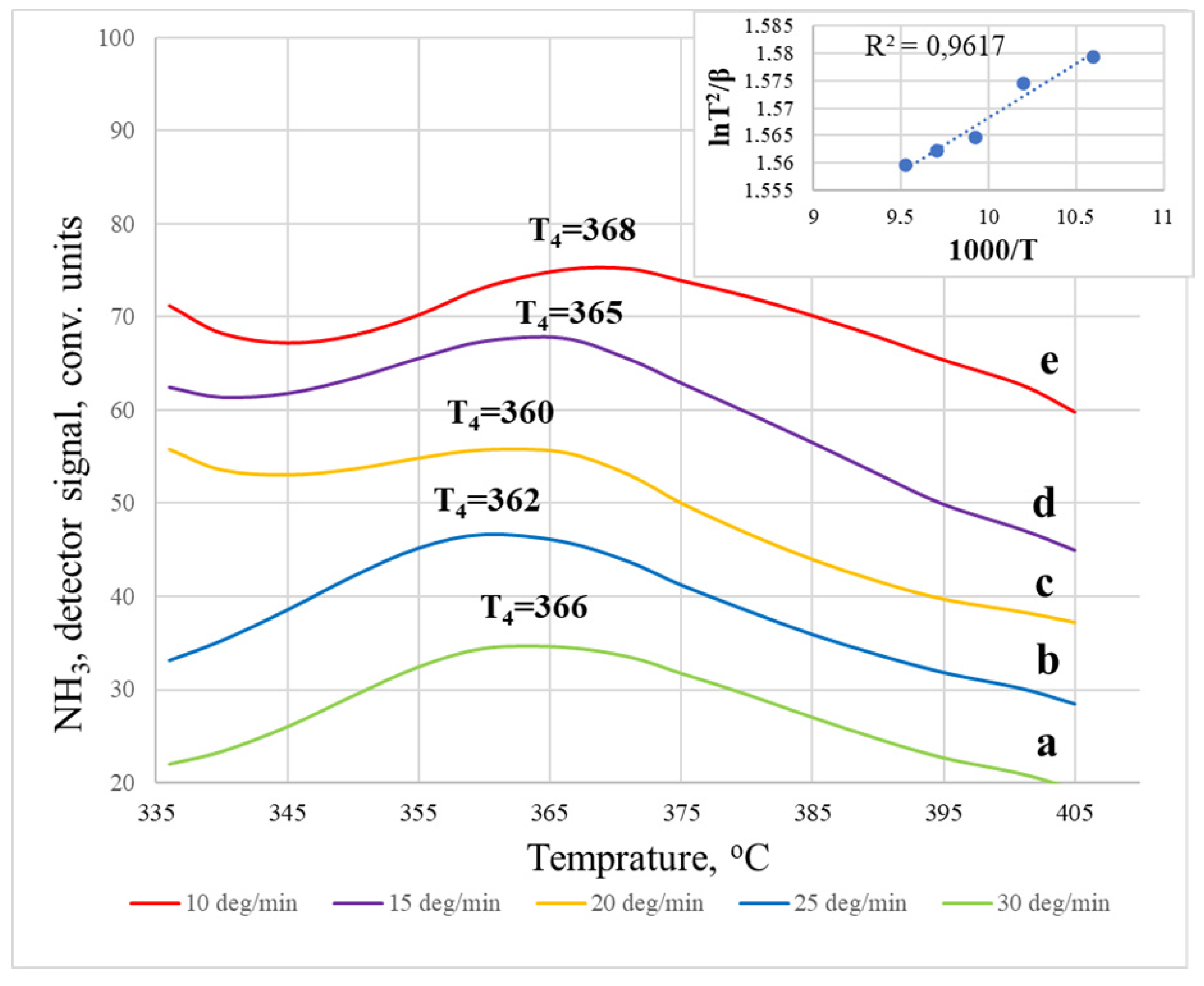
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riboldi, L.; Bolland, O. Overview on pressure swing adsorption (PSA) as CO2 capture technology: State-of-the-art, limits and potentials. Energy Procedia 2017, 114, 2390–2400. [Google Scholar] [CrossRef]
- Jacobs, J.H.; Deering, C.E.; Lesage, K.L.; Stashick, M.J.; Marriott, R.A. Rapid cycling thermal swing adsorption apparatus: Commissioning and data analyses for water adsorption of zeolites 4A and 13X over 2000 cycles. Ind. Eng. Chem. Res. 2021, 60, 7487–7494. [Google Scholar] [CrossRef]
- Daniel, F.; Marta, B.; Patrick, B.; Roger, D.W.; Adélio, M. Two-Stage Vacuum Pressure Swing Adsorption Using AgLiLSX Zeolite for Producing 99.5+% Oxygen from Air. Ind. Eng. Chem. Res. 2016, 55, 722–736. [Google Scholar] [CrossRef]
- Cai, L.X.; Wen, P.C.; Wen, M.H.; Jing, H.M.; Rui, F.L. CH4/N2 Adsorptive Separation on Zeolite X/AC Composites. J. Chem. 2019, 2019, 2078360. [Google Scholar] [CrossRef]
- Kosinov, N. High-Silica Zeolite Membranes for Gas and Liquid Separation High-Silica Zeolite Membranes for Gas and Liquid Separation; Eindhoven University of Technology: Eindhoven, The Netherlands, 2014. [Google Scholar]
- Melkon, T.; Gunther, M.; Stefan, K. Henninger, Relation of water adsorption capacities of zeolites with their structural properties. Microporous Mesoporous Mater. 2018, 264, 70–75. [Google Scholar] [CrossRef]
- Kakavandi, I.A.; Shokroo, E.J.; Baghbani, M.; Farniaei, M. Dynamic modeling of nitrogen adsorption on zeolite 13x bed. Fluid Mech. Res. Int. 2017, 1, 20–24. [Google Scholar] [CrossRef][Green Version]
- Gordina, N.E.; Rumyantsev, R.N.; Borisova, T.N.; Kolobkova, A.E.; Tsvetova, E.V.; Afanas’evaa, E.E.; Severgina, E.S.; Prokof’ev, V.Y. Use of combinations of ultrasonic treatment and microwave crystallization to intensify the synthesis of LTA zeolite membranes. Pet. Chem. 2021, 3, 292–298. [Google Scholar] [CrossRef]
- Prokof’ev, V.Y.; Gordina, N.E. Preparation of granulated LTA and SOD zeolites from mechanically activated mixtures of metakaolin and sodium hydroxide. Appl. Clay Sci. 2014, 101, 44–51. [Google Scholar] [CrossRef]
- Fang, J.; Yang, C.F.; Guiying, W.; Ming, Y. Modified Brønsted type equation with ammonia as probe molecule: Quantitative acidity-activity relationship for pyridine synthesis with ZSM-5 catalyst. React. Kinet. Mech. Catal. 2018, 123, 517–527. [Google Scholar] [CrossRef]
- Hisao, I.; Chie, A.; Yasuhisa, H. Development of Ammonia Selectively Permeable Zeolite Membrane for Sensor in Sewer System. Membranes 2021, 11, 348. [Google Scholar] [CrossRef]
- Prokof’ev, V.Y.; Gordina, N.E.; Zhidkova, A.B. Investigation of mechanochemical synthesis of zeolite NaA made of metakaolin in the mills with shock-shear type of strain. Russ. J. Appl. Chem. 2012, 85, 1077–1082. [Google Scholar] [CrossRef]
- Miki, N.; Naonobu, K. New Method for the Temperature-Programmed Desorption (TPD) of Ammonia Experiment for Characterization of Zeolite Acidity: A Review. Chem. Rec. 2013, 13, 432–455. [Google Scholar]
- Gordina, N.E.; Prokof’ev, V.Y.; Borisova, T.N.; Elizarova, A.M. Synthesis of granular low-modulus zeolites from metakaolin using mechanochemical activation and ultrasonic treatment. Izv. Vyss. Uchebnykh Zaved. Khimiya I Khimicheskaya Tekhnol. 2019, 62, 99–106. [Google Scholar] [CrossRef]
- Ahmad, M.; Mohammad, R.F.; Mohammad, R.M. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, J.; Dong, L.; Huang, M.; Li, B.; Jin, G.; Gao, J.; Zhang, F.; Fan, M.; Zhang, L.; et al. The effect of positioning cations on acidity and stability of the framework structure of Y zeolite. Sci. Rep. 2016, 6, 23382. [Google Scholar] [CrossRef] [PubMed]
- Abee, M.W.; Cox, D.F. NH3 chemisorption on stoichiometric and oxygen deficient SnO2(110) surfaces. Surf. Sci. 2002, 520, 65–77. [Google Scholar] [CrossRef]
- Bernd, H.; Matthias, H.; Louis, A.C.; Randall, Q. Snurr Characterization of Acidic OH Groups in Zeolites of Different Types: An Interpretation of NH3-TPD Results in the Light of Confinement Effects. J. Phys. Chem. B 2002, 106, 3882–3889. [Google Scholar] [CrossRef]
- Miki, N.; Shinji, N.; Naonobu, K. IRMS–TPD of ammonia for characterization of acid site in b-zeolite. Microporous Mesoporous Mater. 2005, 82, 105–112. [Google Scholar] [CrossRef]
- Rabo, J.A. Zeolite Chemistry and Catalysis; American Chemical Society: Washington, DC, USA, 1976; p. 506. ISBN 0841202761 9780841202764. [Google Scholar]
- Shu, Y.; Ma, D.; Xu, L.; Xu, Y.; Bao, X. Methane dehydro-aromatization over Mo/MCM-22 catalysts: A highly selective catalyst for the formation of benzene. Catal. Lett. 2000, 70, 67–73. [Google Scholar] [CrossRef]
- Verdoliva, V.; Saviano, M.; De Luca, S. Zeolites as Acid/Basic Solid Catalysts: Recent Synthetic Developments. Catalysts 2019, 9, 248. [Google Scholar] [CrossRef]
- Kouva, S.; Kanervo, J.; Schubler, F.; Olindo, R. Sorption and diffusion parameters fromacuumm-TPD of ammonia on H-ZMS-5. Chem. Eng. Sci. 2013, 89, 40–48. [Google Scholar] [CrossRef]
- Ben Younes, N.; Ortigosa, J.M.; Marie, O.; Blasco, T.; Mhamdi, M. Effect of zeolite structure on the selective catalytic reduction of NO with ammonia over Mn-Fe supported on ZSM-5, BEA, MOR and FER. Res. Chem. Intermed. 2021, 47, 2003–2028. [Google Scholar] [CrossRef]
- Suhasaria, T.; Thrower, J.D.; Zacharias, H. Thermal desorption of astrophysically relevant molecules from forsterite (010). Mon. Not. R. Astron. Soc. 2017, 472, 389–399. [Google Scholar] [CrossRef]
- Holba, P.; Šesták, J. Imperfections of Kissinger evaluation method and crystallization kinetics. Glass Phys. Chem. 2014, 40, 486–495. [Google Scholar] [CrossRef]
- Li, X.; Guo, H.; Chen, H.-M. Determination of the Activation Energy for Desorption by Derivative Thermogravimetric Analysis. Adsorpt. Sci. Technol. 2006, 24, 907–914. [Google Scholar] [CrossRef]
- Nechaev, Y.S. On the nature, kinetics and limiting values of hydrogen sorption by carbon nanostructures. Phys. Usp. 2006, 49, 563–591. [Google Scholar] [CrossRef]
- Klepel, O.; Hunger, B. Temperature-programmed desorption (TPD) of carbon dioxide on alkali-metal cation-exchanged faujasite type zeolites. J. Therm. Anal. Calorim. 2005, 80, 201–206. [Google Scholar] [CrossRef]
- Denisova, K.O.; Il’in, A.A.; Rumyantsev, R.N.; Il’in, A.P.; Smirnov, N.N. Acid-Base Properties of Cobalt Ferrite Surface Examined by Different Physicochemical Methods. Theor. Found. Chem. Eng. 2020, 54, 1306–1313. [Google Scholar] [CrossRef]
- Takeuchi, M.; Tsukamoto, T.; Kondo, A.; Matsuoka, M. Investigation of NH3 and NH4+ adsorbed on ZSM-5 zeolites by near and middle infrared spectroscopy. Catal. Sci. Technol. 2015, 5, 4587–4593. [Google Scholar] [CrossRef]
- Borodin, D.; Rahinov, I.; Galparsoro, O.; Fingerhut, J.; Schwarzer, M.; Golibrzuch, K.; Skoulatakis, G.; Auerbach, D.J.; Kandratsenka, A.; Schwarzer, D.; et al. Kinetics of NH3 Desorption and Diffusion on Pt: Implications for the Ostwald Process. J. Am. Chem. Soc. 2021, 143, 18305–18316. [Google Scholar] [CrossRef]
- Prokof’eva, V.Y.; Gordina, N.E.; Zakharova, O.N.; Borisova, T.N.; Kolobkova, A.E.; Tsvetova, E.V. Study of the kinetics of adsorption and desorption of water vapor on low-silica zeolites. Pet. Chem. 2020, 60, 550–556. [Google Scholar] [CrossRef]
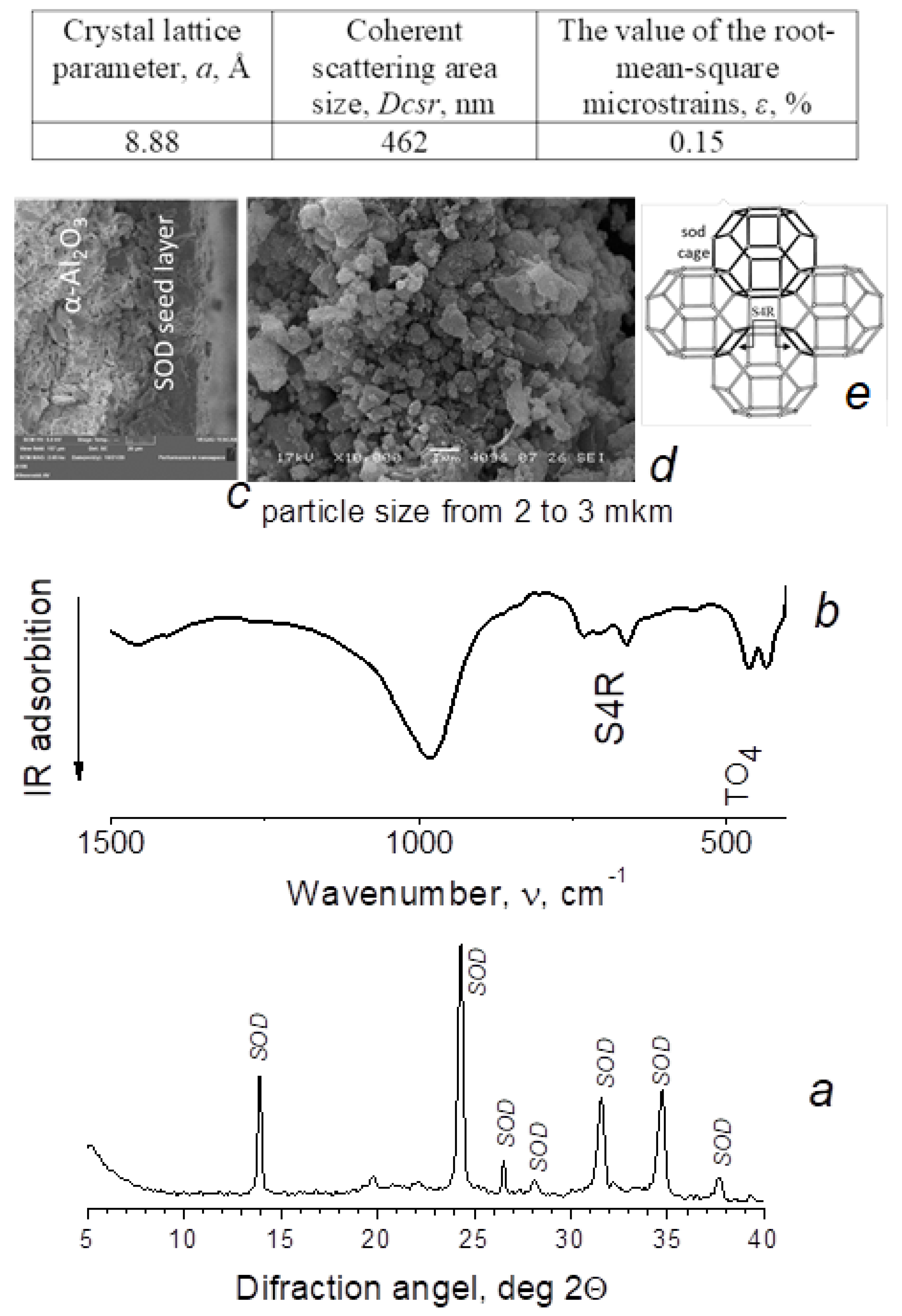
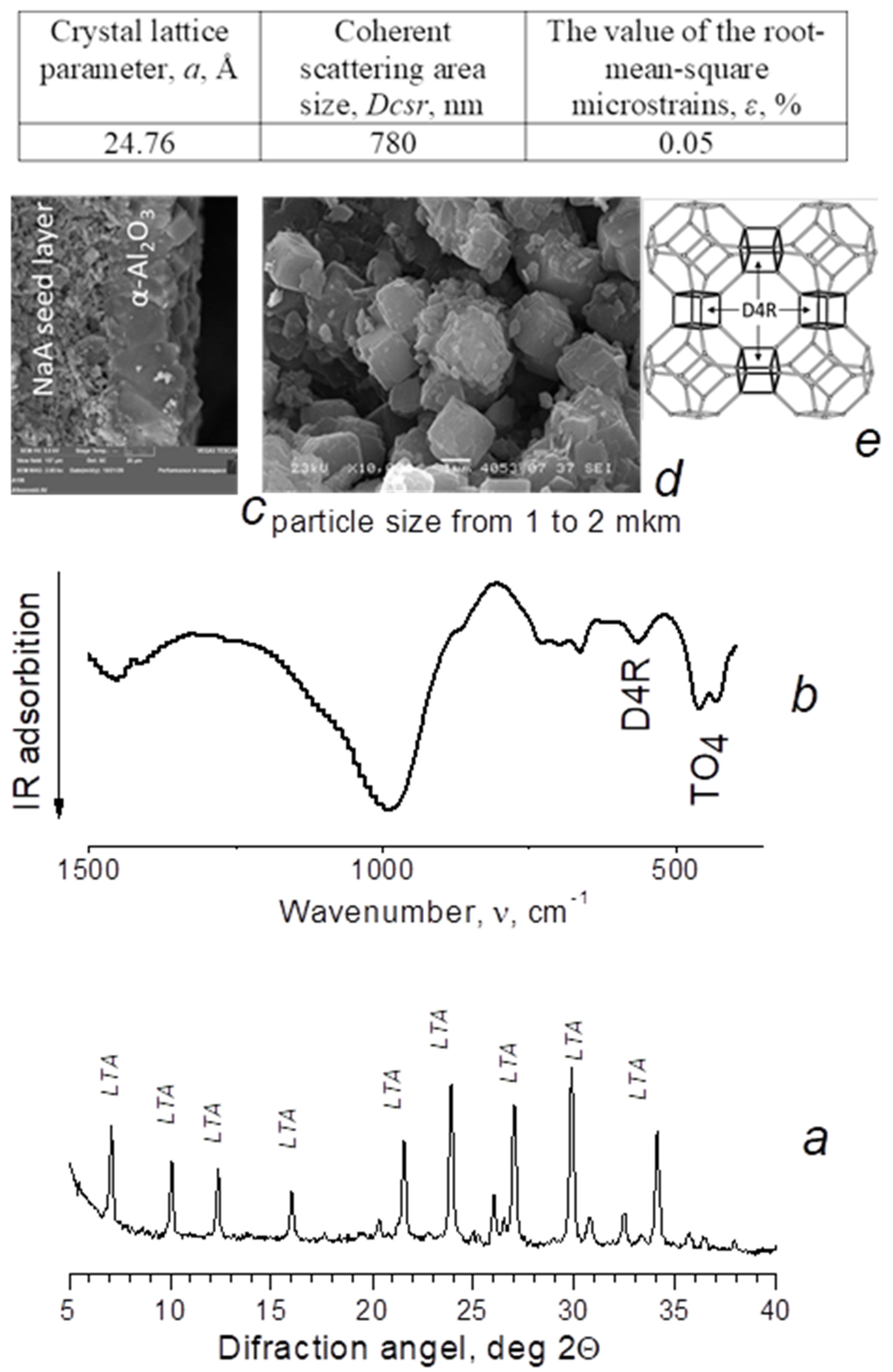
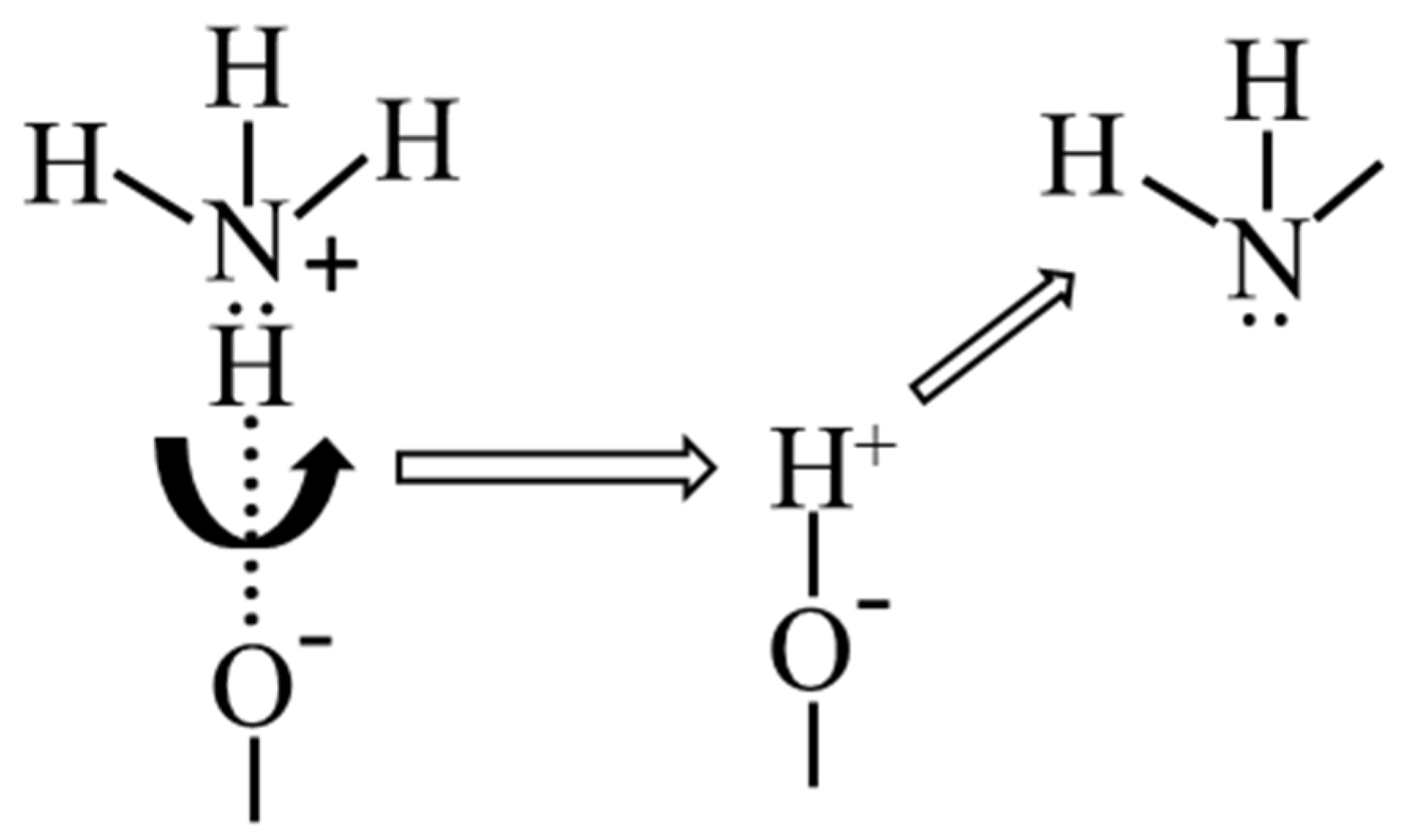
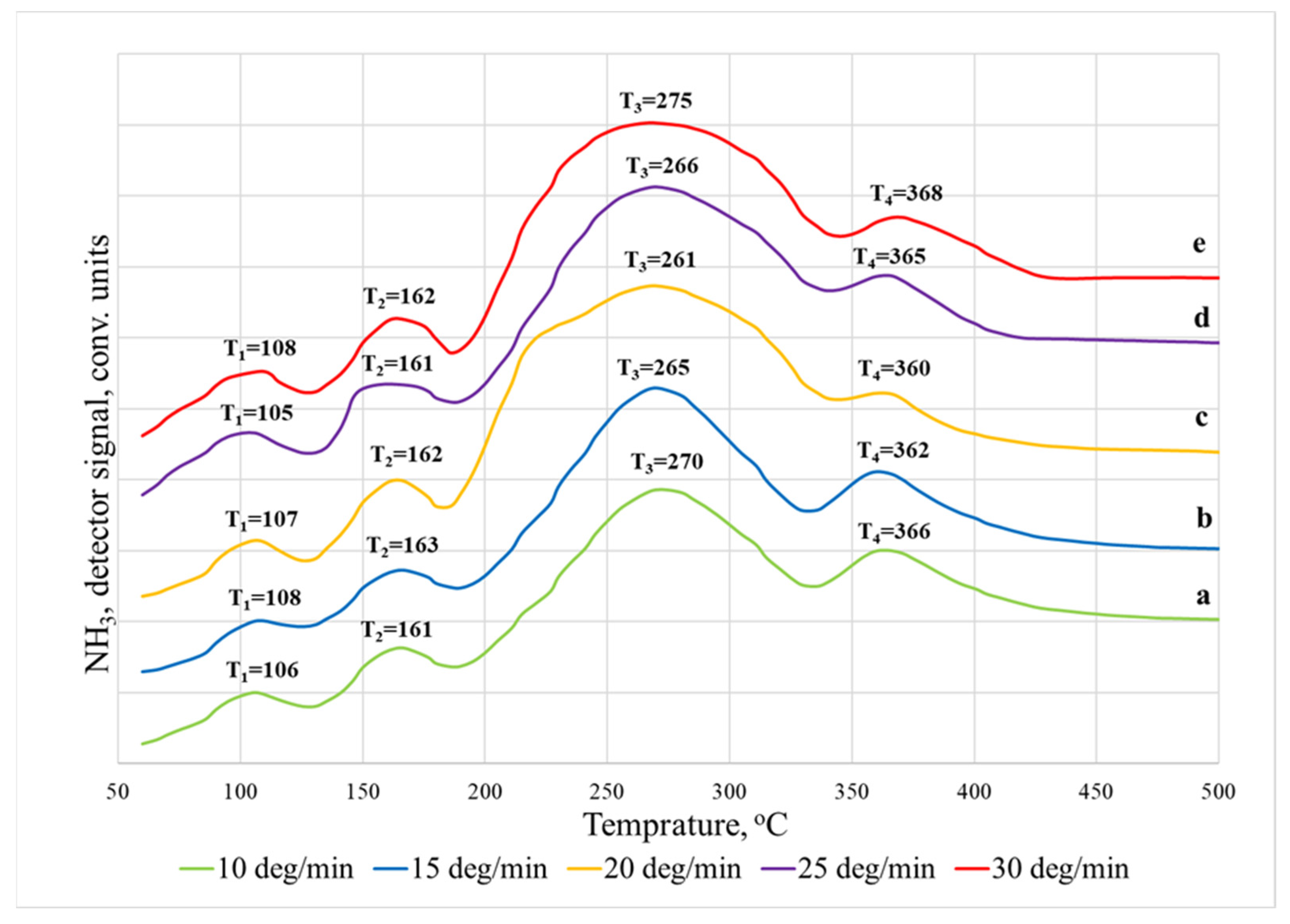


| Heating Rate β, °C/min | Temperature Maximum, Ti | Acidity Corresponding to Ni × 10−19, Units/m2 | Total Acidity, Corresponding to ⅀N(i−j) × 10−19, Units/m2 | ||
|---|---|---|---|---|---|
| 10 | T1 | 106 | N1 | 0.00012 ± 0.000012 | 0.006 ± 0.0006 |
| T2 | 161 | N2 | 0.00029 ± 0.000029 | ||
| T3 | 270 | N3 | 0.0033 ± 0.00033 | ||
| T4 | 366 | N4 | 0.00229 ± 0.000229 | ||
| 15 | T1 | 108 | N1 | 0.0001 ± 0.00001 | 0.0065 ± 0.00065 |
| T2 | 163 | N2 | 0.0003 ± 0.00003 | ||
| T3 | 265 | N3 | 0.0036 ± 0.00036 | ||
| T4 | 362 | N4 | 0.0024 ± 0.00024 | ||
| 20 | T1 | 107 | N1 | 0.0001 ± 0.00001 | 0.0063 ± 0.00063 |
| T2 | 162 | N2 | 0.0003 ± 0.00003 | ||
| T3 | 261 | N3 | 0.0035 ± 0.00035 | ||
| T4 | 360 | N4 | 0.0023 ± 0.00023 | ||
| 25 | T1 | 105 | N1 | 0.0001 ± 0.00001 | 0.0059 ± 0.00059 |
| T2 | 161 | N2 | 0.0003 ± 0.00003 | ||
| T3 | 266 | N3 | 0.0033 ± 0.00033 | ||
| T4 | 365 | N4 | 0.0022 ± 0.00022 | ||
| 30 | T1 | 108 | N1 | 0.0001 ± 0.00001 | 0.0062 ± 0.00062 |
| T2 | 162 | N2 | 0.0003 ± 0.00003 | ||
| T3 | 275 | N3 | 0.0035 ± 0.00035 | ||
| T4 | 368 | N4 | 0.0023 ± 0.00023 | ||
| Heating Rate β, °C/min | Temperature Maximum, Ti | Acidity Corresponding to Ni × 10−19, Units/m2 | Total Acidity, Corresponding to ⅀N(i−j) × 10−19, Units/m2 | ||
|---|---|---|---|---|---|
| 10 | T1 | 82 | N1 | 0.0120 ± 0.00120 | 0.08 ± 0.008 |
| T2 | 130 | N2 | 0.0210 ± 0.00210 | ||
| T3 | 160 | N3 | 0.0185 ± 0.00185 | ||
| T4 | 251 | N4 | 0.0155 ± 0.00155 | ||
| T5 | 395 | N5 | 0.013 ± 0.0013 | ||
| 15 | T1 | 85 | N1 | 0.0121 ± 0.00121 | 0.079 ± 0.0079 |
| T2 | 135 | N2 | 0.0394 ± 0.00394 | ||
| T3 | 170 | N3 | 0.0118 ± 0.00118 | ||
| T4 | 255 | N4 | 0.0058 ± 0.00058 | ||
| T5 | 405 | N5 | 0.0099 ± 0.00099 | ||
| 20 | T1 | 95 | N1 | 0.0036 ± 0.00036 | 0.0773 ± 0.00773 |
| T2 | 140 | N2 | 0.0333 ± 0.00333 | ||
| T3 | 187 | N3 | 0.0279 ± 0.00279 | ||
| T4 | 263 | N4 | 0.0027 ± 0.00027 | ||
| T5 | 425 | N5 | 0.0098 ± 0.00098 | ||
| 25 | T1 | 101 | N1 | 0.0058 ± 0.00058 | 0.0801 ± 0.00801 |
| T2 | 150 | N2 | 0.0312 ± 0.00312 | ||
| T3 | 200 | N3 | 0.0253 ± 0.00253 | ||
| T4 | 275 | N4 | 0.0081 ± 0.00081 | ||
| T5 | 430 | N5 | 0.0097 ± 0.00097 | ||
| 30 | T1 | 105 | N1 | 0.0067 ± 0.00067 | 0.083 ± 0.0083 |
| T2 | 150 | N2 | 0.0363 ± 0.00363 | ||
| T3 | 201 | N3 | 0.0235 ± 0.00235 | ||
| T4 | 280 | N4 | 0.0076 ± 0.00076 | ||
| T5 | 435 | N5 | 0.0089 ± 0.00089 | ||
| SOD Membrane Heating Rate, °C/min | Temperature Maximum | Tmax, °C | Ea, kJ/mol |
|---|---|---|---|
| 10 | T1 | 106 | 12.74 ± 1.02 |
| 15 | 108 | ||
| 20 | 107 | ||
| 25 | 105 | ||
| 30 | 108 | ||
| 10 | T2 | 161 | 18.04 ± 1.26 |
| 15 | 163 | ||
| 20 | 162 | ||
| 25 | 161 | ||
| 30 | 162 | ||
| 10 | T3 | 270 | 32.3 ± 1.715 |
| 15 | 265 | ||
| 20 | 261 | ||
| 25 | 266 | ||
| 30 | 275 | ||
| 10 | T4 | 366 | 69.94 ± 3.99 |
| 15 | 362 | ||
| 20 | 360 | ||
| 25 | 365 | ||
| 30 | 368 |
| LTA Membrane Heating Rate, °C/min | Temperature Maximum | Tmax, °C | Ea, kJ/mol |
|---|---|---|---|
| 10 | T1 | 82 | 20.24 ± 1.21 |
| 15 | 85 | ||
| 20 | 95 | ||
| 25 | 101 | ||
| 30 | 105 | ||
| 10 | T2 | 130 | 28.31 ± 1.98 |
| 15 | 135 | ||
| 20 | 140 | ||
| 25 | 150 | ||
| 30 | 150 | ||
| 10 | T3 | 160 | 35.72 ± 1.88 |
| 15 | 170 | ||
| 20 | 187 | ||
| 25 | 200 | ||
| 30 | 201 | ||
| 10 | T4 | 251 | 74.26 ± 4.91 |
| 15 | 255 | ||
| 20 | 263 | ||
| 25 | 275 | ||
| 30 | 280 | ||
| 10 | T5 | 395 | 78.18 ± 2.42 |
| 15 | 405 | ||
| 20 | 425 | ||
| 25 | 430 | ||
| 30 | 435 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordina, N.E.; Borisova, T.N.; Klyagina, K.S.; Astrakhantseva, I.A.; Ilyin, A.A.; Rumyantsev, R.N. Investigation of NH3 Desorption Kinetics on the LTA and SOD Zeolite Membranes. Membranes 2022, 12, 147. https://doi.org/10.3390/membranes12020147
Gordina NE, Borisova TN, Klyagina KS, Astrakhantseva IA, Ilyin AA, Rumyantsev RN. Investigation of NH3 Desorption Kinetics on the LTA and SOD Zeolite Membranes. Membranes. 2022; 12(2):147. https://doi.org/10.3390/membranes12020147
Chicago/Turabian StyleGordina, Natalya E., Tatyana N. Borisova, Ksenya S. Klyagina, Irina A. Astrakhantseva, Alexander A. Ilyin, and Ruslan N. Rumyantsev. 2022. "Investigation of NH3 Desorption Kinetics on the LTA and SOD Zeolite Membranes" Membranes 12, no. 2: 147. https://doi.org/10.3390/membranes12020147
APA StyleGordina, N. E., Borisova, T. N., Klyagina, K. S., Astrakhantseva, I. A., Ilyin, A. A., & Rumyantsev, R. N. (2022). Investigation of NH3 Desorption Kinetics on the LTA and SOD Zeolite Membranes. Membranes, 12(2), 147. https://doi.org/10.3390/membranes12020147









