LCZ696 Protects against Diabetic Cardiomyopathy-Induced Myocardial Inflammation, ER Stress, and Apoptosis through Inhibiting AGEs/NF-κB and PERK/CHOP Signaling Pathways
Abstract
1. Introduction
2. Results
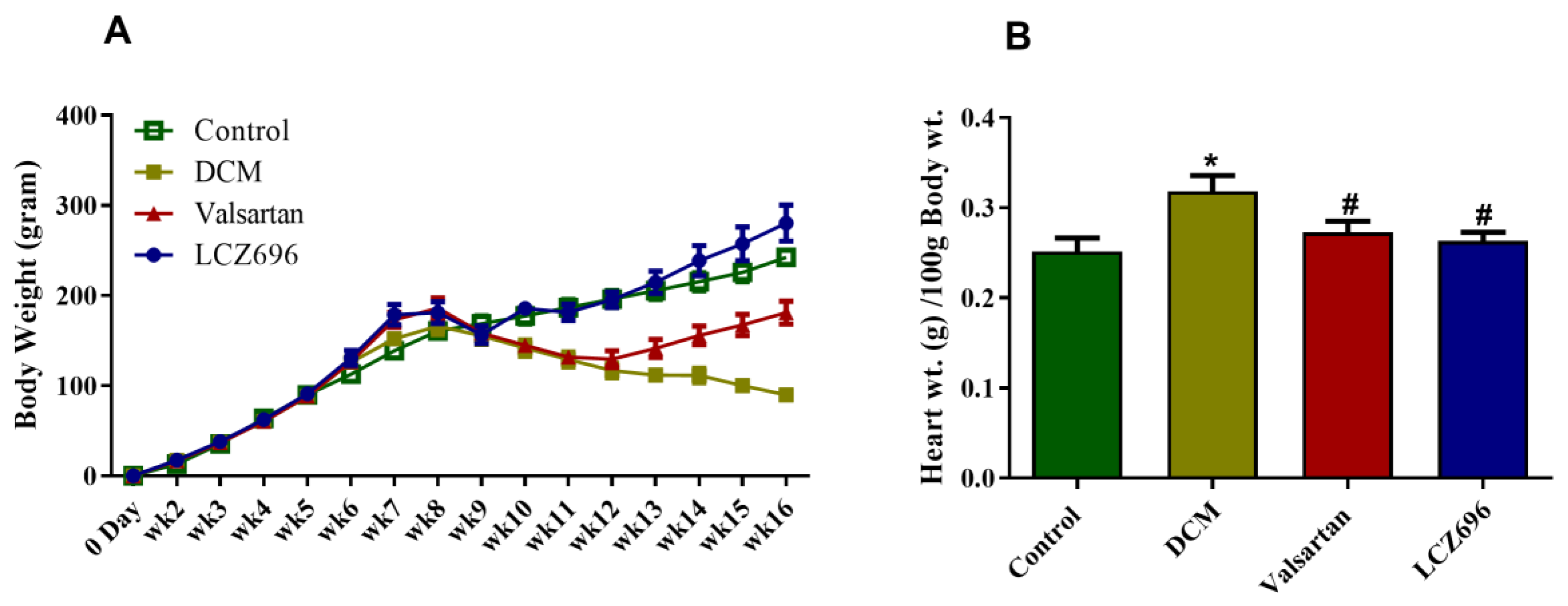
2.1. Effect of LCZ696 and Valsartan Treatments on Weekly Body Growth and Heart Weight
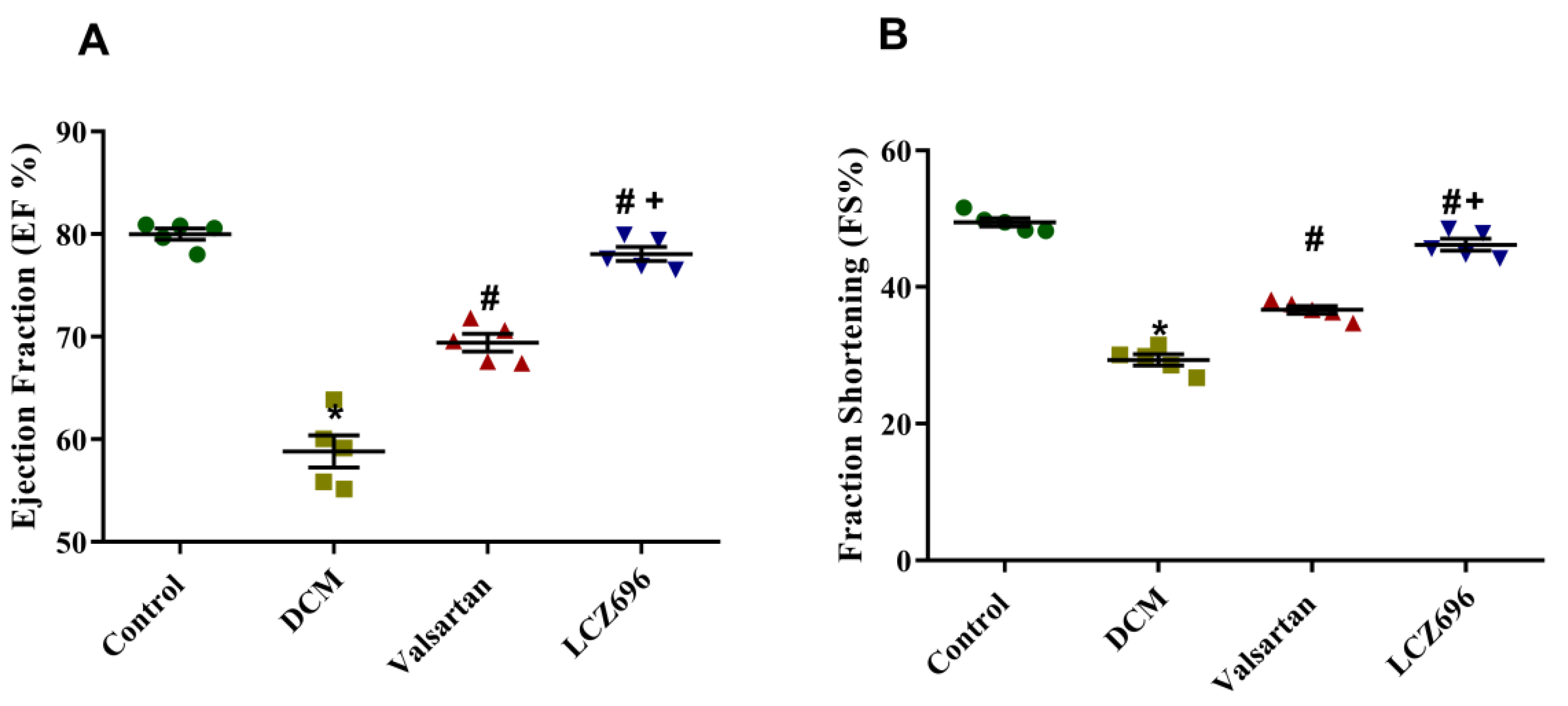
2.2. Effect of LCZ696 and Valsartan Treatment on Blood Pressure and Cardiac Function in Diabetic Cardiomyopathy Rats
2.3. Effects of LCZ696 and Valsartan on Serum Glucose, Insulin, and Cardiac Enzymes
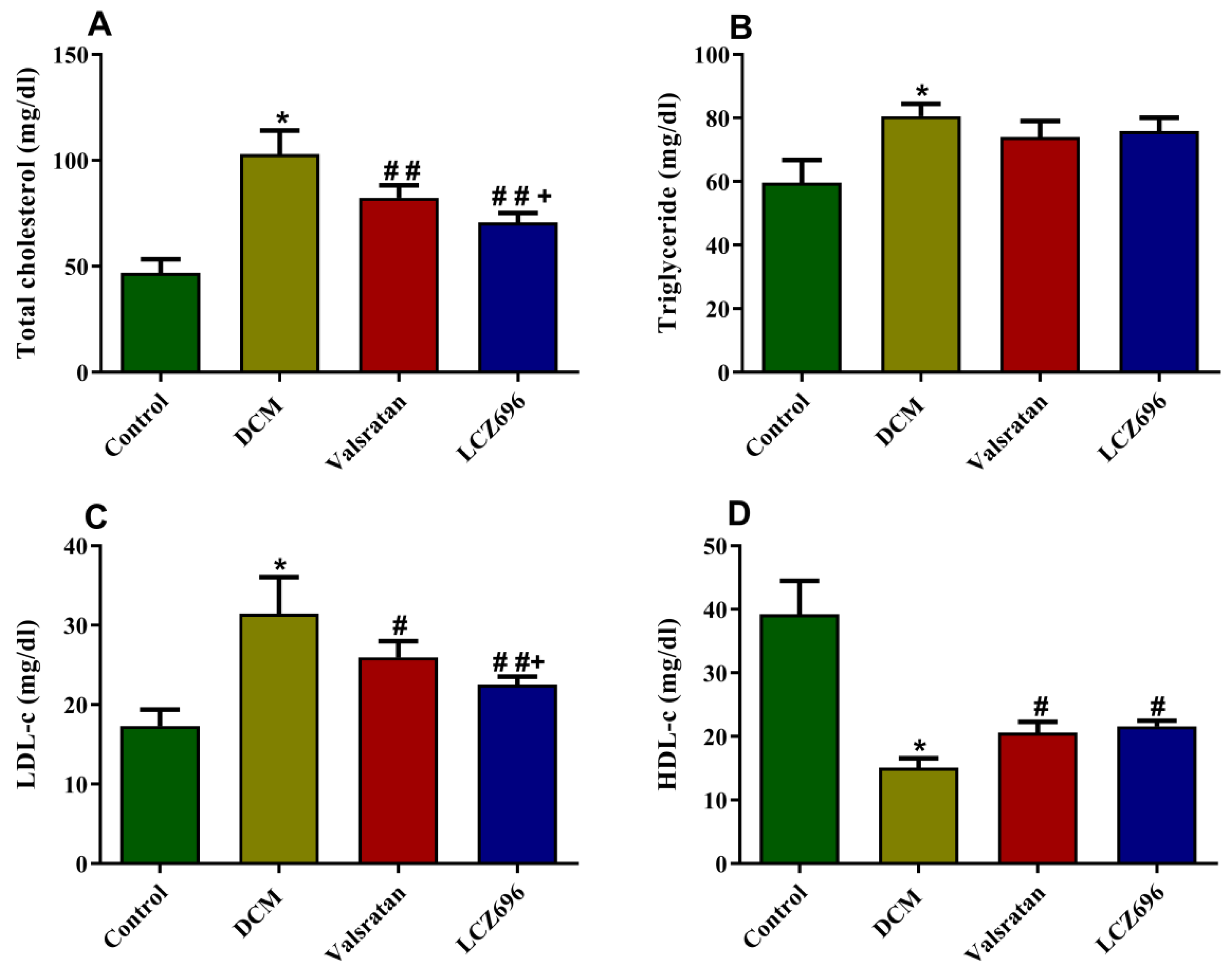
2.4. Effect of LCZ696 and Valsartan Treatments on Serum Lipid Profile
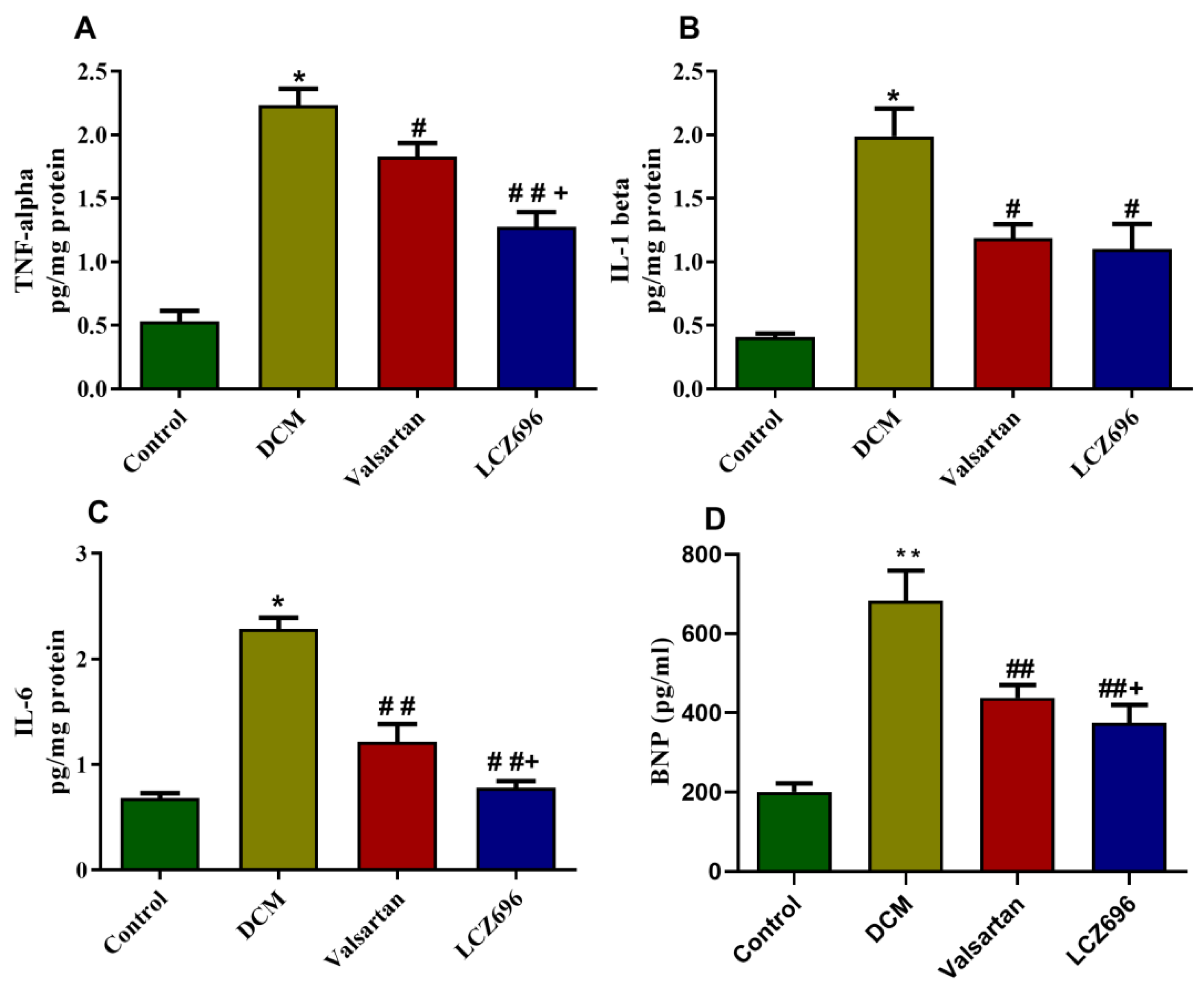
2.5. Effect of LCZ696 and Valsartan Treatment on Inflammatory Biomarkers
2.6. Effect of LCZ696 and Valsartan Treatments on the mRNA and Protein Expressions of Apoptotic Genes including BAX, Bcl2, and Caspase-3 in Cardiac Tissues
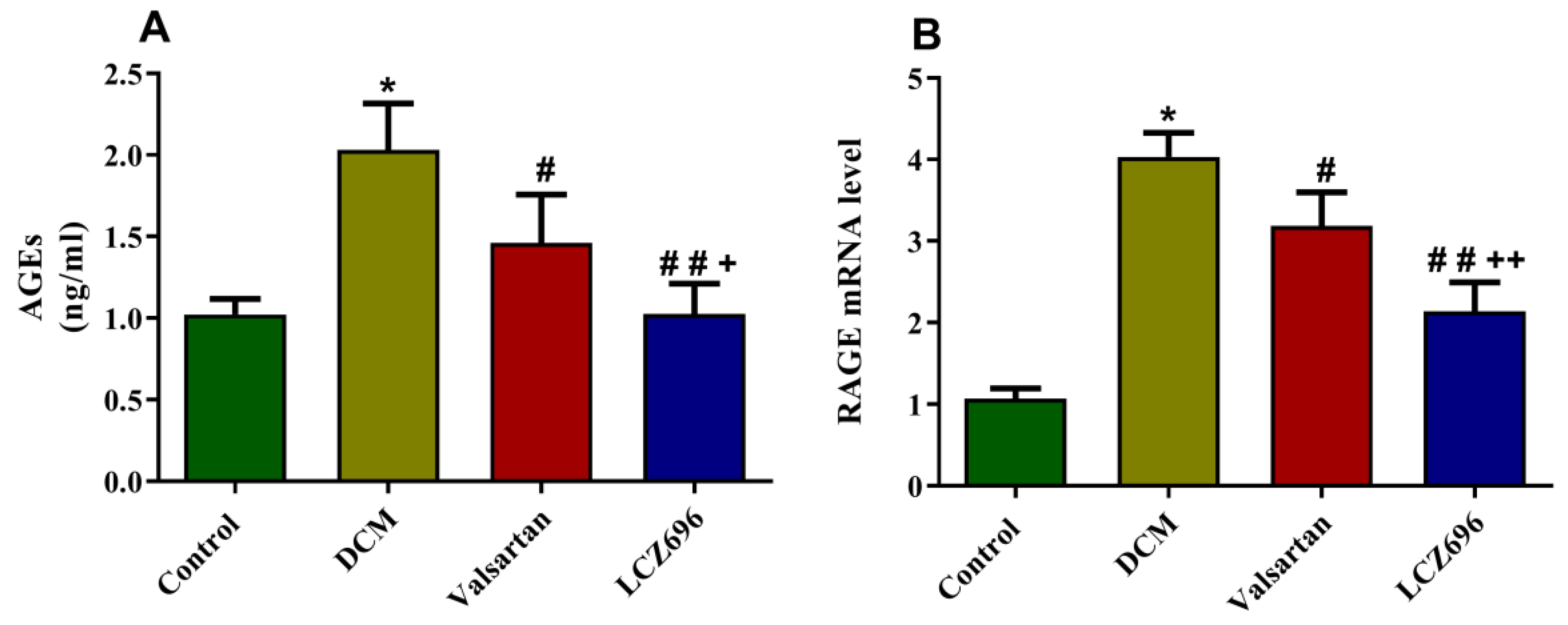
2.7. Effect of LCZ696 and Valsartan on AGEs Accumulation and RAGE Expression Levels
2.8. Effect of LCZ696 and Valsartan on NF-κB mRNA and Protein Expressions in Cardiac Tissues
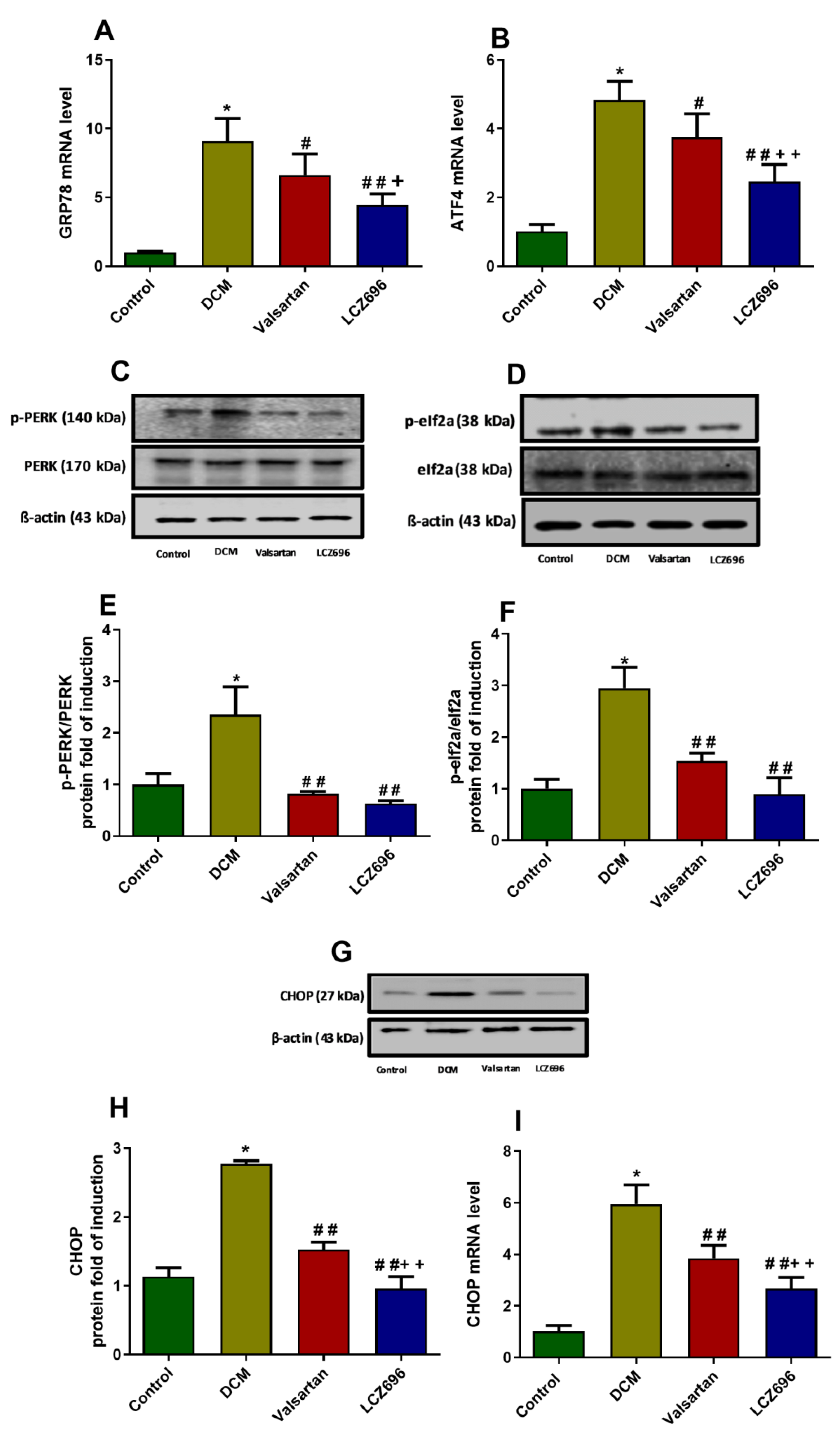
2.9. Effect of LCZ696 and Valsartan on Expressions of Endoplasmic Reticulum Stress Parameters
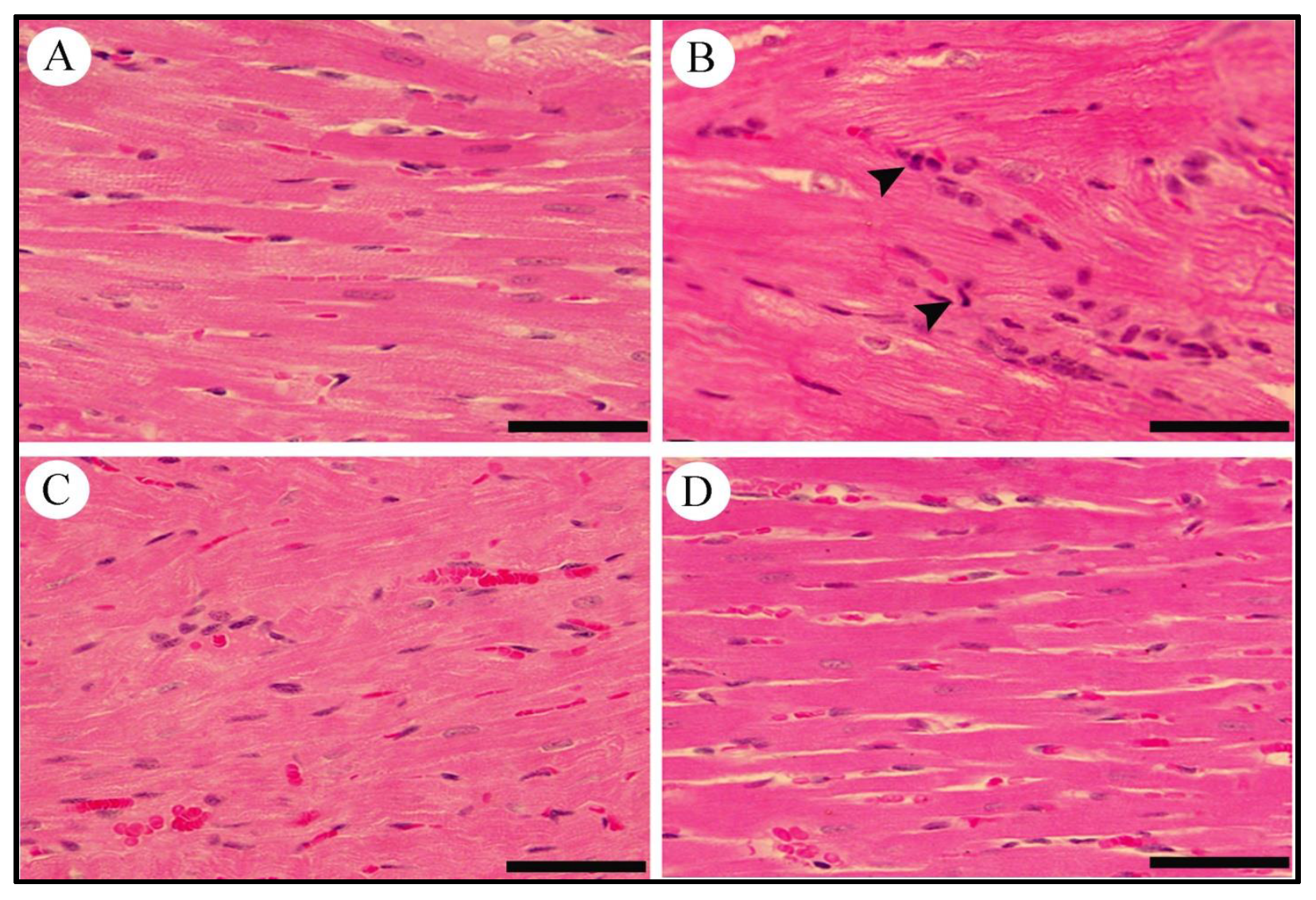
2.10. Effect of LCZ696 and Valsartan on Histopathological Changes in Heart Tissues
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Approval
4.2. Experimental Design
4.3. Serum Biochemical Assays
4.4. Non-Invasive (Tail-Cuff) Blood Pressure Measurements
4.5. Echocardiography
4.6. RNA Extraction and cDNA Synthesis
4.7. Quantification of mRNA Expression Levels by Real-Time Polymerase Chain Reaction (RT-PCR)
4.8. Western Blot Analysis
4.9. Measurement of Advanced Glycation Products Activity and Interleukins in Cardiac Tissues
4.10. Cardiac Histopathology
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.d.; Da Rocha Fernandes, J.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Fotheringham, A.K. Vascular complications in diabetes: Old messages, new thoughts. Diabetologia 2017, 60, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Pant, T.; Dhanasekaran, A.; Bai, X.; Zhao, M.; Thorp, E.B.; Forbess, J.M.; Bosnjak, Z.J.; Ge, Z.-D. Genome-wide differential expression profiling of lncRNAs and mRNAs associated with early diabetic cardiomyopathy. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Parim, B.; Uddandrao, V.S.; Saravanan, G. Diabetic cardiomyopathy: Molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail. Rev. 2019, 24, 279–299. [Google Scholar] [CrossRef]
- Miki, T.; Yuda, S.; Kouzu, H.; Miura, T. Diabetic cardiomyopathy: Pathophysiology and clinical features. Heart Fail. Rev. 2013, 18, 149–166. [Google Scholar] [CrossRef]
- Xing, R.; Liu, D.; Cheng, X.; Tian, X.; Yan, C.; Han, Y.J.B. Communications, b.r. MiR-207 inhibits autophagy and promotes apoptosis of cardiomyocytes by directly targeting LAMP2 in type 2 diabetic cardiomyopathy. Biochem. Biophys. Res. Commun. 2019, 520, 27–34. [Google Scholar] [CrossRef]
- Song, S.; Tan, J.; Miao, Y.; Li, M.; Zhang, Q. Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J. Cell. Physiol. 2017, 232, 2977–2984. [Google Scholar] [CrossRef]
- Yu, W.; Gao, B.; Li, N.; Wang, J.; Qiu, C.; Zhang, G.; Liu, M.; Zhang, R.; Li, C.; Ji, G.; et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2017, 1863, 1973–1983. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef]
- Aragno, M.; Mastrocola, R.; Medana, C.; Catalano, M.G.; Vercellinatto, I.; Danni, O.; Boccuzzi, G. Oxidative stress-dependent impairment of cardiac-specific transcription factors in experimental diabetes. Endocrinology 2006, 147, 5967–5974. [Google Scholar] [CrossRef] [PubMed]
- Norton, G.R.; Candy, G.; Woodiwiss, A.J. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation 1996, 93, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 2018, 9, 3083. [Google Scholar] [CrossRef] [PubMed]
- Song, X.J.; Yang, C.Y.; Liu, B.; Wei, Q.; Korkor, M.T.; Liu, J.Y.; Yang, P. Atorvastatin inhibits myocardial cell apoptosis in a rat model with post-myocardial infarction heart failure by downregulating ER stress response. Int. J. Med. Sci. 2011, 8, 564. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Packer, M.; McMurray, J.J.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015, 131, 54–61. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Correia-Santos, A.M.; Suzuki, A.; Anjos, J.S.; Rêgo, T.S.; Almeida, K.C.; Boaventura, G.T.J.M. Indução de Diabetes Tipo 2 por dieta hiperlipídica e baixa dose de estreptozotocina em ratas wistar. Med. Ribeirão Preto 2012, 45, 436–444. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, C.; Luo, X.; Wang, L.; Zhang, R.; Wang, Z.; Fan, D.; Yang, H.; Deng, J.J.N. Effect of stay-green wheat, a novel variety of wheat in China, on glucose and lipid metabolism in high-fat diet induced type 2 diabetic rats. Nutrients 2015, 7, 5143–5155. [Google Scholar] [CrossRef]
- Abdulmalek, S.; Eldala, A.; Awad, D.; Balbaa, M.J.S.R. Ameliorative effect of curcumin and zinc oxide nanoparticles on multiple mechanisms in obese rats with induced type 2 diabetes. Sci. Rep. 2021, 11, 1–22. [Google Scholar] [CrossRef]
- Magalhães, D.A.; Kume, W.T.; Correia, F.S.; Queiroz, T.S.; Allebrandt, E.W.; Santos, M.P.; Kawashita, N.H.; França, S.A. High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: A new proposal. An. Da Acad. Bras. De Ciências 2019, 91, 1678–2690. [Google Scholar] [CrossRef] [PubMed]
- Rankin, M.M.; Kushner, J.A. Adaptive β-cell proliferation is severely restricted with advanced age. Diabetes 2009, 58, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Gohlke, P.; Kuwer, I.; Bartenbach, S.; Schnell, A.; Unger, T. Effect of low-dose treatment with perindopril on cardiac function in stroke-prone spontaneously hypertensive rats: Role of bradykinin. J. Cardiovasc. Pharmacol. 1994, 24, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Badole, S.L.; Chaudhari, S.M.; Jangam, G.B.; Kandhare, A.D.; Bodhankar, S.L. Cardioprotective activity of Pongamia pinnata in streptozotocin-nicotinamide induced diabetic rats. BioMed Res. Int. 2015, 2015, 403291. [Google Scholar] [CrossRef] [PubMed]
- Malek, V.; Sharma, N.; Gaikwad, A.B. Simultaneous inhibition of neprilysin and activation of ACE2 prevented diabetic cardiomyopathy. Pharmacol. Rep. 2019, 71, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-H.; Lu, J.; Yu, X.-J.; Sun, L.; Zang, W.-J. Ameliorative effect of Captopril and Valsartan on an animal model of diabetic cardiomyopathy. Biol. Pharm. Bull. 2008, 31, 2045–2049. [Google Scholar] [CrossRef]
- Malek, V.; Gaikwad, A.B. Telmisartan and thiorphan combination treatment attenuates fibrosis and apoptosis in preventing diabetic cardiomyopathy. Cardiovasc. Res. 2019, 115, 373–384. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S. Endoplasmic reticulum stress signalling–from basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Khan, S.; Zhang, D.; Zhang, Y.; Li, M.; Wang, C. Wogonin attenuates diabetic cardiomyopathy through its anti-inflammatory and anti-oxidative properties. Mol. Cell. Endocrinol. 2016, 428, 101–108. [Google Scholar] [CrossRef]
- Frustaci, A.; Kajstura, J.; Chimenti, C.; Jakoniuk, I.; Leri, A.; Maseri, A.; Nadal-Ginard, B.; Anversa, P. Myocardial cell death in human diabetes. Circ. Res. 2000, 87, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, W.; Wang, G.; Guo, L.; Jiang, Y.; Kang, Y.J. Hyperglycemia-induced apoptosis in mouse myocardium: Mitochondrial cytochrome C–mediated caspase-3 activation pathway. Diabetes 2002, 51, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Van Linthout, S.; Dhayat, S.; Dhayat, N.; Schmidt, A.; Noutsias, M.; Song, X.-Y.; Spillmann, F.; Riad, A.; Schultheiss, H.-P. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res. Cardiol. 2007, 102, 500. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, G.; von Lewinski, D.; Eaton, D.M.; Sourij, H.; Houser, S.R.; Wallner, M. Diabetic cardiomyopathy: Current and future therapies. Beyond glycemic control. Front. Physiol. 2018, 9, 1514. [Google Scholar] [CrossRef]
- Adamopoulos, C.; Mihailidou, C.; Grivaki, C.; Papavassiliou, K.A.; Kiaris, H.; Piperi, C.; Papavassiliou, A.G. Systemic effects of AGEs in ER stress induction in vivo. Glycoconj. J. 2016, 33, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Piperi, C.; Adamopoulos, C.; Dalagiorgou, G.; Diamanti-Kandarakis, E.; Papavassiliou, A.G. Crosstalk between advanced glycation and endoplasmic reticulum stress: Emerging therapeutic targeting for metabolic diseases. J. Clin. Endocrinol. Metab. 2012, 97, 2231–2242. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.P.; Xu, K.F.; Mao, X.D.; Lu, Y.B.; Fang, L.; Yang, J.W.; Liu, C. Effect of taurine-conjugated ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am. J. Nephrol. 2008, 28, 1014–1022. [Google Scholar] [CrossRef]
- Rong, G.; Tang, X.; Guo, T.; Duan, N.; Wang, Y.; Yang, L.; Zhang, J.; Liang, X. Advanced oxidation protein products induce apoptosis in podocytes through induction of endoplasmic reticulum stress. J. Physiol. Biochem. 2015, 71, 455–470. [Google Scholar] [CrossRef]
- Montagnani, M. Diabetic cardiomyopathy: How much does it depend on AGE? Br. J. Pharmacol. 2008, 154, 725–726. [Google Scholar] [CrossRef]
- Komiya, N.; Hirose, H.; Saisho, Y.; Saito, I.; Itoh, H. Effects of 12-month valsartan therapy on glycation and oxidative stress markers in type 2 diabetic subjects with hypertension. Int. Heart J. 2008, 49, 681–689. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Zhou, Q.; Xu, W.; Cai, L. Endoplasmic reticulum stress and diabetic cardiomyopathy. Exp. Diabetes Res. 2012, 2012, 827971. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dong, Z.; Geng, J.; Sun, Y.; Liu, G.; Kang, W.; Zhang, Y.; Ge, Z. Valsartan protects against ER stress-induced myocardial apoptosis via CHOP/Puma signaling pathway in streptozotocin-induced diabetic rats. Eur. J. Pharm. Sci. 2011, 42, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Korennykh, A.; Walter, P. Structural basis of the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2012, 28, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, Q.; Song, X.; Wang, Y.; Wang, Y.; Song, E.; Song, Y. Activating transcription factor 4 (ATF4)-ATF3-C/EBP homologous protein (CHOP) cascade shows an essential role in the ER stress-induced sensitization of tetrachlorobenzoquinone-challenged PC12 cells to ROS-mediated apoptosis via death receptor 5 (DR5) signaling. Chem. Res. Toxicol. 2016, 29, 1510–1518. [Google Scholar] [PubMed]
- Chen, Y.; Gui, D.; Chen, J.; He, D.; Luo, Y.; Wang, N. Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside IV is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats. Cell. Physiol. Biochem. 2014, 33, 1975–1987. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Dai, D.-L.; Yao, L.; Yu, H.-H.; Ning, B.; Zhang, Q.; Chen, J.; Cheng, W.-H.; Shen, W.; Yang, Z.-X. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol. Cell. Biochem. 2012, 364, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.-H.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, T.; Dai, H.; Liu, G.; Wang, H.; Sun, Y.; Zhang, Y.; Ge, Z. Involvement of endoplasmic reticulum stress in myocardial apoptosis of streptozocin-induced diabetic rats. J. Clin. Biochem. Nutr. 2007, 41, 58–67. [Google Scholar] [CrossRef]
- Ouyang, C.; You, J.; Xie, Z. The interplay between autophagy and apoptosis in the diabetic heart. J. Mol. Cell. Cardiol. 2014, 71, 71–80. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Cai, L. Diabetic cardiomyopathy and its mechanisms: Role of oxidative stress and damage. J. Diabetes Investig. 2014, 5, 623–634. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Ghebeh, H.; Alhaider, A.A.; El-Kadi, A.O.; Soshilov, A.A.; Denison, M.S.; Ansari, M.A.; Korashy, H.M. Metformin inhibits 7,12-dimethylbenz[a]anthracene-induced breast carcinogenesis and adduct formation in human breast cells by inhibiting the cytochrome P4501A1/aryl hydrocarbon receptor signaling pathway. Toxicol Appl Pharm. 2015, 284, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Korashy, H.M.; El-Kadi, A.O. Differential effects of mercury, lead and copper on the constitutive and inducible expression of aryl hydrocarbon receptor (AHR)-regulated genes in cultured hepatoma Hepa 1c1c7 cells. Toxicology 2004, 201, 153–172. [Google Scholar] [CrossRef] [PubMed]



| Day 0 | 8th Week | 16th Week | ||||
|---|---|---|---|---|---|---|
| Systolic | Diastolic | Systolic | Diastolic | Systolic | Diastolic | |
| Control | 116.29 ± 8.04 | 83.07 ± 8.83 | 114.73 ± 5.88 | 88.6 ± 5.68 | 121.71 ± 5.72 | 79.93 ± 10.47 |
| DCM | 115.64 ± 7.72 | 83.57 ± 7.55 | 158.57 ± 7.19 * | 110.93 ± 7.84 * | 175.11 ± 7.03 * | 117.21 ± 4.55 * |
| Valsartan | 117.42 ± 3.92 | 77.33 ± 9.03 | 153.53 ± 7.32 | 118.4 ± 8.32 | 135.67 ± 5.92 # | 88 ± 5.89 # |
| LCZ695 | 120.93 ± 2.52 | 80.27 ± 4.32 | 150.73 ± 7.05 | 116.8 ± 4.48 | 134.9 ± 5.22 # | 86 ± 4.48 # |
| Gene | 5′→3′ Forward Primer | 5′→3′ Reverse Primer |
|---|---|---|
| β-actin | TGT TGC CCTAGA CTT CGA GCA | GCCAGTAGAGGCAGGGATGAT |
| Caspase-3 | CGCAGACCTTGTGATATTCCAG | CGTTTCTTCCATCCTTCCAGG |
| BAX | TCCACCAAGAAGCTGAGCGAG | GTCCAGCCCATGATGGTTCT |
| Bcl2 | CATGTGTGTGGAGAGCGTCAA | GCCGGTTCAGGTACTCAGTCA |
| NF-kB | AGTCCTGCTCCTTCCAAAAC | CTTCGGTGTAGCCCATTTGT |
| RAGE | TCCTGGTGGGACCGTGAC | GGGTGTGCCATCTTTTATCCA |
| CHOP | CCAGCAGAGGTCACAAGCAC | CGCACTGACCACTCTGTT TC |
| ATF-4 | CACTAGGTACCGCCAGAAGAAGA | AATCCGCCCTCTCTTTTAGAG |
| GRP78 | TCAGCCCACCGTAACAAT | CAAACTTCTCGGCGTCAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belali, O.M.; Ahmed, M.M.; Mohany, M.; Belali, T.M.; Alotaibi, M.M.; Al-Hoshani, A.; Al-Rejaie, S.S. LCZ696 Protects against Diabetic Cardiomyopathy-Induced Myocardial Inflammation, ER Stress, and Apoptosis through Inhibiting AGEs/NF-κB and PERK/CHOP Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 1288. https://doi.org/10.3390/ijms23031288
Belali OM, Ahmed MM, Mohany M, Belali TM, Alotaibi MM, Al-Hoshani A, Al-Rejaie SS. LCZ696 Protects against Diabetic Cardiomyopathy-Induced Myocardial Inflammation, ER Stress, and Apoptosis through Inhibiting AGEs/NF-κB and PERK/CHOP Signaling Pathways. International Journal of Molecular Sciences. 2022; 23(3):1288. https://doi.org/10.3390/ijms23031288
Chicago/Turabian StyleBelali, Osamah M., Mohammed M. Ahmed, Mohamed Mohany, Tarig M. Belali, Meshal M. Alotaibi, Ali Al-Hoshani, and Salim S. Al-Rejaie. 2022. "LCZ696 Protects against Diabetic Cardiomyopathy-Induced Myocardial Inflammation, ER Stress, and Apoptosis through Inhibiting AGEs/NF-κB and PERK/CHOP Signaling Pathways" International Journal of Molecular Sciences 23, no. 3: 1288. https://doi.org/10.3390/ijms23031288
APA StyleBelali, O. M., Ahmed, M. M., Mohany, M., Belali, T. M., Alotaibi, M. M., Al-Hoshani, A., & Al-Rejaie, S. S. (2022). LCZ696 Protects against Diabetic Cardiomyopathy-Induced Myocardial Inflammation, ER Stress, and Apoptosis through Inhibiting AGEs/NF-κB and PERK/CHOP Signaling Pathways. International Journal of Molecular Sciences, 23(3), 1288. https://doi.org/10.3390/ijms23031288









