Oxysterols Profile in Zebrafish Embryos Exposed to Triclocarban and Propylparaben—A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Zebrafish Maintenance and Eggs Collection
2.3. Zebrafish Embryos Exposure
2.4. Quantification of Oxysterols Levels
2.5. Statistical Analysis
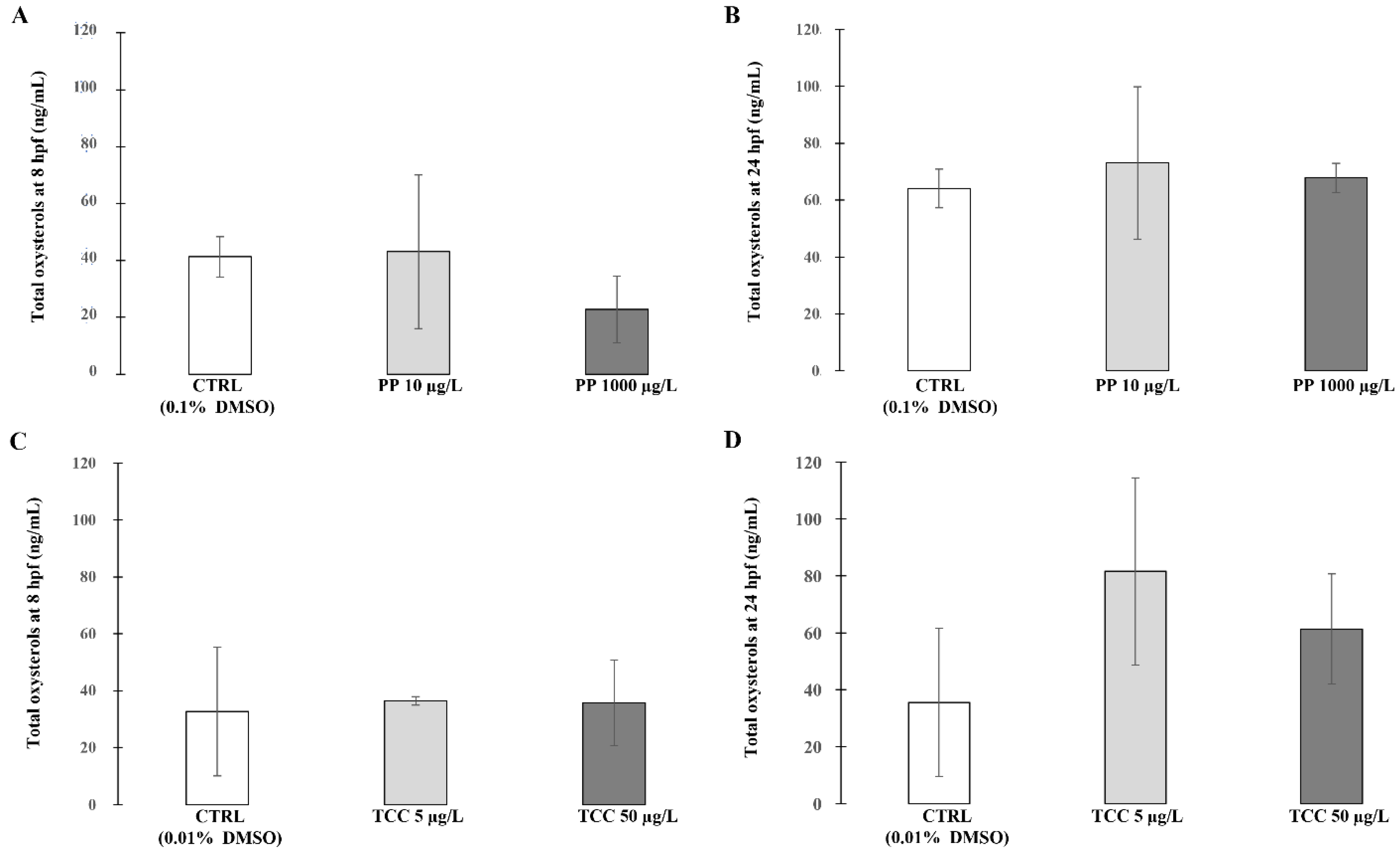
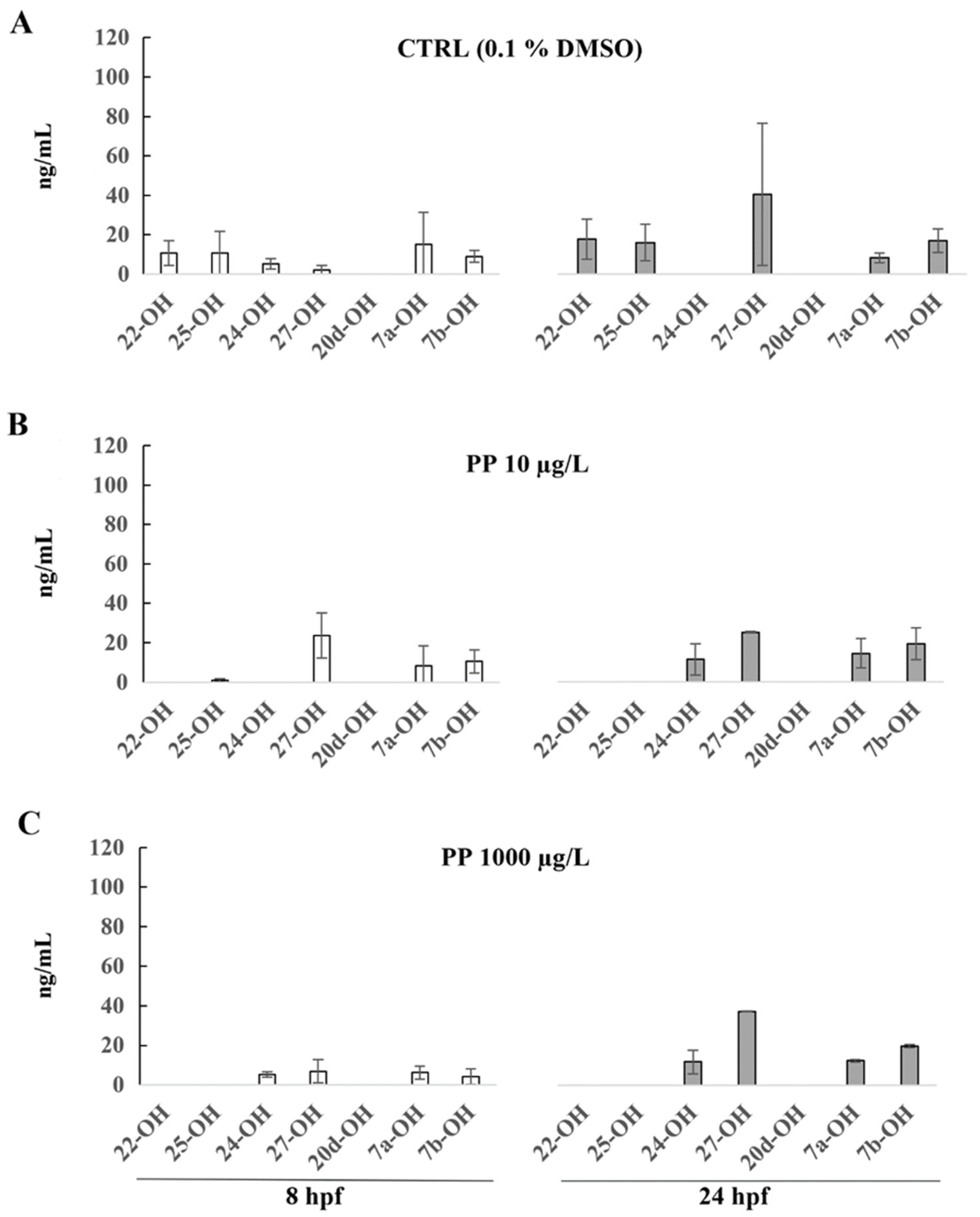
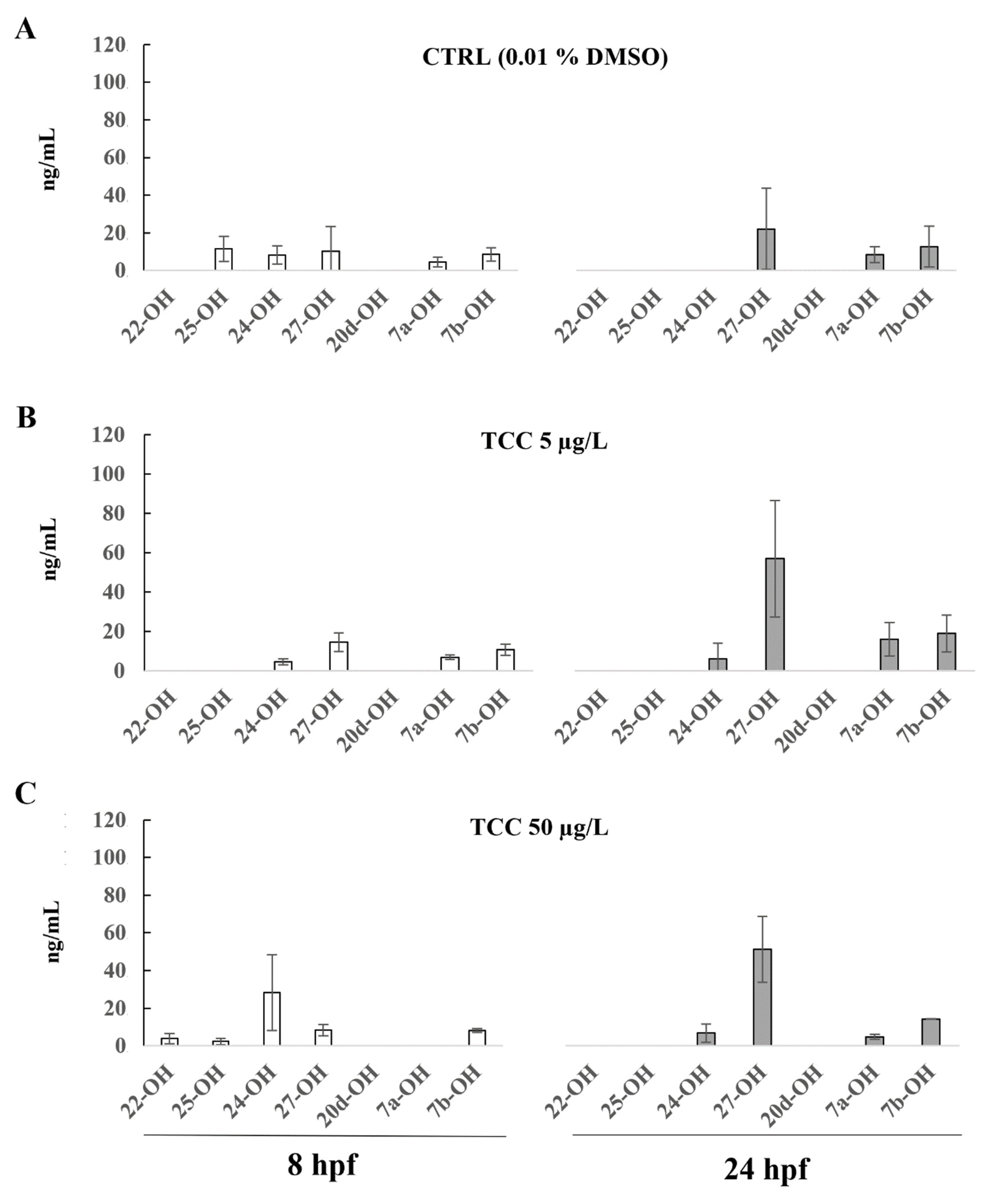
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darbre, P.D. Chemical components of plastics as endocrine disruptors: Overview and commentary. Birth Defects Res. 2020, 112, 1300–1307. [Google Scholar] [CrossRef]
- Merrill, M.A.L.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casals-Casas, C.; Desvergne, B. Endocrine Disruptors: From Endocrine to Metabolic Disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grün, F.; Blumberg, B. Endocrine disrupters as obesogens. Mol. Cell. Endocrinol. 2011, 304, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, A.M.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [Green Version]
- Toporova, L.; Balaguer, P. Nuclear receptors are important targets of environmental disrupting compounds. Mol. Cell. Endocrinol. 2019, 15, 110665. [Google Scholar] [CrossRef]
- Le Magueresse-Battistoni, B.; Labaronne, E.; Vidal, H.; Naville, D. Endocrine disrupting chemicals in mixture and obesity, diabetes and related metabolic disorders. World J. Biol. Chem. 2017, 8, 108–119. [Google Scholar] [CrossRef]
- Küblbeck, J.; Vuorio, T.; Niskanen, J.; Fortino, V.; Braeuning, A.; Abass, K.; Rautio, A.; Hakkola, J.; Honkakoski, P. The EDCMET Project: Metabolic E ff ects of Endocrine Disruptors. Int. J. Mol. Sci. 2020, 21, 3021. [Google Scholar] [CrossRef]
- Guillemot-legris, O.; Mutemberezi, V.; Muccioli, G.G. Oxysterols in Metabolic Syndrome: From Bystander Molecules to Bioactive Lipids. Trends Mol. Med. 2016, 22, 594–614. [Google Scholar] [CrossRef]
- Mutemberezi, V.; Masquelier, J.; Guillemot-Legris, O. Development and validation of an HPLC-MS method for the simultaneous quantification of key oxysterols, endocannabinoids, and ceramides: Variations in metabolic syndrome. Anal. Bioanal. Chem. 2015, 408, 733–745. [Google Scholar] [CrossRef]
- Zmysłowski, A.; Szterk, A. Oxysterols as a biomarker in diseases. Clin. Chim. Acta 2019, 491, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Tremblay-Franco, M.; Zerbinati, C.; Pacelli, A.; Palmaccio, G.; Lubrano, C.; Ducheix, S.; Guillou, H.; Iuliano, L. Effect of obesity and metabolic syndrome on plasma oxysterols and fatty acids in human. Steroids 2015, 99, 287–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Nelson, E.R. Oxysterols, and nuclear receptors. Mol. Cell. Endocrinol. 2019, 484, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Gurlek, A.; Sendur, S.N.; Karahan, S.; Akbiyik, F.; Lay, I. Oxysterol species: Reliable markers of oxidative stress in diabetes mellitus. J. Endocrinol. Investig. 2019, 42, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Alkazemi, D.; Egeland, G.; Vaya, J.; Meltzer, S.; Kubow, S. Oxysterol as a Marker of Atherogenic Dyslipidemia in Adolescence. J. Clin. Endocrinol. Metab. 2008, 93, 4282–4289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perugini, M.; Merola, C.; Amorena, M.; D’Angelo, M.; Cimini, A.; Benedetti, E. Sublethal exposure to propylparaben leads to lipid metabolism impairment in zebrafish early-life stages. J. Appl. Toxicol. 2019, 40, 493–503. [Google Scholar] [CrossRef]
- Zhou, T.; Wei, J.; Su, Y.; Hu, Z.; Li, Y.; Yuan, H.; Zhao, K.; Liu, C.; Zhang, H. Triclocarban at environmentally relevant concentrations induces the endoplasmic reticulum stress in zebrafish. Environ. Toxicol. 2018, 34, 223–232. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Bugos, J. Assessing the Public Health Implications of the Food Preservative Propylparaben: Has This Chemical Been Safely Used for Decades. Curr. Environ. Health Rep. 2021, 8, 54–70. [Google Scholar] [CrossRef]
- Petric, Z.; Ružić, J.; Žuntar, I. The controversies of parabens—An overview nowadays. Acta Pharm. 2021, 71, 17–32. [Google Scholar] [CrossRef]
- Haman, C.; Dauchy, X.; Rosin, C.; Munoz, J.F. Occurrence, fate and behavior of parabens I aquatic environments: A review. Water Res. 2015, 1, 1–11. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Picò, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia river basin: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Calma, M.L.; Medina, P.M.B. Acute and chronic exposure of the holometabolous life cycle of Aedes aegypti L. to emerging contaminants naproxen and propylparaben. Environ. Pollut. 2020, 266 Pt 3, 115275. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Kim, M.S.; Hwang, U.K.; Jeong, C.B.; Lee, J.S. Effects of methylparaben, ethylparaben, and propylparaben on life parameters and sex ratio in the marine copepod Tigriopus japonicus. Chemosphere 2019, 226, 388–394. [Google Scholar] [CrossRef]
- Bereketoglu, C.; Pradhan, A. Science of the Total Environment Comparative transcriptional analysis of methylparaben and propylparaben in zebra fish. Sci. Total Environ. 2019, 671, 129–139. [Google Scholar] [CrossRef]
- Gomez, E.; Pillon, A.; Fenet, H.; Rosain, D.; Duchesne, M.J.; Nicolas, J.C.; Balaguer, P.; Casellas, C. Estrogenic activity of cosmetic components in reporter cell lines: Parabens, UV screens, and musks. J. Toxicol. Environ Health B Crit Rev. A 2005, 68, 239–251. [Google Scholar] [CrossRef]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Saturnino, C.; Salvagno, L.; Ielo, I.; Drommi, D.; Scali, E.; Plutino, M.R.; Rosace, G.; et al. The Different Facets of Triclocarban: A Review. Molecules 2021, 26, 2811. [Google Scholar] [CrossRef] [PubMed]
- Chalew, T.E.; Halden, R.U. Environmental Exposure of Aquatic and Terrestrial Biota to Triclosan and Tricocarban. J. Am. Water Work. Assoc. 2009, 45, 4–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pycke, B.F.; Geer, L.A.; Dalloul, M.; Abulafia, O.; Jenck, A.M.; Halden, R.U. Human fetal exposure to triclosan and triclocarban in an urban population from Brooklyn, New York. Environ. Sci. Technol. 2014, 48, 8831–8838. [Google Scholar] [CrossRef]
- Hinther, A.; Bromba, C.M.; Wulff, J.E.; Helbing, C.C. Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ. Sci. Technol. 2011, 45, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.C.; Zhao, B.; Chen, J.; Cherednichenko, G.; Sanmarti, E.; Denison, M.S.; Lasley, B.; Pessah, I.N.; Kültz, D.; Chang, D.P.; et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ. Health Perspect. 2008, 116, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Freeman, L.J. Toxicogenomics to Evaluate Endocrine Disrupting Effects of Environmental Chemicals Using the Zebrafish Model. Curr. Genom. 2016, 17, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Benchoula, K.; Khatib, A.; Jaffar, A.; Ahmed, Q.U.; Sulaiman, W.M.A.W.; Wahab, R.A.; El-Seedi, H.R. The promise of zebrafish as a model of metabolic syndrome. Exp. Anim. 2019, 68, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seth, A.; Stemple, D.L.; Barroso, I. The emerging use of zebrafish to model metabolic disease. Dis. Models Mech. 2013, 6, 1080–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Archer, A.; Lauter, G.; Hauptmann, G.; Mode, A.; Gustafsson, J.A. Transcriptional Activity and Developmental Expression of Liver X Receptor ( lxr ) in Zebrafish. Dev. Dyn. 2008, 237, 1090–1098. [Google Scholar] [CrossRef]
- Jamadagni, P.; Patten, S.A. Neurotoxicology 25-hydroxycholesterol impairs neuronal and muscular development in zebra fish. Neurotoxicology 2019, 75, 14–23. [Google Scholar] [CrossRef]
- Pereiro, P.; Forn-cuní, G.; Dios, S.; Coll, J.; Figueras, A.; Novoa, B. Interferon-independent antiviral activity of 25-hydroxycholesterol in a teleost fish. Antivir. Res. 2017, 145, 146–159. [Google Scholar] [CrossRef]
- OECD Test No. 203: Fish, Acute Toxicity Test. OECD, Guideline for Testing of Chemicals. OECD. 1992. Available online: http://www.oecd.org/chemicalsafety/risk-assessment/1948241.pdf (accessed on 12 December 2021).
- Caioni, G.; Panella, G.; Merola, C.; Cimini, A.; Amorena, M.; Benedetti, E.; Perugini, M. Environmentally relevant concentrations of triclocarban affect morphological traits and melanogenesis in zebrafish larvae. Aquat. Toxicol. 2021, 236, 105842. [Google Scholar] [CrossRef]
- Ashrap, P.; Watkins, D.J.; Calafat, A.M.; Ye, X.; Rosario, Z.; Brown, P.; Vélez-vega, C.M.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in Northern Puerto Rico: Predictors and trends. Environ. Int. 2018, 121, 990–1002. [Google Scholar] [CrossRef]
- Fanti, F.; Merola, C.; Vremere, A.; Oliva, E.; Perugini, M.; Amorena, M.; Compagnone, D.; Sergi, M. Quantitative analysis of oxysterols in zebrafish embryos by HPLC-MS/MS. Talanta 2020, 220, 121393. [Google Scholar] [CrossRef]
- Enright, H.A.; Falso, M.J.S.; Malfatti, M.A.; Lao, V.; Kuhn, E.A.; Hum, N.; Shi, Y.; Sales, A.P.; Haack, K.W.; Kulp, K.S.; et al. Maternal exposure to an environmentally relevant dose of triclocarban results in perinatal exposure and potential alterations in offspring development in the mouse model. PLoS ONE 2017, 12, e0181996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neier, K.; Marchlewicz, E.H.; Dolinoy, D.C.; Neier, K.; Marchlewicz, E.H.; Dana, C. Assessing Human Health Risk to Endocrine Disrupting Chemicals: A Focus on Prenatal Exposures and Oxidative Stress. Endocr. Disruptors 2015, 3, e1069916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikamo, E.; Harada, S.; Nishikawa, J.; Nishihara, T. Endocrine disruptors induce cytochrome P450 by affecting transcriptional regulation via pregnane X receptor. Toxicol. Appl. Pharmacol. 2003, 193, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Xu, Y.; He, S.; Ren, X.; Yang, Y.; Luo, S.; Xie, X.; Luo, L. Antimicrobial Triclocarban Exhibits Higher Agonistic Activity on Estrogen-Related Receptor γ than Triclosan at Human Exposure Levels: A Novel Estrogenic Disruption Mechanism. Environ. Sci. Technol. 2020, 7, 434–439. [Google Scholar] [CrossRef]
- Chen, J.; Brown, T.R.; Russo, J. Regulation of energy metabolism pathways by estrogens and estrogenic chemicals and potential implications in obesity associated with increased exposure to endocrine disruptors. Biochim. Biophys. Acta 2009, 1793, 1128–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grayaa, S.; Zerbinati, C.; Messedi, M.; Hadjkacem, I.; Chtourou, M.; Touhemi, D.B.; Naifar, M.; Ayadi, H.; Ayedi, F.; Iuliano, L. Plasma oxysterol profiling in children reveals 24-hydroxycholesterol as a potential marker for Autism Spectrum Disorders. Biochimie 2018, 153, 80–85. [Google Scholar] [CrossRef]
- Thisse, B.; Thisse, C. Fast Release Clones: A High Throughput Expression Analysis. ZFIN Direct Data Submission. 2004. Available online: http://zfin.org (accessed on 12 December 2021).
- Björkhem, I.; Cedazo-minguez, A.; Leoni, V.; Meaney, S. Molecular Aspects of Medicine Oxysterols and neurodegenerative diseases. Mol. Asp. Med. 2009, 30, 171–179. [Google Scholar] [CrossRef]
- Paul, S.M.; Doherty, J.J.; Robichaud, A.J.; Belfort, G.M.; Chow, B.Y.; Hammond, R.S.; Crawford, D.C.; Linsenbardt, A.J.; Shu, H.; Izumi, Y.; et al. The Major Brain Cholesterol Metabolite 24 (S)-Hydroxycholesterol Is a Potent Allosteric Modulator of N-Methyl-D-Aspartate Receptors. J. Neurosci. 2013, 33, 17290–17300. [Google Scholar] [CrossRef] [Green Version]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fantò, F.; Poli, G.; Gamba, P. Changes in brain oxysterols at different stages of Alzheimer’s disease: Their involvement in neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Kajta, M.; Rzemieniec, J.; Wnuk, A.; Lasoń, W. Triclocarban impairs autophagy in neuronal cells and disrupts estrogen receptor signaling via hypermethylation of specific genes. Sci. Total Environ. 2019, 701, 134818. [Google Scholar] [CrossRef]
- Merola, C.; Perugini, M.; Lucon-Xiccato, T.; Bertolucci, C. Behavioural effects of early-life exposure to parabens in zebrafish larvae. J. Appl. Toxicol. 2021, 41, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhou, T.; Hu, Z.; Li, Y.; Yuan, H.; Zhao, K.; Zhang, H.; Liu, C. Effects of triclocarban on oxidative stress and innate immune response in zebrafish embryos. Chemosphere 2018, 210, 93–101. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merola, C.; Vremere, A.; Fanti, F.; Iannetta, A.; Caioni, G.; Sergi, M.; Compagnone, D.; Lorenzetti, S.; Perugini, M.; Amorena, M. Oxysterols Profile in Zebrafish Embryos Exposed to Triclocarban and Propylparaben—A Preliminary Study. Int. J. Environ. Res. Public Health 2022, 19, 1264. https://doi.org/10.3390/ijerph19031264
Merola C, Vremere A, Fanti F, Iannetta A, Caioni G, Sergi M, Compagnone D, Lorenzetti S, Perugini M, Amorena M. Oxysterols Profile in Zebrafish Embryos Exposed to Triclocarban and Propylparaben—A Preliminary Study. International Journal of Environmental Research and Public Health. 2022; 19(3):1264. https://doi.org/10.3390/ijerph19031264
Chicago/Turabian StyleMerola, Carmine, Anton Vremere, Federico Fanti, Annamaria Iannetta, Giulia Caioni, Manuel Sergi, Dario Compagnone, Stefano Lorenzetti, Monia Perugini, and Michele Amorena. 2022. "Oxysterols Profile in Zebrafish Embryos Exposed to Triclocarban and Propylparaben—A Preliminary Study" International Journal of Environmental Research and Public Health 19, no. 3: 1264. https://doi.org/10.3390/ijerph19031264
APA StyleMerola, C., Vremere, A., Fanti, F., Iannetta, A., Caioni, G., Sergi, M., Compagnone, D., Lorenzetti, S., Perugini, M., & Amorena, M. (2022). Oxysterols Profile in Zebrafish Embryos Exposed to Triclocarban and Propylparaben—A Preliminary Study. International Journal of Environmental Research and Public Health, 19(3), 1264. https://doi.org/10.3390/ijerph19031264








