Laser-Induced Generation of Hydrogen in Water by Using Graphene Target
Abstract
:1. Introduction
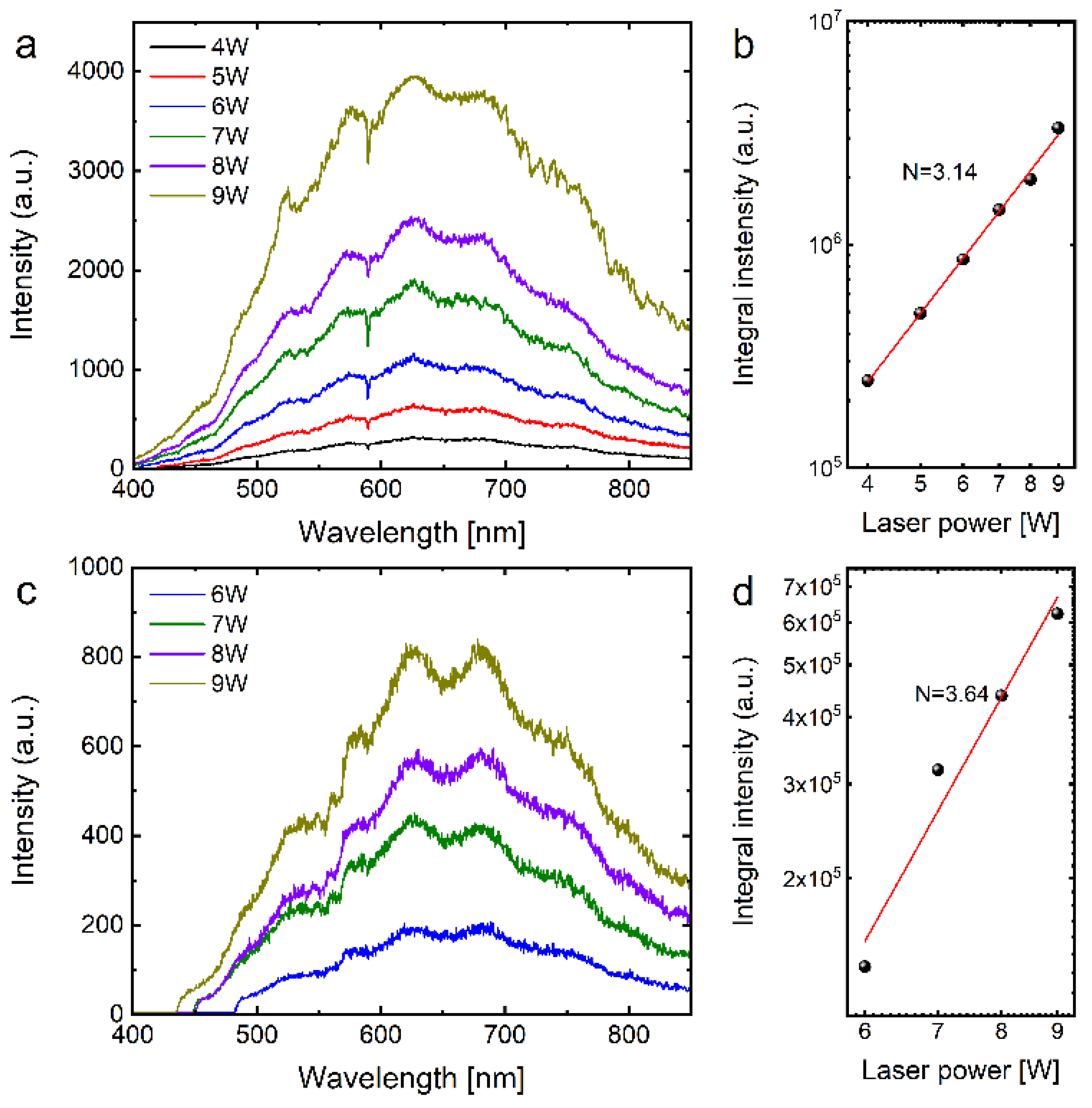
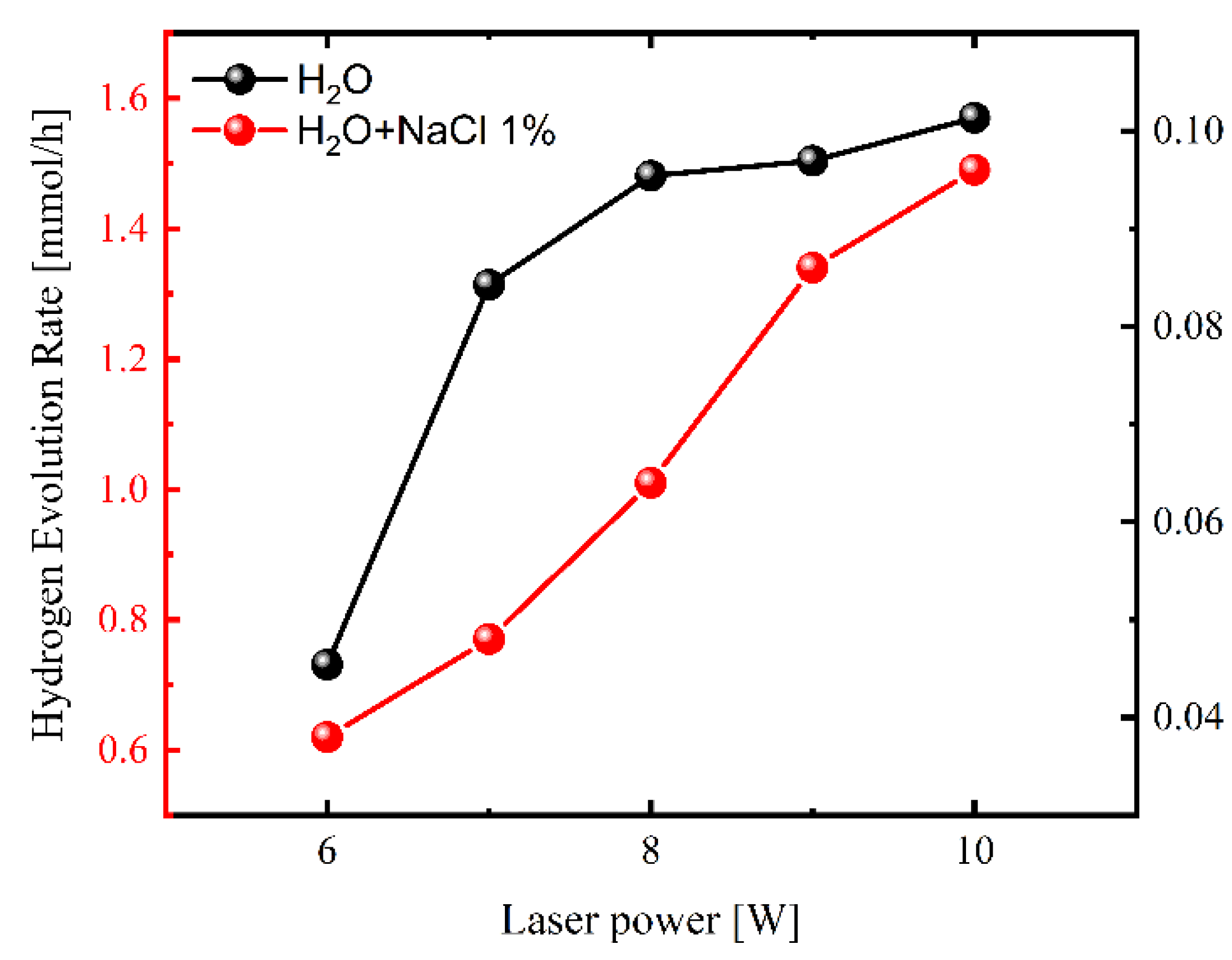
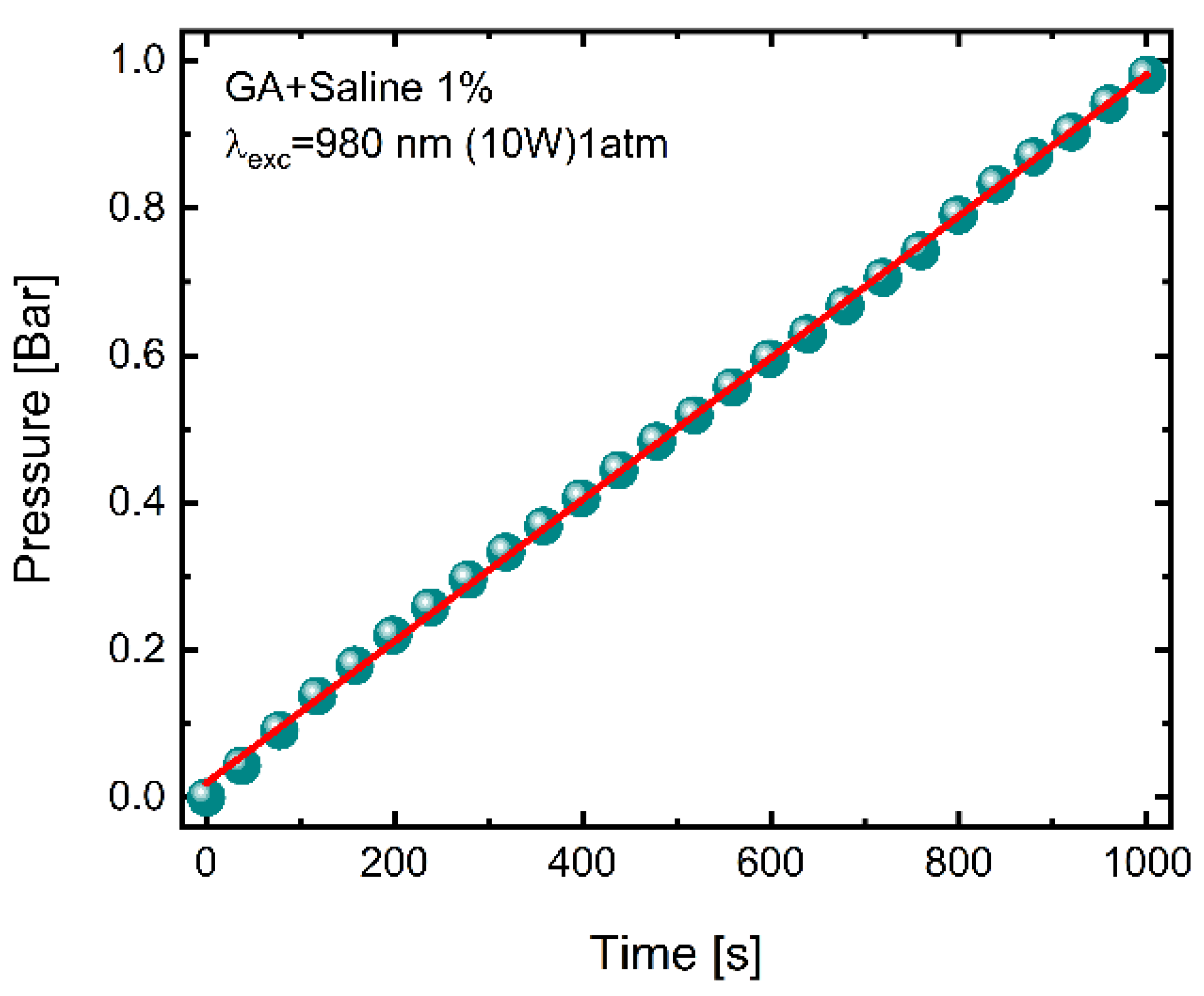
2. Results and Discussion
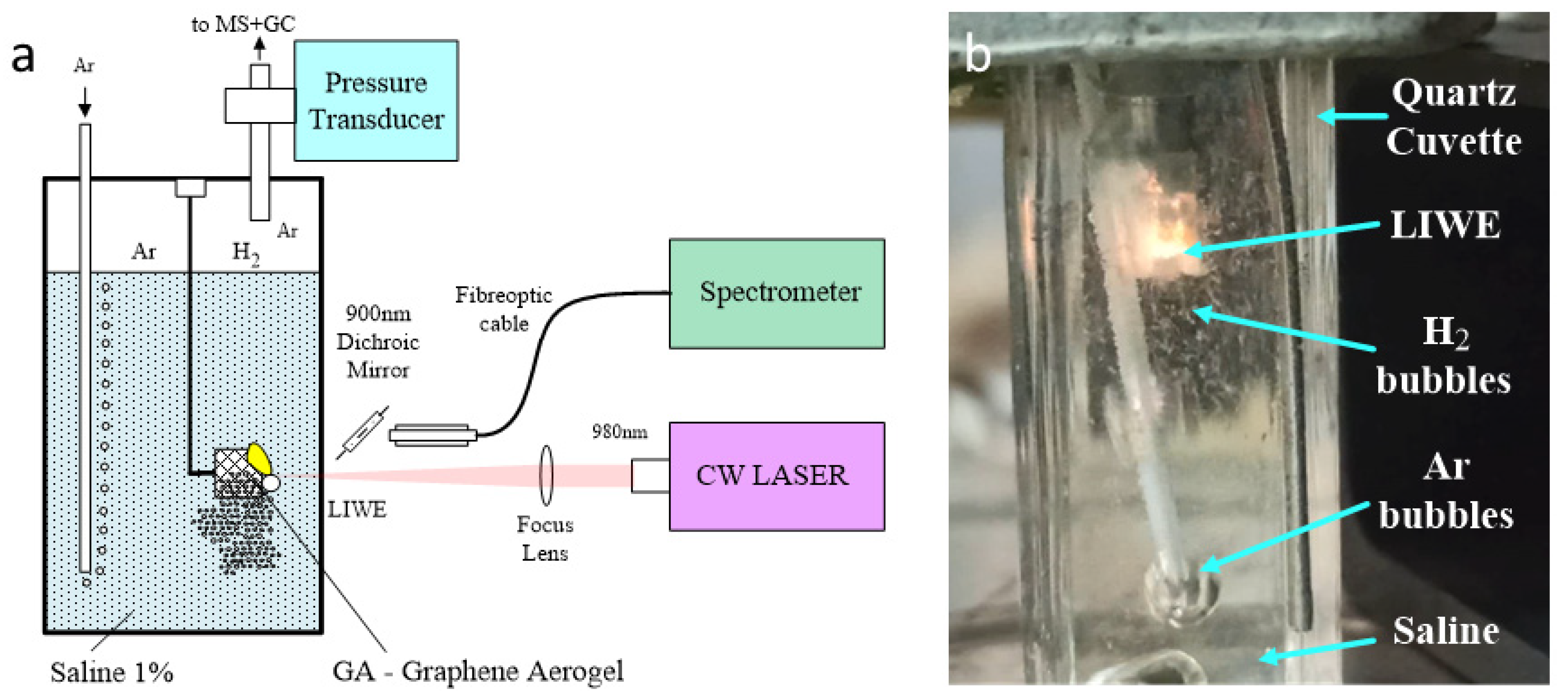
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abuadala, A.; Dincer, I. A review on biomass-based hydrogen production and potential applications. Int. J. Energy Res. 2012, 36, 415–455. [Google Scholar] [CrossRef]
- Mujeebu, M.A. Hydrogen and syngas production by superadiabatic combustion—A review. Appl. Energy 2016, 173, 210–224. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Biomass-based hydrogen production: A review and analysis. Int. J. Hydrogen Energy 2009, 34, 8799–8817. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Jafari, M.; Iranshahi, D. Progress in catalytic naphtha reforming process: A review. Appl. Energy 2013, 109, 79–93. [Google Scholar] [CrossRef]
- Trane, R.; Dahl, S.; Skjøth-Rasmussen, M.S.; Jensen, A.D. Catalytic steam reforming of bio-oil. Int. J. Hydrogen Energy 2012, 37, 6447–6472. [Google Scholar] [CrossRef]
- Iranshahi, D.; Pourazadi, E.; Paymooni, K.; Rahimpour, M.R.; Jahanmiri, A.; Moghtaderi, B. A dynamic membrane reactor concept for naphtha reforming, considering radial-flow patterns for both sweeping gas and reacting materials. Chem. Eng. J. 2011, 178, 264–275. [Google Scholar] [CrossRef]
- Ligthart, D.A.J.M.; Van Santen, R.A.; Hensen, E.J.M. Influence of particle size on the activity and stability in steam methane reforming of supported Rh nanoparticles. J. Catal. 2011, 280, 206–220. [Google Scholar] [CrossRef]
- Boyano, A.; Blanco-Marigorta, A.M.; Morosuk, T.; Tsatsaronis, G. Exergoenvironmental analysis of a steam methane reforming process for hydrogen production. Energy 2011, 36, 2202–2214. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Tan, K.F.; Borgna, A.; Saeys, M. Effect of boron on the stability of Ni catalysts during steam methane reforming. J. Catal. 2009, 261, 158–165. [Google Scholar] [CrossRef]
- Elsharnouby, O.; Hafez, H.; Nakhla, G.; El Naggar, M.H. A critical literature review on biohydrogen production by pure cultures. Int. J. Hydrogen Energy 2013, 38, 4945–4966. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Das, D.; Veziroǧlu, T.N. Hydrogen production by biological processes: A survey of literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Burmistrz, P.; Chmielniak, T.; Czepirski, L.; Gazda-Grzywacz, M. Carbon footprint of the hydrogen production process utilizing subbituminous coal and lignite gasification. J. Clean. Prod. 2016, 139, 858–865. [Google Scholar] [CrossRef]
- Huang, J.; Dincer, I. Parametric analysis and assessment of a coal gasification plant for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 3294–3303. [Google Scholar] [CrossRef]
- Seyitoglu, S.S.; Dincer, I.; Kilicarslan, A. Energy and exergy analyses of hydrogen production by coal gasification. Int. J. Hydrogen Energy 2017, 42, 2592–2600. [Google Scholar] [CrossRef]
- Slama, R. Ben Production of Hydrogen by Electrolysis of Water: Effects of the Electrolyte Type on the Electrolysis Performances. Comput. Water Energy Environ. Eng. 2013, 2, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Atlam, O.; Kolhe, M. Equivalent electrical model for a proton exchange membrane (PEM) electrolyser. Energy Convers. Manag. 2011, 52, 2952–2957. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Briguglio, N.; Brunaccini, G.; Di Blasi, A.; Stassi, A.; Ornelas, R.; Trifoni, E.; Antonucci, V.; Aricò, A.S. An electrochemical study of a PEM stack for water electrolysis. Int. J. Hydrogen Energy 2012, 37, 1939–1946. [Google Scholar] [CrossRef]
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Grubb, W.T. Batteries with Solid Ion Exchange Electrolytes.1. Secondary Cells Employing Metal Electrodes. J. Electrochem. Soc. 1959, 106, 275–278. [Google Scholar] [CrossRef]
- Xu, W.; Scott, K. The effects of ionomer content on PEM water electrolyser membrane electrode assembly performance. Int. J. Hydrogen Energy 2010, 35, 12029–12037. [Google Scholar] [CrossRef]
- Dönitz, W.; Erdle, E. High-temperature electrolysis of water vapor-status of development and perspectives for application. Int. J. Hydrogen Energy 1985, 10, 291–295. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef] [Green Version]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically assisted microbial production of hydrogen from acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Burnat, D.; Schlupp, M.; Wichser, A.; Lothenbach, B.; Gorbar, M.; Züttel, A.; Vogt, U.F. Composite membranes for alkaline electrolysis based on polysulfone and mineral fillers. J. Power Sources 2015, 291, 163–172. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Hydrogen evolution from water using solid carbon and light energy. Nature 1979, 282, 283–284. [Google Scholar] [CrossRef]
- Akimoto, I.; Maeda, K.; Ozaki, N. Hydrogen generation by laser irradiation of carbon powder in water. J. Phys. Chem. C 2013, 117, 18281–18285. [Google Scholar] [CrossRef]
- Maeda, K.; Ozaki, N.; Akimoto, I. Alcohol additive effect in hydrogen generation from water with carbon by photochemical reaction. Jpn. J. Appl. Phys. 2014, 53, 05FZ03. [Google Scholar] [CrossRef]
- Seyitliyev, D.; Kholikov, K.; Grant, B.; San, O.; Er, A.O. Laser-induced hydrogen generation from graphite and coal. Int. J. Hydrogen Energy 2017, 42, 26277–26288. [Google Scholar] [CrossRef]
- Coughlin, R.W.; Farooque, M. Hydrogen production from coal, water and electrons. Nature 1979, 279, 301–303. [Google Scholar] [CrossRef]
- Barmina, E.V.; Simakin, A.V.; Shafeev, G.A. Hydrogen emission under laser exposure of colloidal solutions of nanoparticles. Chem. Phys. Lett. 2016, 655–656, 35–38. [Google Scholar] [CrossRef]
- Kierzkowska-Pawlak, H.; Tyczkowski, J.; Jarota, A.; Abramczyk, H. Hydrogen production in liquid water by femtosecond laser-induced plasma. Appl. Energy 2019, 247, 24–31. [Google Scholar] [CrossRef]
- Strek, W.; Mista, W.; Wiewiorski, P.; Tomala, R. Laser induced hydrogen emission from ethanol with dispersed graphene particles. Chem. Phys. Lett. 2021, 775, 138649. [Google Scholar] [CrossRef]
- Strek, W.; Wiewiórski, P.; Mista, W.; Hanulia, T.; Tomala, R. Laser-Induced Hydrogen Generation from Methanol with Graphene Aerogel as the Target. ACS Omega 2021, 6, 3711–3716. [Google Scholar] [CrossRef] [PubMed]
- Strek, W.; Cichy, B.; Radosinski, L.; Gluchowski, P.; Marciniak, L.; Lukaszewicz, M.; Hreniak, D. Laser-induced white-light emission from graphene ceramics–opening a band gap in graphene. Light Sci. Appl. 2015, 4, e237. [Google Scholar] [CrossRef] [Green Version]
- Strek, W.; Tomala, R.; Lukaszewicz, M.; Cichy, B.; Gerasymchuk, Y.; Gluchowski, P.; Marciniak, L.; Bednarkiewicz, A.; Hreniak, D. Laser induced white lighting of graphene foam. Sci. Rep. 2017, 7, 41281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widodo, C.S.; Sela, H.; Santosa, D.R. The effect of NaCl concentration on the ionic NaCl solutions electrical impedance value using electrochemical impedance spectroscopy methods. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 2021, p. 050003. [Google Scholar]
- Yu, Z.; Xu, J.; Meng, L.; Liu, L. Efficient hydrogen production by saline water electrolysis at high current densities without the interfering chlorine evolution. J. Mater. Chem. A 2021, 9, 22248–22253. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]

| Ar 20 mL/min H2O-Distilled Water | ||||
|---|---|---|---|---|
| Laser Power [W] | Gas Products | |||
| H2 [%] | O2 [%] | CO2 [%] | CO [%] | |
| 10.0 | 47.00 | 10.44 | 11.23 | 31.33 |
| 9.0 | 54.42 | 6.80 | 11.56 | 27.21 |
| 8.0 | 54.30 | 9.05 | 9.50 | 27.15 |
| 7.0 | 53.25 | 11.83 | 11.24 | 23.67 |
| 6.0 | 55.56 | 7.94 | 12.70 | 23.81 |
| Ar 20 mL/min H2O + 1% NaCl | ||||
|---|---|---|---|---|
| Laser Power [W] | Gas Products | |||
| H2 [%] | O2 [%] | CO2 [%] | CO [%] | |
| 10.0 | 79.95 | 8.13 | 3.66 | 8.27 |
| 9.0 | 78.99 | 8.52 | 4.27 | 8.21 |
| 8.0 | 78.48 | 8.43 | 4.85 | 8.23 |
| 7.0 | 78.21 | 8.93 | 5.10 | 7.75 |
| 6.0 | 77.81 | 9.07 | 5.42 | 7.70 |
| 5.5 | 80.91 | 8.17 | 4.84 | 6.08 |
| 5.0 | 59.21 | 14.47 | 6.58 | 19.74 |
| 4.5 | 57.14 | 22.86 | 8.57 | 11.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strek, W.; Wiewiórski, P.; Miśta, W.; Tomala, R.; Stefanski, M. Laser-Induced Generation of Hydrogen in Water by Using Graphene Target. Molecules 2022, 27, 718. https://doi.org/10.3390/molecules27030718
Strek W, Wiewiórski P, Miśta W, Tomala R, Stefanski M. Laser-Induced Generation of Hydrogen in Water by Using Graphene Target. Molecules. 2022; 27(3):718. https://doi.org/10.3390/molecules27030718
Chicago/Turabian StyleStrek, Wieslaw, Przemysław Wiewiórski, Włodzimierz Miśta, Robert Tomala, and Mariusz Stefanski. 2022. "Laser-Induced Generation of Hydrogen in Water by Using Graphene Target" Molecules 27, no. 3: 718. https://doi.org/10.3390/molecules27030718






