How Microbes Affect Depression: Underlying Mechanisms via the Gut–Brain Axis and the Modulating Role of Probiotics
Abstract
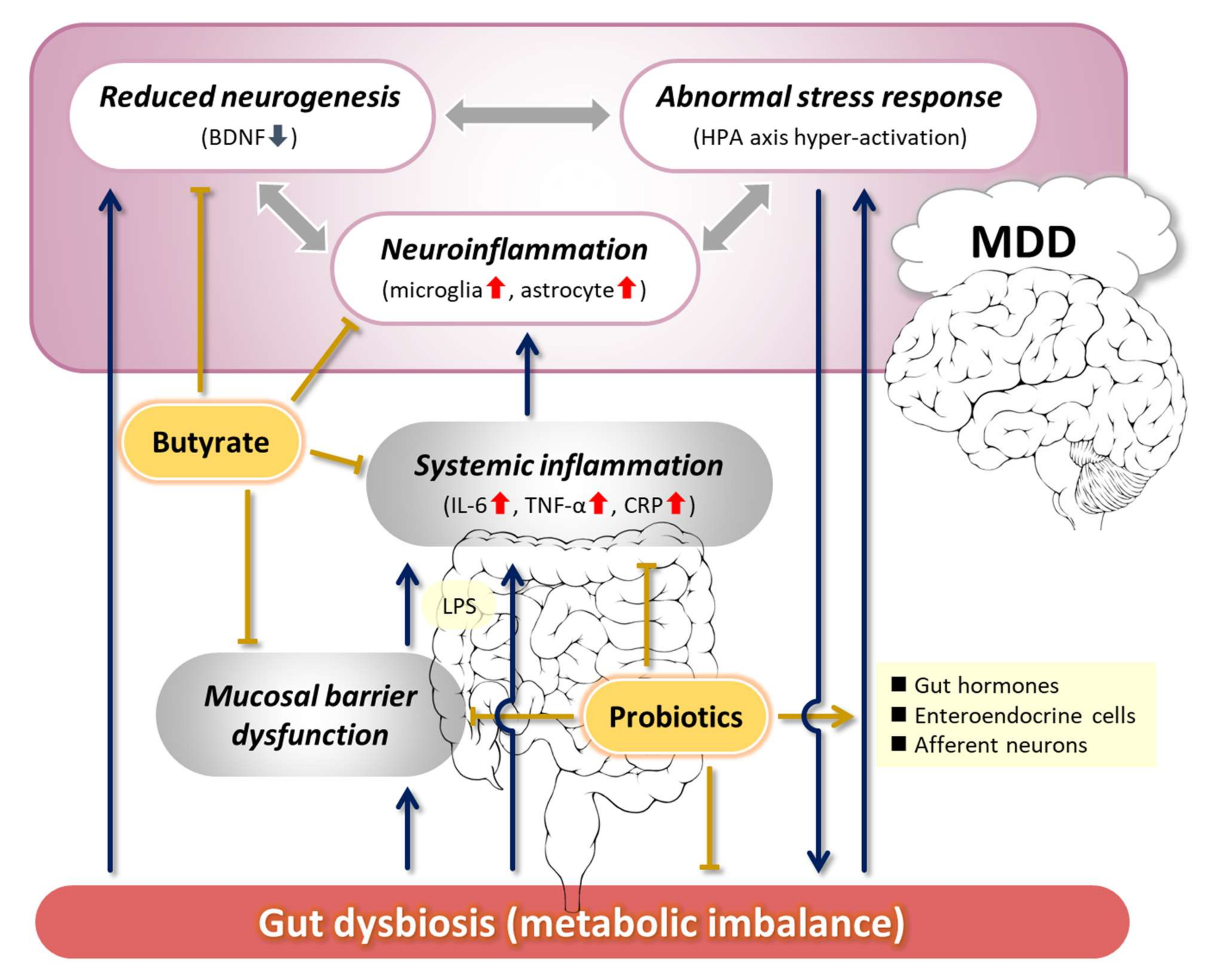
1. Introduction
2. Pathogenesis of Depression Related to the MGB Axis
2.1. Abnormal Stress Response
2.2. Decreased Neurogenesis and Its Association with BDNF
2.3. Neuroinflammation
2.4. Other MDD-Related Factors
2.4.1. Sleep Disorders
2.4.2. Metabolic Disorders
2.4.3. Dysregulation of Monoamines and Gamma-Aminobutyric Acid
3. Proposals for Improving the Pathophysiology of MDD via the MGB Axis
3.1. Ameliorating the Stress Response
3.2. Maintenance of BDNF Expression and Neurogenesis
3.3. Anti-Inflammatory Effect
4. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/en/news-room/fact-sheets/detail/depression (accessed on 3 December 2021).
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [CrossRef]
- Owens, M.J. Selectivity of antidepressants: From the monoamine hypothesis of depression to the SSRI revolution and beyond. J. Clin. Psychiatry 2004, 65, 5–10. [Google Scholar]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Gui, S.; Zeng, B.; Pu, J.; Zheng, P.; Zeng, L.; Luo, Y.; Wu, Y.; Zhou, C.; et al. Proteomics analysis of the gut–brain axis in a gut microbiota-dysbiosis model of depression. Transl. Psychiatry 2021, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.K.; Bundgaard-Nielsen, C.; Hjerrild, S.; Nielsen, R.E.; Leutscher, P.; Sørensen, S. Gut microbiota variations in patients diagnosed with major depressive disorder-A systematic review. Brain Behav. 2021, 11, e02177. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Marin, I.A.; Goertz, J.E.; Ren, T.; Rich, S.S.; Onengut-Gumuscu, S.; Farber, E.; Wu, M.; Overall, C.C.; Kipnis, J.; Gaultier, A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017, 7, 43859. [Google Scholar] [CrossRef]
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef]
- Hori, T.; Matsuda, K.; Oishi, K. Probiotics: A dietary factor to modulate the gut microbiome, host immune system, and gut-brain interaction. Microorganisms 2020, 8, 1401. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; Feinberg, M.; Greden, J.F.; Tarika, J.; Albala, A.A.; Haskett, R.F.; James, N.M.; Kronfol, Z.; Lohr, N.; Steiner, M.; et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch. Gen. Psychiatry 1981, 38, 15–22. [Google Scholar] [CrossRef]
- Heuser, I.; Yassouridis, A.; Holsboer, F. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. J. Psychiatr. Res. 1994, 28, 341–356. [Google Scholar] [CrossRef]
- Wróbel, A.; Serefko, A.; Rechberger, E.; Banczerowska-Górska, M.; Poleszak, E.; Dudka, J.; Skorupska, K.; Miotła, P.; Semczuk, A.; Kulik-Rechberger, B.; et al. Inhibition of Rho kinase by GSK 269962 reverses both corticosterone-induced detrusor overactivity and depression-like behaviour in rats. Eur. J. Pharmacol. 2018, 837, 127–136. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Hu, K.; Yang, J. Evidence of the moderating role of hair cortisol and hair cortisone in the relationship between work stress and depression symptoms among Chinese fishermen. J. Affect. Disord. 2021, 294, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Green, C.; Stolicyn, A.; Harris, M.A.; Shen, X.; Romaniuk, L.; Barbu, M.C.; Hawkins, E.L.; Wardlaw, J.M.; Steele, J.D.; Waiter, G.D.; et al. Hair glucocorticoids are associated with childhood adversity, depressive symptoms and reduced global and lobar grey matter in Generation Scotland. Transl. Psychiatry 2021, 11, 523. [Google Scholar] [CrossRef]
- Gu, F.; Wu, Y.; Liu, Y.; Dou, M.; Jiang, Y.; Liang, H. Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the BDNF-TrkB signal pathway and the intestinal microbiota. Food Funct. 2020, 11, 6148–6157. [Google Scholar] [CrossRef]
- Huang, P.; Gao, T.; Dong, Z.; Zhou, C.; Lai, Y.; Pan, T.; Liu, Y.; Zhao, X.; Sun, X.; Hua, H.; et al. Neural circuitry among connecting the hippocampus, prefrontal cortex and basolateral amygdala in a mouse depression model: Associations correlations between BDNF levels and BOLD—fMRI signals. Brain Res. Bull. 2018, 142, 107–115. [Google Scholar] [CrossRef]
- Chen, F.; Chen, S.; Liu, J.; Amin, N.; Jin, W.; Fang, M. Agomelatine softens depressive-like behavior through the regulation of autophagy and apoptosis. Biomed. Res. Int. 2021, 2021, 6664591. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wu, X.; Wang, J.; Guan, Y.; Zhang, Y.; Gong, M.; Wang, Y.; Li, B. Antidepressant effect of catalpol on corticosterone-induced depressive-like behavior involves the inhibition of HPA axis hyperactivity, central inflammation and oxidative damage probably via dual regulation of NF-κB and Nrf2. Brain Res. Bull. 2021, 177, 81–91. [Google Scholar] [CrossRef]
- De Castro Chaves, R.; Mallmann, A.S.V.; Oliveira, N.F.; Oliveira, I.C.M.; Capibaribe, V.C.C.; da Silva, D.M.A.; Lopes, I.S.; Valentim, J.T.; de Carvalho, A.M.R.; Macêdo, D.S.; et al. Reversal effect of Riparin IV in depression and anxiety caused by corticosterone chronic administration in mice. Pharmacol. Biochem. Behav. 2019, 180, 44–51. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Z.; Song, S.; Zhang, H.; Zhang, W.; Liu, L.; Chen, Y.; Sun, J. Gut microbiota modulates stress-induced hypertension through the HPA axis. Brain Res. Bull. 2020, 162, 49–58. [Google Scholar] [CrossRef]
- Wu, W.-L.; Adame, M.D.; Liou, C.-W.; Barlow, J.T.; Lai, T.-T.; Sharon, G.; Schretter, C.E.; Needham, B.D.; Wang, M.I.; Tang, W.; et al. Microbiota regulate social behaviour via stress response neurons in the brain. Nature 2021, 595, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Patterson, E.; Meunier, J.; Roman, F.J.; Lehtinen, M.J. Strain specific stress-modulating effects of candidate probiotics: A systematic screening in a mouse model of chronic restraint stress. Behav. Brain Res. 2020, 379, 112376. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.K.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, R.; Madempudi, R.S.; Neelamraju, J.; Ahire, J.J.; Vinay, H.R.; Lal, A.; Thomas, G.; Stephen, S. Effect of multi-strain probiotic formulation on students facing examination stress: A double-blind, placebo-controlled study. Probiotics Antimicrob. Proteins 2021, 13, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Lew, L.C.; Hor, Y.Y.; Yusoff, N.A.A.; Choi, S.B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Quero, C.D.; Manonelles, P.; Fernández, M.; Abellán-Aynés, O.; López-Plaza, D.; Andreu-Caravaca, L.; Hinchado, M.D.; Gálvez, I.; Ortega, E. Differential health effects on inflammatory, immunological and stress parameters in professional soccer players and sedentary individuals after consuming a synbiotic. A triple-blinded, randomized, placebo-controlled pilot study. Nutrients 2021, 13, 1321. [Google Scholar] [CrossRef]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut–brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Agrimi, J.; Spalletti, C.; Baroni, C.; Keceli, G.; Zhu, G.; Caragnano, A.; Matteucci, M.; Chelko, S.; Ramirez-Correa, G.A.; Bedja, D.; et al. Obese mice exposed to psychosocial stress display cardiac and hippocampal dysfunction associated with local brain-derived neurotrophic factor depletion. EBioMedicine 2019, 47, 384–401. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.B.; Williamson, R.; Santini, M.A.; Clemmensen, C.; Ettrup, A.; Rios, M.; Knudsen, G.M.; Aznar, S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacol. 2011, 14, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Perret, G.; Bondolfi, G.; Schwald, M.; Bertschy, G.; Aubry, J.M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002, 109, 143–148. [Google Scholar] [CrossRef]
- Satomura, E.; Baba, H.; Nakano, Y.; Maeshima, H.; Suzuki, T.; Arai, H. Correlations between brain-derived neurotrophic factor and clinical symptoms in medicated patients with major depression. J. Affect. Disord. 2011, 135, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Frodl, T.; Meisenzahl, E.M.; Zill, P.; Baghai, T.; Rujescu, D.; Leinsinger, G.; Bottlender, R.; Schüle, C.; Zwanzger, P.; Engel, R.R.; et al. Reduced hippocampal volumes associated with the long variant of the serotonin transporter polymorphism in major depression. Arch. Gen. Psychiatry 2004, 61, 177–183. [Google Scholar] [CrossRef]
- Bouckaert, F.; Dols, A.; Emsell, L.; de Winter, F.L.; Vansteelandt, K.; Claes, L.; Sunaert, S.; Stek, M.; Sienaert, P.; Vandenbulcke, M. Relationship between Hippocampal Volume, Serum BDNF, and depression severity following electroconvulsive therapy in late-life depression. Neuropsychopharmacology 2016, 41, 2741–2748. [Google Scholar] [CrossRef]
- Ji, M.; Niu, S.; Mi, H.; Jang, P.; Li, Y.; Hu, W. Antidepressant functions of Jie Yu Chu Fan capsule in promoting hippocampal nerve cell neurogenesis in a mouse model of chronic unpredictable mild stress. Ann. Transl. Med. 2020, 8, 1020. [Google Scholar] [CrossRef]
- Aydemir, O.; Deveci, A.; Taneli, F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: A preliminary study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 261–265. [Google Scholar] [CrossRef]
- Shimizu, E.; Hashimoto, K.; Okamura, N.; Koike, K.; Komatsu, N.; Kumakiri, C.; Nakazato, M.; Watanabe, H.; Shinoda, N.; Okada, S.I.; et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry 2003, 54, 70–75. [Google Scholar] [CrossRef]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; MacRi, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain. Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress–induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Jang, H.M.; Kim, J.K.; Joo, M.K.; Shin, Y.J.; Lee, C.K.; Kim, H.J.; Kim, D.H. Transplantation of fecal microbiota from patients with inflammatory bowel disease and depression alters immune response and behavior in recipient mice. Sci. Rep. 2021, 11, 20406. [Google Scholar] [CrossRef]
- Scott, G.A.; Terstege, D.J.; Vu, A.P.; Law, S.; Evans, A.; Epp, J.R. Disrupted neurogenesis in germ-free mice: Effects of age and sex. Front. Cell Dev. Biol. 2020, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; de Preter, V.; Hamer, H.M.; van den Mooter, G.; de Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ling, Z.; Wang, F.; Chen, W.; Li, H.; Jin, J.; Zhang, H.; Pang, M.; Yu, J.; Liu, J. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci. Lett. 2016, 613, 30–35. [Google Scholar] [CrossRef]
- Tu, F.; Pang, Q.; Huang, T.; Zhao, Y.; Liu, M.; Chen, X. Apigenin ameliorates post-stroke cognitive deficits in rats through histone acetylation- mediated neurochemical alterations. Med. Sci. Monit. 2017, 23, 4004–4013. [Google Scholar] [CrossRef]
- Tian, P.; Zhu, H.; Qian, X.; Chen, Y.; Wang, Z.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Consumption of butylated starch alleviates the chronic restraint stress-induced neurobehavioral and gut barrier deficits through reshaping the gut microbiota. Front. Immunol. 2021, 12, 755481. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.K.; Miller, B.J. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder, and depression. Schizophr. Bull. 2018, 44, 75–83. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Cavanagh, J.; de Boer, P.; Mondelli, V.; Jones, D.N.C.; Drevets, W.C.; Cowen, P.J.; Harrison, N.A.; Pointon, L.; Pariante, C.M.; et al. Treatment-resistant depression and peripheral C-reactive protein. Br. J. Psychiatry 2019, 214, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Haroon, E.; Patel, T.A.; Goldsmith, D.R.; Wommack, E.C.; Woolwine, B.J.; Le, N.-A.; Feinberg, R.; Tansey, M.G.; Miller, A.H. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry 2020, 25, 1301–1311. [Google Scholar] [CrossRef]
- Nie, X.; Kitaoka, S.; Tanaka, K.; Segi-Nishida, E.; Imoto, Y.; Ogawa, A.; Nakano, F.; Tomohiro, A.; Nakayama, K.; Taniguchi, M.; et al. The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron 2018, 99, 464–479. [Google Scholar] [CrossRef]
- Weng, L.; Dong, S.; Wang, S.; Yi, L.; Geng, D. Macranthol attenuates lipopolysaccharide-induced depressive-like behaviors by inhibiting neuroinflammation in prefrontal cortex. Physiol. Behav. 2019, 204, 33–40. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, J.; Zhai, L.; Gao, C.; Xie, L.; Zhao, L.; Yin, X. Inhibition of activated astrocyte ameliorates lipopolysaccharide- induced depressive-like behaviors. J. Affect. Disord. 2019, 242, 52–59. [Google Scholar] [CrossRef]
- Virmani, G.; D’almeida, P.; Nandi, A.; Marathe, S. Subfield-specific effects of chronic mild unpredictable stress on hippocampal astrocytes. Eur. J. Neurosci. 2021, 54, 5730–5746. [Google Scholar] [CrossRef]
- Ryan, K.M.; Allers, K.A.; McLoughlin, D.M.; Harkin, A. Tryptophan metabolite concentrations in depressed patients before and after electroconvulsive therapy. Brain. Behav. Immun. 2020, 83, 153–162. [Google Scholar] [CrossRef]
- Öztürk, M.; Yalın Sapmaz, Ş.; Kandemir, H.; Taneli, F.; Aydemir, Ö. The role of the kynurenine pathway and quinolinic acid in adolescent major depressive disorder. Int. J. Clin. Pract. 2021, 75, e13739. [Google Scholar] [CrossRef]
- Hassanain, H.H.; Chon, S.Y.; Gupta, S.L. Differential regulation of human indoleamine 2,3-dioxygenase gene expression by interferons-γ and -α. Analysis of the regulatory region of the gene and identification of an interferon-γ-inducible DNA-binding factor. J. Biol. Chem. 1993, 268, 5077–5084. [Google Scholar] [CrossRef]
- Pariante, C.M.; Pearce, B.D.; Pisell, T.L.; Sanchez, C.I.; Po, C.; Su, C.; Miller, A.H. The proinflammatory cytokine, interleukin-1α, reduces glucocorticoid receptor translocation and function. Endocrinology 1999, 140, 4359–4366. [Google Scholar] [CrossRef]
- Besedovsky, H.O.; del Rey, A.; Klusman, I.; Furukawa, H.; Monge Arditi, G.; Kabiersch, A. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J. Steroid Biochem. Mol. Biol. 1991, 40, 613–618. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Araujo, D.M.; Hefti, F. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience 1993, 53, 297–301. [Google Scholar] [CrossRef]
- Kaneko, N.; Kudo, K.; Mabuchi, T.; Takemoto, K.; Fujimaki, K.; Wati, H.; Iguchi, H.; Tezuka, H.; Kanba, S. Suppression of cell proliferation by interferon-alpha through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology 2006, 31, 2619–2626. [Google Scholar] [CrossRef]
- Zhang, J.; Hoedt, E.C.; Liu, Q.; Berendsen, E.; Teh, J.J.; Hamilton, A.; O’Brien, A.W.; Ching, J.Y.L.; Wei, H.; Yang, K.; et al. Elucidation of Proteus mirabilis as a key bacterium in Crohn’s disease inflammation. Gastroenterology 2021, 160, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Engevik, M.A.; Danhof, H.A.; Ruan, W.; Engevik, A.C.; Chang-Graham, A.L.; Engevik, K.A.; Shi, Z.; Zhao, Y.; Brand, C.K.; Krystofiak, E.S.; et al. Fusobacterium nucleatum secretes outer membrane vesicles and promotes intestinal inflammation. MBio 2021, 12, e02706-20. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hara, T.; Hori, T.; Mitsuyama, K.; Nagaoka, M.; Tomiyasu, N.; Suzuki, A.; Sata, M. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin. Exp. Immunol. 2005, 140, 417–426. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, W.; Tian, F.; Zhao, J.; Zhang, H.; Hong, K.; Yu, L. Akkermansia muciniphila exerts strain-specific effects on DSS-induced ulcerative colitis in mice. Front. Cell. Infect. Microbiol. 2021, 11, 698914. [Google Scholar] [CrossRef] [PubMed]
- Segain, J.P.; Raingeard de la Blétière, D.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Huang, L.; Zhang, C.; Zhang, L.; Xia, X.; Zhong, Z.; Wang, B.; Wang, Y.; Man Hoi, M.P.; Ding, W.; et al. Gut commensal-derived butyrate reverses obesity-induced social deficits and anxiety-like behaviors via regulation of microglial homeostasis. Eur. J. Pharmacol. 2021, 908, 174338. [Google Scholar] [CrossRef]
- Wang, W.; Lv, S.; Zhou, Y.; Fu, J.; Li, C.; Liu, P. Tumor necrosis factor-α affects blood-brain barrier permeability in acetaminophen-induced acute liver failure. Eur. J. Gastroenterol. Hepatol. 2011, 23, 552–558. [Google Scholar] [CrossRef]
- Rochfort, K.D.; Collins, L.E.; Murphy, R.P.; Cummins, P.M. Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: Consequences for interendothelial adherens and tight junctions. PLoS ONE 2014, 9, e101815. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Li, X.; Liu, E.; Wang, X.; Zhou, Q.; Ye, J.; Wang, J.Z. Peripheral inflammation promotes brain tau transmission via disrupting blood-brain barrier. Biosci. Rep. 2020, 40, BSR20193629. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Wilson, S.J.; Bailey, M.L.; Andridge, R.; Peng, J.; Jaremka, L.M.; Fagundes, C.P.; Malarkey, W.B.; Laskowski, B.; Belury, M.A. Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 2018, 98, 52–60. [Google Scholar] [CrossRef]
- Spiegelhalder, K.; Regen, W.; Nanovska, S.; Baglioni, C.; Riemann, D. Comorbid sleep disorders in neuropsychiatric disorders across the life cycle. Curr. Psychiatry Rep. 2013, 15, 364. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Zhang, H.; Zhang, D. Sleep duration and depression among adults: A meta-analysis of prospective studies. Depress. Anxiety 2015, 32, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, R.; Zhang, Y.; Zhang, R.; Liang, L.; Wang, Y.; Wei, Y.; Zhu, R.; Wang, F. Association between perceived stress and depression among medical students during the outbreak of COVID-19: The mediating role of insomnia. J. Affect. Disord. 2021, 292, 89–94. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyoshi, C.; Obana, N.; Yajima, K.; Hotta-Hirashima, N.; Ikkyu, A.; Kanno, S.; Soga, T.; Fukuda, S.; Yanagisawa, M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 2020, 10, 19554. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Gondo, Y.; Kikuchi-Hayakawa, H.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Kuwano, Y.; Miyazaki, K.; et al. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: A double-blind, randomised, placebo-controlled trial. Benef. Microbes 2017, 8, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Tsai, Y.C.; Kuo, T.B.J.; Yang, C.C.H. Effects of Lactobacillus plantarum ps128 on depressive symptoms and sleep quality in self-reported insomniacs: A randomized, double-blind, placebo-controlled pilot trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef]
- Silva, D.A.; da Silva Freire Coutinho, E.; Ferriani, L.O.; Viana, M.C. Depression subtypes and obesity in adults: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e12966. [Google Scholar] [CrossRef]
- Rao, W.W.; Zong, Q.Q.; Zhang, J.W.; An, F.R.; Jackson, T.; Ungvari, G.S.; Xiang, Y.; Su, Y.Y.; D’Arcy, C.; Xiang, Y.T. Obesity increases the risk of depression in children and adolescents: Results from a systematic review and meta-analysis. J. Affect. Disord. 2020, 267, 78–85. [Google Scholar] [CrossRef]
- Duarte-Silva, E.; de Melo, M.G.; Maes, M.; Filho, A.J.M.C.; Macedo, D.; Peixoto, C.A. Shared metabolic and neuroimmune mechanisms underlying Type 2 Diabetes Mellitus and Major Depressive Disorder. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2021, 111, 110351. [Google Scholar] [CrossRef]
- Nascimento, J.C.; Matheus, V.A.; Oliveira, R.B.; Tada, S.F.S.; Collares-Buzato, C.B. High-fat diet induces disruption of the tight junction-mediated paracellular barrier in the proximal small intestine before the onset of type 2 diabetes and endotoxemia. Dig. Dis. Sci. 2021, 66, 3359–3374. [Google Scholar] [CrossRef]
- Horne, R.; Foster, J.A. Metabolic and microbiota measures as peripheral biomarkers in major depressive disorder. Front. psychiatry 2018, 9, 513. [Google Scholar] [CrossRef]
- Caroleo, M.; Carbone, E.A.; Primerano, A.; Foti, D.; Brunetti, A.; Segura-Garcia, C. The role of hormonal, metabolic and inflammatory biomarkers on sleep and appetite in drug free patients with major depression: A systematic review. J. Affect. Disord. 2019, 250, 249–259. [Google Scholar] [CrossRef]
- Kluge, M.; Schüssler, P.; Dresler, M.; Schmidt, D.; Yassouridis, A.; Uhr, M.; Steiger, A. Effects of ghrelin on psychopathology, sleep and secretion of cortisol and growth hormone in patients with major depression. J. Psychiatr. Res. 2011, 45, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Jerlhag, E.; Egecioglu, E.; Landgren, S.; Salomé, N.; Heilig, M.; Moechars, D.; Datta, R.; Perrissoud, D.; Dickson, S.L.; Engel, J.A. Requirement of central ghrelin signaling for alcohol reward. Proc. Natl. Acad. Sci. USA 2009, 106, 11318–11323. [Google Scholar] [CrossRef]
- Santos, V.V.; Stark, R.; Rial, D.; Silva, H.B.; Bayliss, J.A.; Lemus, M.B.; Davies, J.S.; Cunha, R.A.; Prediger, R.D.; Andrews, Z.B. Acyl ghrelin improves cognition, synaptic plasticity deficits and neuroinflammation following amyloid β (Aβ1-40) administration in mice. J. Neuroendocrinol. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Weikel, J.C.; Wichniak, A.; Ising, M.; Brunner, H.; Friess, E.; Held, K.; Mathias, S.; Schmid, D.A.; Uhr, M.; Steiger, A. Ghrelin promotes slow-wave sleep in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E407–E415. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.C.; Anderson, G.M. Dopamine neuron-restricted leptin receptor signaling reduces some aspects of food reward but exacerbates the obesity of leptin receptor-deficient male mice. Endocrinology 2017, 158, 4246–4256. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Fisher, J.; Brown, J. Chronic subcutaneous leptin infusion diminishes the responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis in female rhesus monkeys. Physiol. Behav. 2005, 84, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.A.; Held, K.; Ising, M.; Uhr, M.; Weikel, J.C.; Steiger, A. Ghrelin stimulates appetite, imagination of food, GH, ACTH, and cortisol, but does not affect leptin in normal controls. Neuropsychopharmacology 2005, 30, 1187–1192. [Google Scholar] [CrossRef]
- Huang, H.-J.; Zhu, X.-C.; Han, Q.-Q.; Wang, Y.-L.; Yue, N.; Wang, J.; Yu, R.; Li, B.; Wu, G.-C.; Liu, Q.; et al. Ghrelin alleviates anxiety- and depression-like behaviors induced by chronic unpredictable mild stress in rodents. Behav. Brain Res. 2017, 326, 33–43. [Google Scholar] [CrossRef]
- Morimoto, I.; Yamamoto, S.; Kai, K.; Fujihira, T.; Morita, E.; Eto, S. Centrally administered murine-leptin stimulates the hypothalamus-pituitary-adrenal axis through arginine-vasopressin. Neuroendocrinology 2000, 71, 366–374. [Google Scholar] [CrossRef]
- Björklund, A.; Wiklund, L. Mechanisms of regrowth of the bulbospinal serotonin system following 5,6-dihydroxytryptamine induced axotomy. I. Biochemical correlates. Brain Res. 1980, 191, 109–127. [Google Scholar] [CrossRef]
- Leibowitz, S.F.; Shor-Posner, G. Brain serotonin and eating behavior. Appetite 1986, 7, 1–14. [Google Scholar] [CrossRef]
- Cameron, O.G.; Nesse, R.M. Systemic hormonal and physiological abnormalities in anxiety disorders. Psychoneuroendocrinology 1988, 13, 287–307. [Google Scholar] [CrossRef]
- Hoebel, B.G.; Hernandez, L.; Schwartz, D.H.; Mark, G.P.; Hunter, G.A. Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior. Theoretical and clinical implications. Ann. N. Y. Acad. Sci. 1989, 575, 171–191, discussion 192–193. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.; Williams, D.C. The serotonin transporter: A primary target for antidepressant drugs. J. Psychopharmacol. 1998, 12, 115–121. [Google Scholar] [CrossRef]
- Gallopin, T.; Fort, P.; Eggermann, E.; Cauli, B.; Luppi, P.H.; Rossier, J.; Audinat, E.; Mühlethaler, M.; Serafin, M. Identification of sleep-promoting neurons in vitro. Nature 2000, 404, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Morishima, M.; Harada, N.; Hara, S.; Sano, A.; Seno, H.; Takahashi, A.; Morita, Y.; Nakaya, Y. Monoamine oxidase A activity and norepinephrine level in hippocampus determine hyperwheel running in SPORTS rats. Neuropsychopharmacology 2006, 31, 2627–2638. [Google Scholar] [CrossRef]
- Lammel, S.; Lim, B.K.; Malenka, R.C. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 2014, 76, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; O’Dwyer, G.; Teleki, Z.; Stockmeier, C.A.; Miguel-Hidalgo, J.J. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 2007, 32, 471–482. [Google Scholar] [CrossRef]
- Mann, J.J.; Oquendo, M.A.; Watson, K.T.; Boldrini, M.; Malone, K.M.; Ellis, S.P.; Sullivan, G.; Cooper, T.B.; Xie, S.; Currier, D. Anxiety in major depression and cerebrospinal fluid free gamma-aminobutyric acid. Depress. Anxiety 2014, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Hong, X.; Li, X.; Meshul, C.K.; Moore, C.; Yang, Y.; Han, Y.; Li, W.G.; Qi, X.; et al. NG2 glia-derived GABA release tunes inhibitory synapses and contributes to stress-induced anxiety. Nat. Commun. 2021, 12, 5740. [Google Scholar] [CrossRef] [PubMed]
- Lowes, D.C.; Chamberlin, L.A.; Kretsge, L.N.; Holt, E.S.; Abbas, A.I.; Park, A.J.; Yusufova, L.; Bretton, Z.H.; Firdous, A.; Enikolopov, A.G.; et al. Ventral tegmental area GABA neurons mediate stress-induced blunted reward-seeking in mice. Nat. Commun. 2021, 12, 3539. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.R.; Esteban, J.S.D.; Faget, L.; Floresco, S.B.; Hnasko, T.S.; Mahler, S.V. Ventral pallidum GABA neurons mediate motivation underlying risky choice. J. Neurosci. 2021, 41, 4500–4513. [Google Scholar] [CrossRef]
- Petty, F.; Trivedi, M.H.; Fulton, M.; John Rush, A. Benzodiazepines as antidepressants: Does GABA play a role in depression? Biol. Psychiatry 1995, 38, 578–591. [Google Scholar] [CrossRef]
- Shima, T.; Fukushima, K.; Setoyama, H.; Imaoka, A.; Matsumoto, S.; Hara, T.; Suda, K.; Umesaki, Y. Differential effects of two probiotic strains with different bacteriological properties on intestinal gene expression, with special reference to indigenous bacteria. FEMS Immunol. Med. Microbiol. 2008, 52, 69–77. [Google Scholar] [CrossRef]
- Mandić, A.D.; Woting, A.; Jaenicke, T.; Sander, A.; Sabrowski, W.; Rolle-Kampcyk, U.; von Bergen, M.; Blaut, M. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci. Rep. 2019, 9, 1177. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luck, B.; Visuthranukul, C.; Ihekweazu, F.D.; Engevik, A.C.; Shi, Z.; Danhof, H.A.; Chang-Graham, A.L.; Hall, A.; Endres, B.T.; et al. Human-derived Bifidobacterium dentium modulates the mammalian serotonergic system and gut–brain axis. Cmgh 2021, 11, 221–248. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Asano, Y.; Yoshihara, K.; Kimura-Todani, T.; Miyata, N.; Zhang, X.T.; Takakura, S.; Aiba, Y.; Koga, Y.; Sudo, N. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS ONE 2017, 12, e0180745. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Yogeswara, I.B.A.; Maneerat, S.; Haltrich, D. Glutamate decarboxylase from lactic acid bacteria—A key enzyme in GABA synthesis. Microorganisms 2020, 8, 1923. [Google Scholar] [CrossRef]
- Mizuno, S.; Masaoka, T.; Naganuma, M.; Kishimoto, T.; Kitazawa, M.; Kurokawa, S.; Nakashima, M.; Takeshita, K.; Suda, W.; Mimura, M.; et al. Bifidobacterium-rich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Digestion 2017, 96, 29–38. [Google Scholar] [CrossRef]
- Huang, H.L.; Chen, H.T.; Luo, Q.L.; Xu, H.M.; He, J.; Li, Y.Q.; Zhou, Y.L.; Yao, F.; Nie, Y.Q.; Zhou, Y.J. Relief of irritable bowel syndrome by fecal microbiota transplantation is associated with changes in diversity and composition of the gut microbiota. J. Dig. Dis. 2019, 20, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 2016, 19, 387–395. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology 2017, 153, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Otaka, M.; Kikuchi-Hayakawa, H.; Ogura, J.; Ishikawa, H.; Yomogida, Y.; Ota, M.; Hidese, S.; Ishida, I.; Aida, M.; Matsuda, K.; et al. Effect of Lacticaseibacillus paracasei strain Shirota on improvement in depressive symptoms, and its association with abundance of Actinobacteria in gut microbiota. Microorganisms 2021, 9, 1026. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Sugawara, T.; Aoki, Y.; Fujiwara, S.; Rokutan, K. Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct. Foods 2017, 36, 112–121. [Google Scholar] [CrossRef]
- Tanida, M.; Yamano, T.; Maeda, K.; Okumura, N.; Fukushima, Y.; Nagai, K. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci. Lett. 2005, 389, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Tanida, M.; Takada, M.; Kato-Kataoka, A.; Kawai, M.; Miyazaki, K.; Shibamoto, T. Intragastric injection of Lactobacillus casei strain Shirota suppressed spleen sympathetic activation by central corticotrophin-releasing factor or peripheral 2-deoxy-d-glucose in anesthetized rats. Neurosci. Lett. 2016, 619, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Nakaita, Y.; Kaneda, H.; Shigyo, T. Heat-killed Lactobacillus brevis SBC8803 induces serotonin release from intestinal cells. Food Nutr. Sci. 2013, 4, 767–771. [Google Scholar] [CrossRef][Green Version]
- Sun, J.; Wang, F.; Hu, X.; Yang, C.; Xu, H.; Yao, Y.; Liu, J. Clostridium butyricum attenuates chronic unpredictable mild stress-induced depressive-like behavior in mice via the gut-brain axis. J. Agric. Food Chem. 2018, 66, 8415–8421. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, W.; Guo, R.; Liu, H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 2019, 104, 132–142. [Google Scholar] [CrossRef]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H.; et al. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: A prospective open-label trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef]
- Shetty, S.A.; Boeren, S.; Bui, T.P.N.; Smidt, H.; de Vos, W.M. Unravelling lactate-acetate and sugar conversion into butyrate by intestinal Anaerobutyricum and Anaerostipes species by comparative proteogenomics. Environ. Microbiol. 2020, 22, 4863–4875. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef]
- Romo-Araiza, A.; Gutiérrez-Salmeán, G.; Galván, E.J.; Hernández-Frausto, M.; Herrera-López, G.; Romo-Parra, H.; García-Contreras, V.; Fernández-Presas, A.M.; Jasso-Chávez, R.; Borlongan, C.V.; et al. Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front. Aging Neurosci. 2018, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, S.; Neupane, S.; Bhurtel, S.; Katila, N.; Maharjan, S.; Choi, H.; Hong, J.T.; Choi, D.Y. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem. 2019, 69, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.Z.; Martin, K.A.; Xing, P.Y.; Agrawal, R.; Whiley, L.; Wood, T.K.; Hejndorf, S.; Ng, Y.Z.; Low, J.Z.Y.; Rossant, J.; et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2021, 118, e2021091118. [Google Scholar] [CrossRef]
- Lin, C.-J.; Wu, V.; Wu, P.-C.; Wu, C.-J. Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xu, H.; Chen, S.; He, J.; Zhou, Y.; Nie, Y. Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2021, 36, 320–328. [Google Scholar] [CrossRef]
- Purton, T.; Staskova, L.; Lane, M.M.; Dawson, S.L.; West, M.; Firth, J.; Clarke, G.; Cryan, J.F.; Berk, M.; O’Neil, A.; et al. Prebiotic and probiotic supplementation and the tryptophan-kynurenine pathway: A systematic review and meta analysis. Neurosci. Biobehav. Rev. 2021, 123, 1–13. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Khalyfa, A.; Ericsson, A.; Qiao, Z.; Almendros, I.; Farré, R.; Gozal, D. Circulating exosomes and gut microbiome induced insulin resistance in mice exposed to intermittent hypoxia: Effects of physical activity. EBioMedicine 2021, 64, 103208. [Google Scholar] [CrossRef] [PubMed]
- Trindade, L.M.; Torres, L.; Matos, I.D.; Miranda, V.C.; de Jesus, L.C.L.; Cavalcante, G.; de Souza Oliveira, J.J.; Cassali, G.D.; Mancha-Agresti, P.; de Carvalho Azevedo, V.A.; et al. Paraprobiotic Lacticaseibacillus rhamnosus protects intestinal damage in an experimental murine model of mucositis. Probiotics Antimicrob. Proteins 2021. [Google Scholar] [CrossRef]
- Sindhu, K.N.C.; Sowmyanarayanan, T.V.; Paul, A.; Babji, S.; Ajjampur, S.S.R.; Priyadarshini, S.; Sarkar, R.; Balasubramanian, K.A.; Wanke, C.A.; Ward, H.D.; et al. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: A randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2014, 58, 1107–1115. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Zhao, H.; Jia, L.; Yan, Q.; Deng, Q.; Wei, B. Effect of Clostridium butyricum and butyrate on intestinal barrier functions: Study of a rat model of severe acute pancreatitis with intra-abdominal hypertension. Front. Physiol. 2020, 11, 561061. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Han, W.; Lamine, F.; Eutamene, H.; Fioramonti, J.; Bueno, L.; Theodorou, V. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: A possible action through interaction with epithelial cell cytoskeleton contraction. Gut 2006, 55, 1090–1094. [Google Scholar] [CrossRef]
- Seth, A.; Yan, F.; Polk, D.B.; Rao, R.K. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1060–G1069. [Google Scholar] [CrossRef] [PubMed]
- Gotteland, M.; Cruchet, S.; Verbeke, S. Effect of Lactobacillus ingestion on the gastrointestinal mucosal barrier alterations induced by indometacin in humans. Aliment. Pharmacol. Ther. 2001, 15, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.J.M.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakamura, K.; Shimizu, Y.; Yokoi, Y.; Ohira, S.; Hagiwara, M.; Wang, Y.; Song, Y.; Aizawa, T.; Ayabe, T. Decrease of α-defensin impairs intestinal metabolite homeostasis via dysbiosis in mouse chronic social defeat stress model. Sci. Rep. 2021, 11, 9915. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Radtke, F.A.; Chapman, G.; Hall, J.; Syed, Y.A. Modulating neuroinflammation to treat neuropsychiatric disorders. Biomed Res. Int. 2017, 2017, 5071786. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suda, K.; Matsuda, K. How Microbes Affect Depression: Underlying Mechanisms via the Gut–Brain Axis and the Modulating Role of Probiotics. Int. J. Mol. Sci. 2022, 23, 1172. https://doi.org/10.3390/ijms23031172
Suda K, Matsuda K. How Microbes Affect Depression: Underlying Mechanisms via the Gut–Brain Axis and the Modulating Role of Probiotics. International Journal of Molecular Sciences. 2022; 23(3):1172. https://doi.org/10.3390/ijms23031172
Chicago/Turabian StyleSuda, Kazunori, and Kazunori Matsuda. 2022. "How Microbes Affect Depression: Underlying Mechanisms via the Gut–Brain Axis and the Modulating Role of Probiotics" International Journal of Molecular Sciences 23, no. 3: 1172. https://doi.org/10.3390/ijms23031172
APA StyleSuda, K., & Matsuda, K. (2022). How Microbes Affect Depression: Underlying Mechanisms via the Gut–Brain Axis and the Modulating Role of Probiotics. International Journal of Molecular Sciences, 23(3), 1172. https://doi.org/10.3390/ijms23031172





