Effects of Al or Mo Addition on Microstructure and Mechanical Properties of Fe-Rich Nonequiatomic FeCrCoMnNi High-Entropy Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microstructure Observation and Phase Analysis
2.3. Mechanical Properties
3. Results
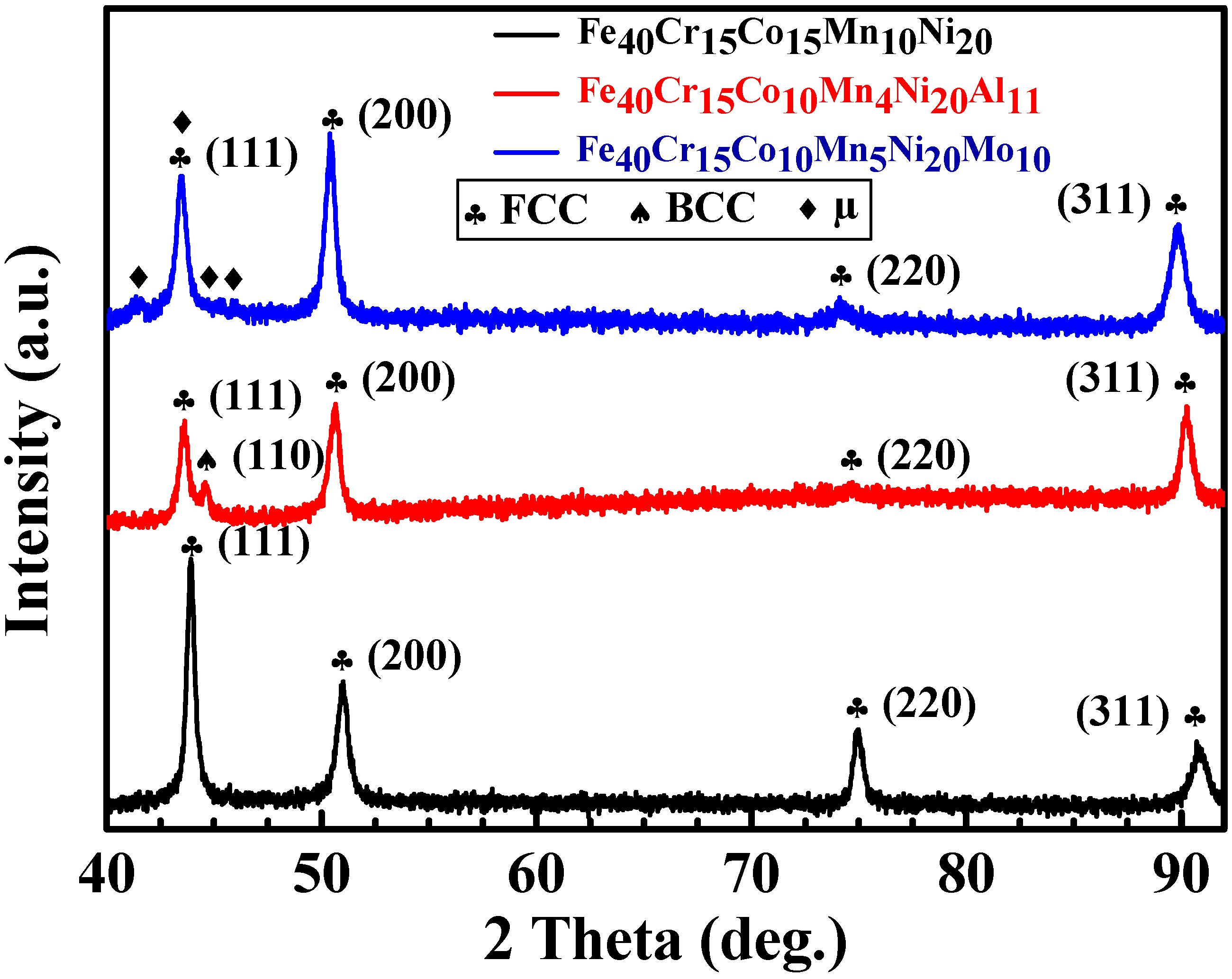
3.1. X-ray Diffraction
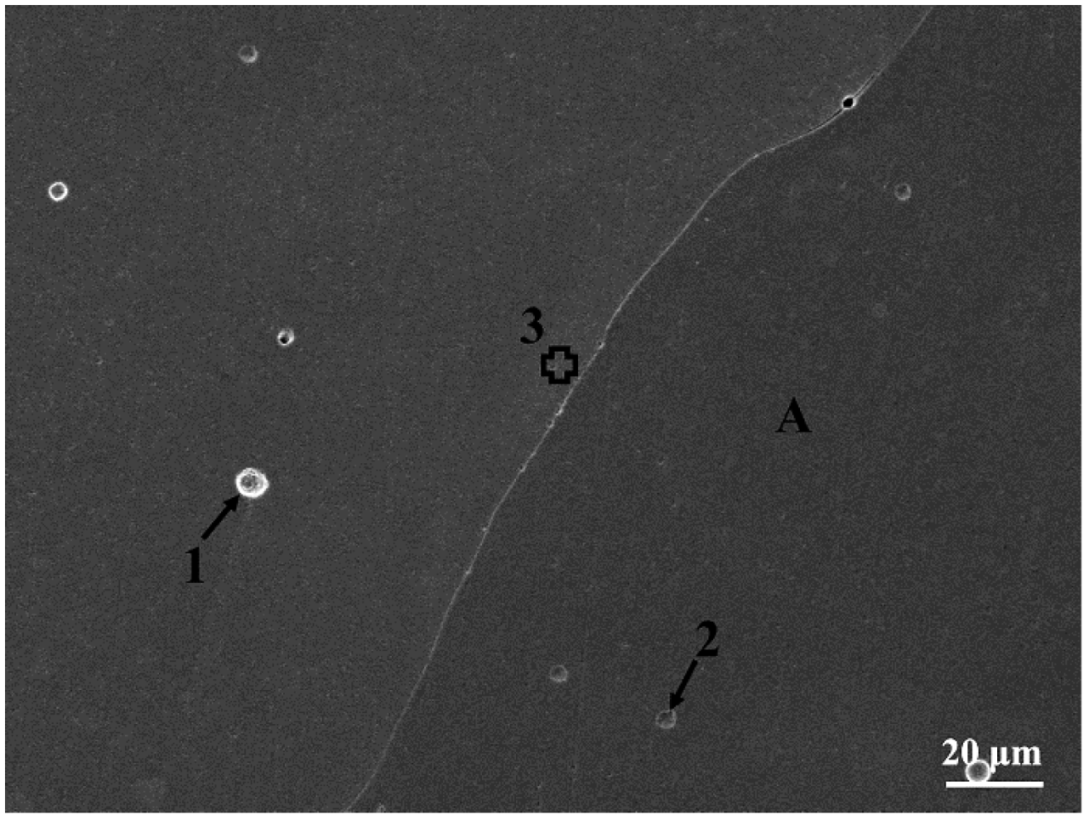
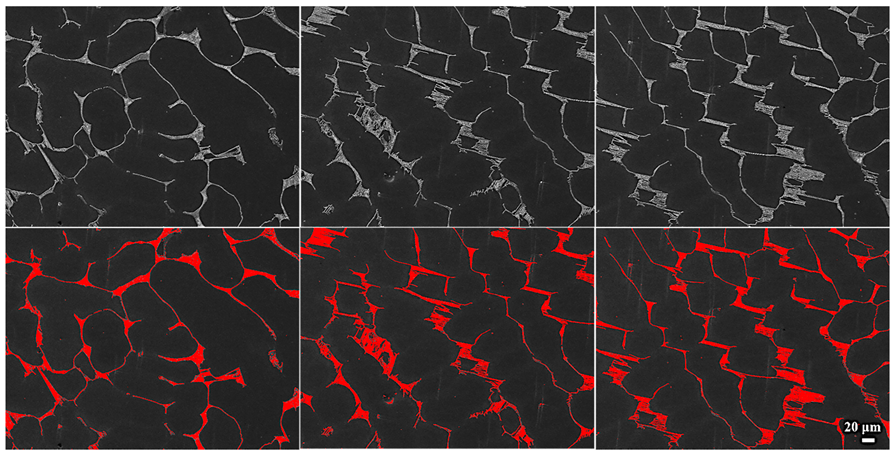
3.2. Metallographic Morphology
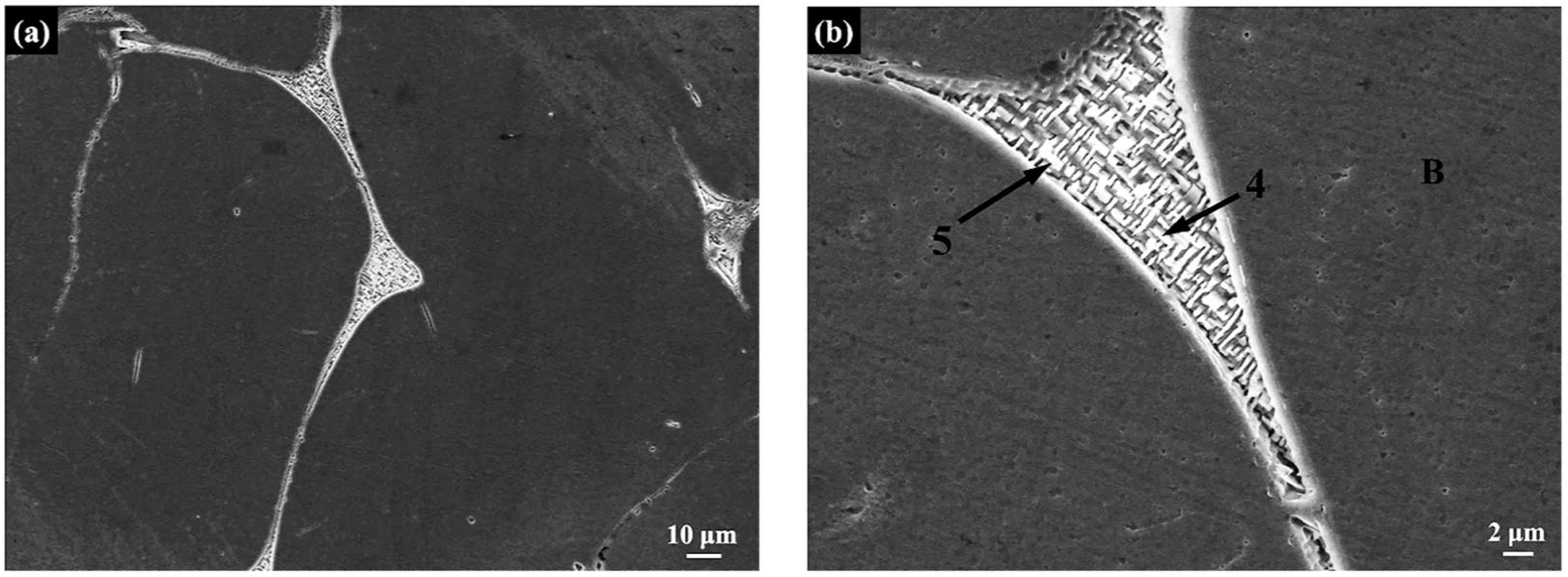
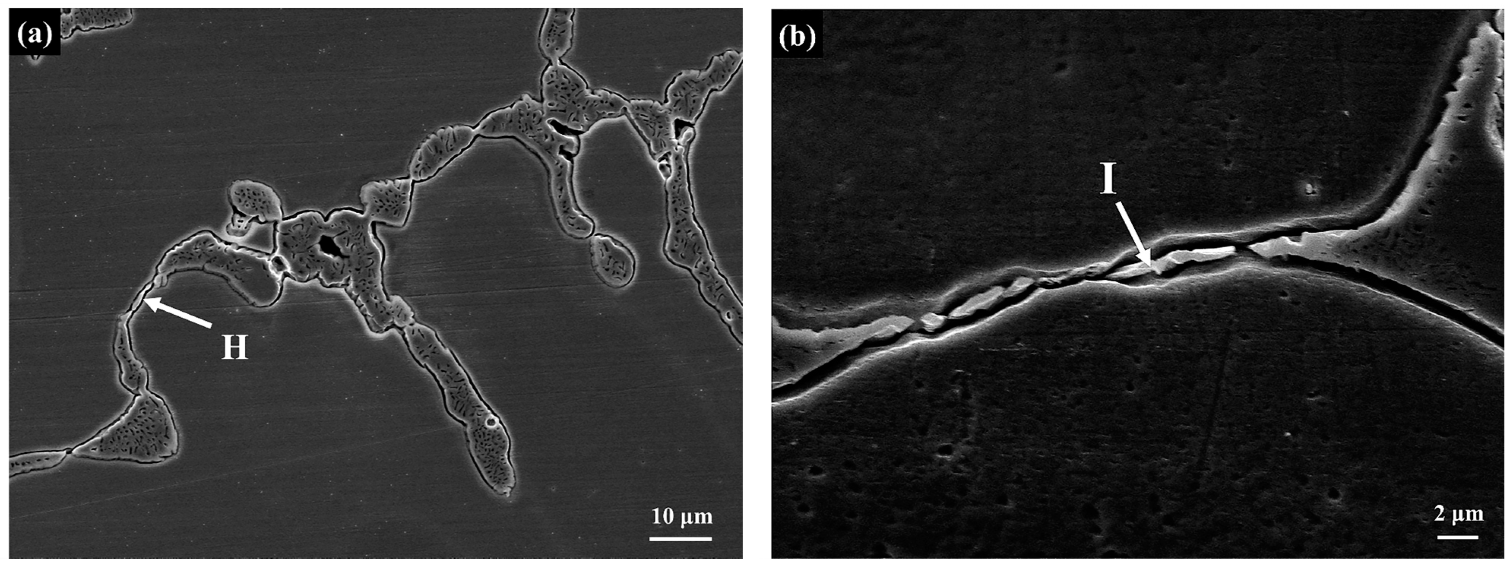
3.3. Scanning Electron Microscopy
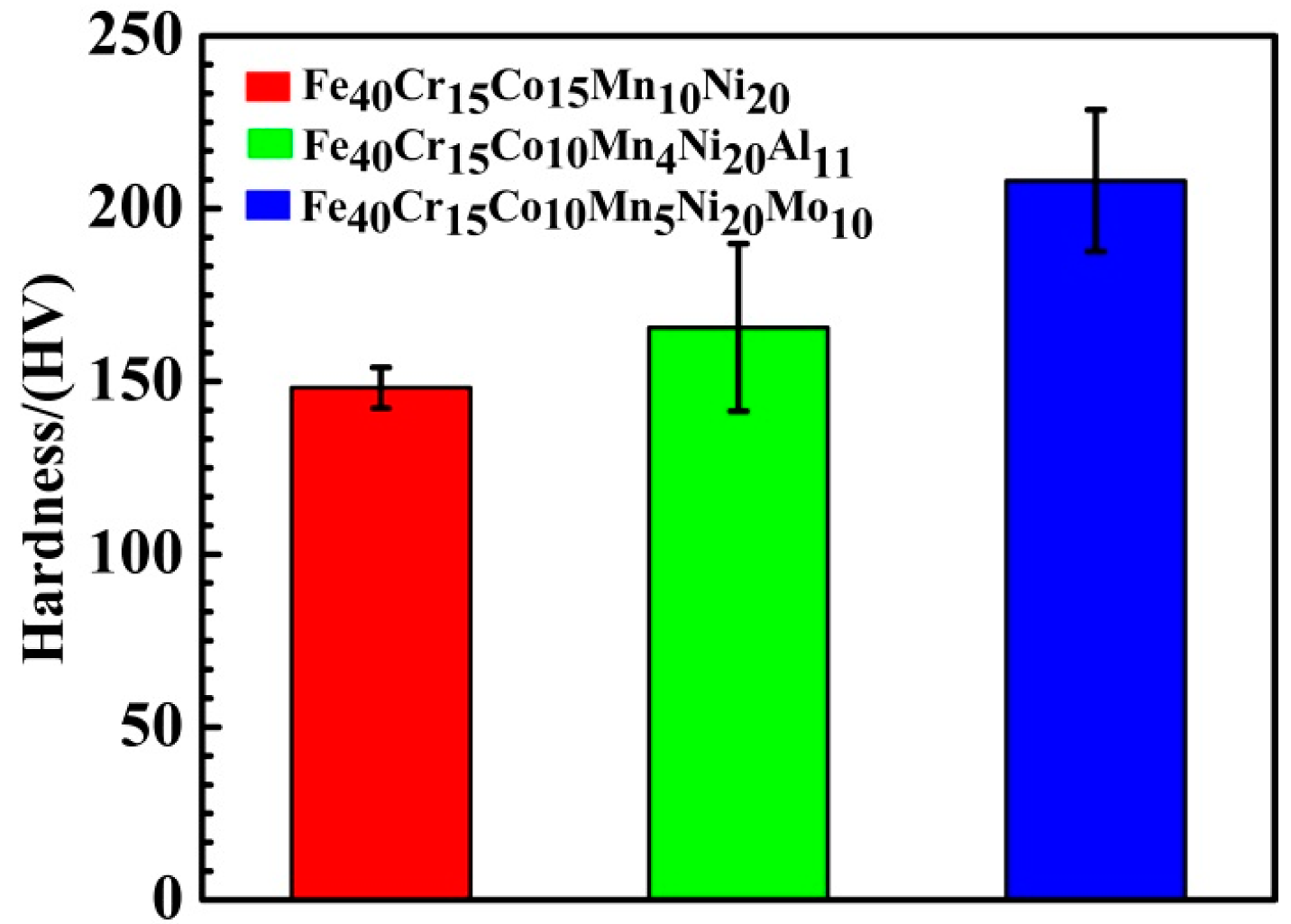
3.4. Vickers Hardness
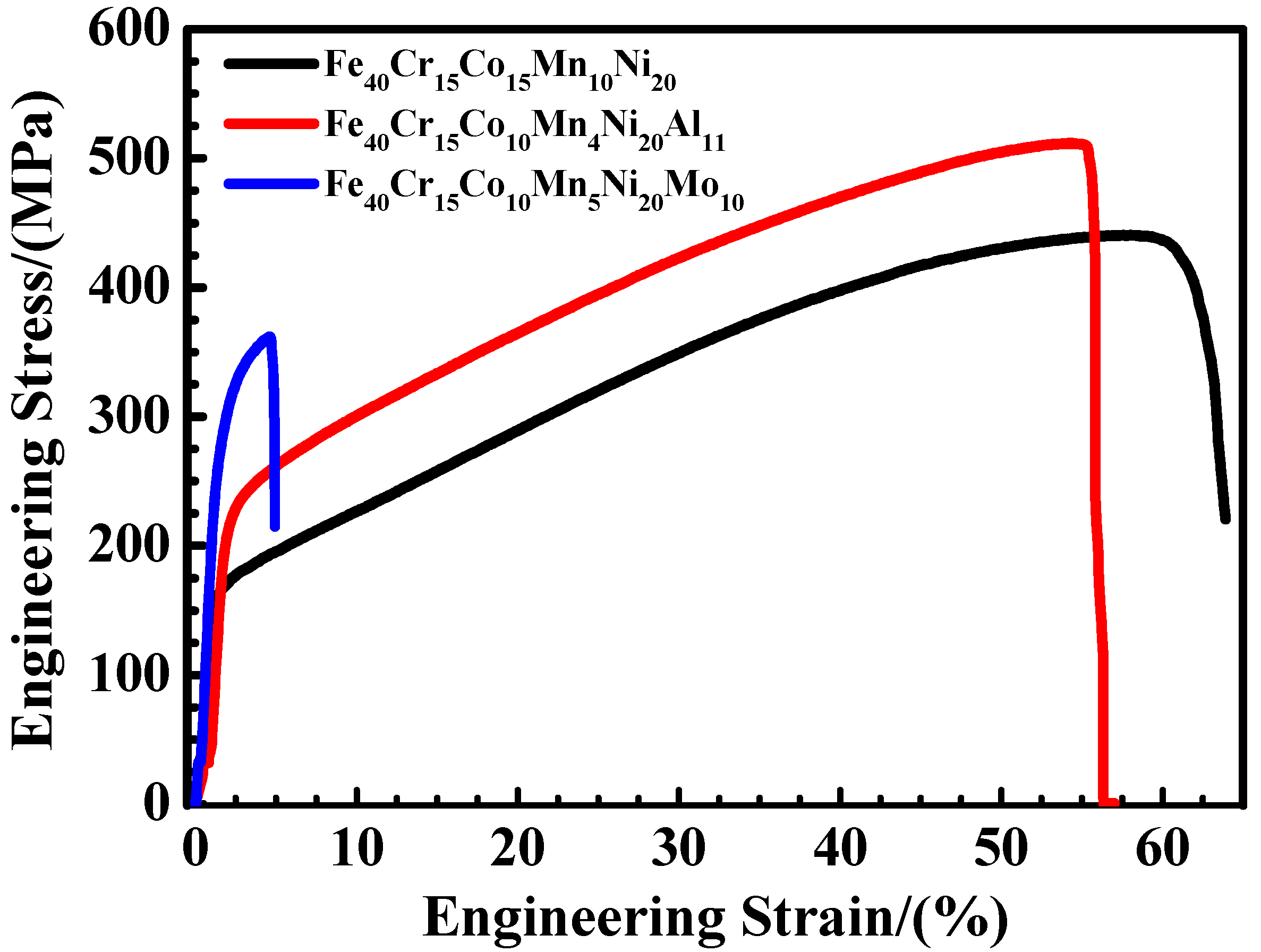
3.5. Tensile Testing
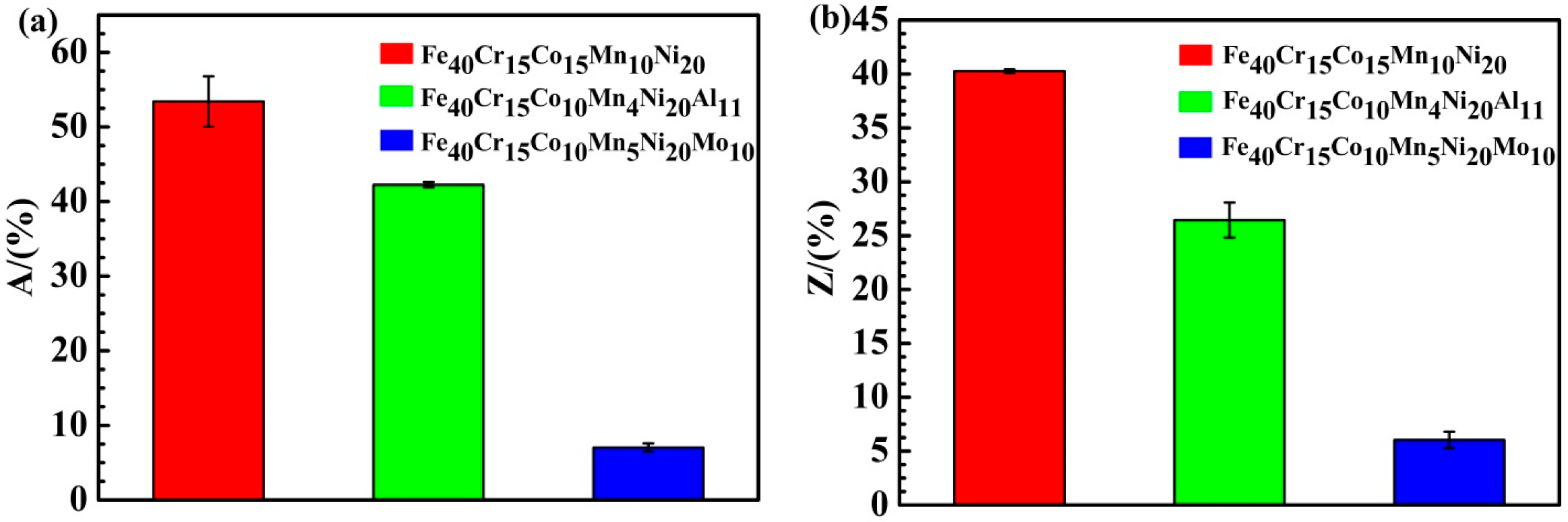
3.5.1. Tensile Strength and Plasticity
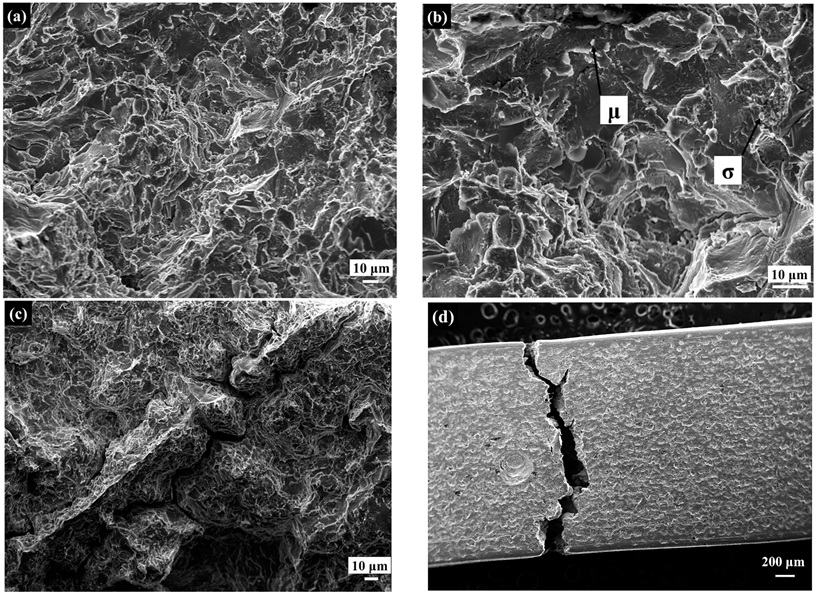
3.5.2. Fractography
4. Discussion
4.1. Phase Formation, Stability, and Transition
4.2. Mechanical Properties
4.2.1. Vickers Hardness
4.2.2. Tensile Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, S.Z.; Choi, E.-A.; Lim, S.H.; Kim, S.; Lee, J. Alloy design strategies to increase strength and its trade-offs together. Prog. Mater. Sci. 2021, 117, 100720. [Google Scholar] [CrossRef]
- Benvenuto, M.A. Metals and Alloys: Industrial Applications; De Gruyter: Berlin, Germany, 2016. [Google Scholar]
- Gupta, M. Changing Wisdom of Metallic Alloys Development. Mater. Sci. Appl. 2018, 9, 1021. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.-W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. Jom 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.Y.; Han, B.; Zhang, S.Y.; Li, Y.; Hu, C.Y. Investigation on microstructures and properties of Al1.5CoCrFeMnNi high entropy alloy coating before and after ultrasonic impact treatment. J. Alloys Compd. 2021, 884, 160989. [Google Scholar] [CrossRef]
- Ming, K.; Bi, X.; Wang, J. Precipitation strengthening of ductile Cr15Fe20Co35Ni20Mo10 alloys. Scr. Mater. 2017, 137, 88–93. [Google Scholar] [CrossRef]
- Klaver, T.P.C.; Simonovic, D.; Sluiter, M.H.F. Brute Force Composition Scanning with a CALPHAD Database to Find Low Temperature Body Centered Cubic High Entropy Alloys. Entropy 2018, 20, 911. [Google Scholar] [CrossRef] [Green Version]
- Sanin, V.N.; Yukhvid, V.I.; Ikornikov, D.M.; Andreev, D.E.; Sachkova, N.V.; Alymov, M.I. SHS metallurgy of high-entropy transition metal alloys. Dokl. Phys. Chem. 2016, 470, 145–149. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, Q.; Wang, D.; Yan, M.; Wang, P.; Jiang, M.; Liu, C.; Diao, D.; Lao, C.; Chen, Z.; et al. Ordered nitrogen complexes overcoming strength–ductility trade-off in an additively manufactured high-entropy alloy. Virtual Phys. Prototyp. 2020, 15, 532–542. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Amar, A.; Li, J.; Xiang, S.; Liu, X.; Zhou, Y.; Le, G.; Wang, X.; Qu, F.; Ma, S.; Dong, W.; et al. Additive manufacturing of high-strength CrMnFeCoNi-based High Entropy Alloys with TiC addition. Intermetallics 2019, 109, 162–166. [Google Scholar] [CrossRef]
- Li, J.-H.; Tsai, M.-H. Theories for predicting simple solid solution high-entropy alloys: Classification, accuracy, and important factors impacting accuracy. Scr. Mater. 2020, 188, 80–87. [Google Scholar] [CrossRef]
- Gorbachev, I.I.; Popov, V.V.; Katz-Demyanetz, A.; Popov, V.; Eshed, E. Prediction of the Phase Composition of High-Entropy Alloys Based on Cr–Nb–Ti–V–Zr Using the Calphad Method. Phys. Met. Metallogr. 2019, 120, 378–386. [Google Scholar] [CrossRef]
- Ren, M.-X.; Li, B.-S.; Fu, H.-Z. Formation condition of solid solution type high-entropy alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 991–995. [Google Scholar] [CrossRef]
- Luan, H.-W.; Shao, Y.; Li, J.-F.; Mao, W.-L.; Han, Z.-D.; Shao, C.; Yao, K.-F. Phase stabilities of high entropy alloys. Scr. Mater. 2020, 179, 40–44. [Google Scholar] [CrossRef]
- Li, S.; Cong, D.; Sun, X.; Zhang, Y.; Chen, Z.; Nie, Z.; Li, R.; Li, F.; Ren, Y.; Wang, Y. Wide-temperature-range perfect superelasticity and giant elastocaloric effect in a high entropy alloy. Mater. Res. Lett. 2019, 7, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.H.; Liang, S.; Yang, J.; Wei, R.; Zhang, C.J. A superior combination of strength-ductility in CoCrFeNiMn high-entropy alloy induced by asymmetric rolling and subsequent annealing treatment. Mater. Charact. 2018, 145, 619–626. [Google Scholar] [CrossRef]
- Jin, X.; Gu, X.; Quan, F.; Ran, X.; Zhang, K.; Mao, A. CoCrFeMnNi high-entropy alloy powder with excellent corrosion resistance and soft magnetic property prepared by gas atomization method. Mater. Werkst. 2019, 50, 837–843. [Google Scholar] [CrossRef]
- Xia, A.; Franz, R. Thermal Stability of MoNbTaVW High Entropy Alloy Thin Films. Coatings 2020, 10, 941. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yue, B.B.; Bhandari, U.; Starovoytov, O.N.; Yang, Y.; Young, D.P.; Yan, J.Y.; Hong, F.; Yang, S.Z. In situ study on the compression deformation of MoNbTaVW high-entropy alloy. J. Alloy. Compd. 2021, 871, 159557. [Google Scholar] [CrossRef]
- Chiu, C.T.; Teng, Y.J.; Dai, B.H.; Tsao, I.Y.; Lin, W.C.; Wang, K.W.; Hsu, L.C.; Chang, Y.C.; Li, C.T.; Nguyen, H.T.T.; et al. Novel high-entropy ceramic/carbon composite materials for the decomposition of organic pollutants. Mater. Chem. Phys. 2022, 275, 125274. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, Y.; Qiao, Y.; Chen, G.L. Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys. Intermetallics 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Han, Y.; Li, H.; Feng, H.; Li, K.; Tian, Y.; Jiang, Z. Enhancing the strength and ductility of CoCrFeMnNi high-entropy alloy by nitrogen addition. Mater. Sci. Eng. A 2020, 789, 139587. [Google Scholar] [CrossRef]
- Borkar, T.; Gwalani, B.; Choudhuri, D.; Mikler, C.V.; Yannetta, C.J.; Chen, X.; Ramanujan, R.V.; Styles, M.J.; Gibson, M.A.; Banerjee, R. A combinatorial assessment of AlxCrCuFeNi2 (0 < x < 1.5) complex concentrated alloys: Microstructure, microhardness, and magnetic properties. Acta Mater. 2016, 116, 63–76. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, X.; Li, L.; Chen, Y.; Liou, F. Fabrication and Characterization of AlxCrCuFeNi2 High-Entropy Alloys Coatings by Laser Metal Deposition. Procedia Manuf. 2019, 39, 509–518. [Google Scholar] [CrossRef]
- Laplanche, G.; Horst, O.; Otto, F.; Eggeler, G.; George, E.P. Microstructural evolution of a CoCrFeMnNi high-entropy alloy after swaging and annealing. J. Alloy. Compd. 2015, 647, 548–557. [Google Scholar] [CrossRef]
- Kwon, H.; Moon, J.; Bae, J.W.; Park, J.M.; Son, S.; Do, H.-S.; Lee, B.-J.; Kim, H.S. Precipitation-driven metastability engineering of carbon-doped CoCrFeNiMo medium-entropy alloys at cryogenic temperature. Scr. Mater. 2020, 188, 140–145. [Google Scholar] [CrossRef]
- Lukianova, O.A.; Rao, Z.; Kulitckii, V.; Li, Z.; Wilde, G.; Divinski, S.V. Impact of interstitial carbon on self-diffusion in CoCrFeMnNi high entropy alloys. Scr. Mater. 2020, 188, 264–268. [Google Scholar] [CrossRef]
- He, J.Y.; Liu, W.H.; Wang, H.; Wu, Y.; Liu, X.J.; Nieh, T.G.; Lu, Z.P. Effects of Al addition on structural evolution and tensile properties of the FeCoNiCrMn high-entropy alloy system. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A Promising New Class of High-Temperature Alloys: Eutectic High-Entropy Alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Lu, Y.P.; Chatterjee, S.; Bhattacharjee, P.P.; Guo, S.; Tsuji, N. Ultrafine-Grained AlCoCrFeNi2.1 Eutectic High-Entropy Alloy. Mater. Res. Lett. 2016, 4, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.S.; Haridas, R.S.; Agrawal, P. High entropy alloys—Tunability of deformation mechanisms through integration of compositional and microstructural domains. Mater. Sci. Eng. A 2021, 812, 141085. [Google Scholar] [CrossRef]
- Chen, C.; Pang, S.; Cheng, Y.; Zhang, T. Microstructure and mechanical properties of Al20−xCr20+0.5xFe20Co20Ni20+0.5x high entropy alloys. J. Alloy. Compd. 2016, 659, 279–287. [Google Scholar] [CrossRef]
- Tasan, C.C.; Deng, Y.; Pradeep, K.G.; Yao, M.J.; Springer, H.; Raabe, D. Composition Dependence of Phase Stability, Deformation Mechanisms, and Mechanical Properties of the CoCrFeMnNi High-Entropy Alloy System. Jom 2014, 66, 1993–2001. [Google Scholar] [CrossRef]
- Bae, J.W.; Park, J.M.; Moon, J.; Choi, W.M.; Lee, B.-J.; Kim, H.S. Effect of μ-precipitates on the microstructure and mechanical properties of non-equiatomic CoCrFeNiMo medium-entropy alloys. J. Alloy. Compd. 2019, 781, 75–83. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Wu, Q.; Niu, S.; Li, J.; Wang, J.; Liu, C.T. Solid solution island of the Co-Cr-Fe-Ni high entropy alloy system. Scr. Mater. 2017, 131, 42–46. [Google Scholar] [CrossRef]
- Bracq, G.; Laurent-Brocq, M.; Perrière, L.; Pirès, R.; Joubert, J.-M.; Guillot, I. The fcc solid solution stability in the Co-Cr-Fe-Mn-Ni multi-component system. Acta Mater. 2017, 128, 327–336. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Wang, Y.; Zhang, L.; Yang, Y.; Liu, F.; Cui, Y. Microstructural evolution, phase formation and mechanical properties of multi-component AlCoCrFeNix alloys. Appl. Phys. A 2019, 125, 699. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Ma, K.H.; Yang, X.; Shek, C.H. Annealing effect on the phase stability and mechanical properties of (FeNiCrMn)(100−)Co high entropy alloys. J. Alloy. Compd. 2017, 695, 2945–2950. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y. Tensile Properties and Impact Toughness of AlCoxCrFeNi3.1–x (x = 0.4, 1) High-Entropy Alloys. Front. Mater. 2020, 7, 92. [Google Scholar] [CrossRef]
- Ke, G.-Y.; Chen, G.-Y.; Hsu, T.; Yeh, J.-W. FCC and BCC equivalents in as-cast solid solutions of AlxCoyCrzCu0.5FevNiw high-entropy alloys. Eur. J. Control 2006, 31, 669–684. [Google Scholar] [CrossRef]
- Wei, S.; He, F.; Tasan, C.C. Metastability in high-entropy alloys: A review. J. Mater. Res. 2018, 33, 2924–2937. [Google Scholar] [CrossRef] [Green Version]
- Kao, Y.-F.; Chen, T.-J.; Chen, S.-K.; Yeh, J.-W. Microstructure and mechanical property of as-cast, -homogenized, and -deformed AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. J. Alloy. Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Rao, J.C.; Diao, H.Y.; Ocelík, V.; Vainchtein, D.; Zhang, C.; Kuo, C.; Tang, Z.; Guo, W.; Poplawsky, J.D.; Zhou, Y.; et al. Secondary phases in AlxCoCrFeNi high-entropy alloys: An in-situ TEM heating study and thermodynamic appraisal. Acta Mater. 2017, 131, 206–220. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.; Stanford, N.; Hodgson, P.; Fabijanic, D.M. Understanding the mechanical behaviour and the large strength/ductility differences between FCC and BCC AlxCoCrFeNi high entropy alloys. J. Alloy. Compd. 2017, 726, 885–895. [Google Scholar] [CrossRef]
- Aizenshtein, M.; Priel, E.; Hayun, S. Effect of pre-deformation and B2 morphology on the mechanical properties of Al0.5CoCrFeNi HEA. Mater. Sci. Eng. A 2020, 788, 139575. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Wang, S.-C.; Tsai, Y.-C.; Lai, C.-H.; Yeh, J.-W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Z.; He, F.; Li, J.; Wang, J. Revealing the Selection of σ and μ Phases in CoCrFeNiMox High Entropy Alloys by CALPHAD. J. Phase Equilibria Diffus. 2018, 39, 446–453. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, Y.; Cui, S.; Yi, Y.; Xing, X.; Wang, X.; Li, W. Effect of Mo Element on the Mechanical Properties and Tribological Responses of CoCrFeNiMox High-Entropy Alloys. Metals 2021, 11, 486. [Google Scholar] [CrossRef]
- Liu, W.H.; Lu, Z.P.; He, J.Y.; Luan, J.H.; Wang, Z.J.; Liu, B.; Liu, Y.; Chen, M.W.; Liu, C.T. Ductile CoCrFeNiMox high entropy alloys strengthened by hard intermetallic phases. Acta Mater. 2016, 116, 332–342. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Z.; Pi, J. Microstructure and wear behavior of the AlCrFeCoNi high-entropy alloy fabricated by additive manufacturing. Mater. Lett. 2020, 261, 127004. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Sun, H.; Shao, Z. Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing. Nanotechnol. Rev. 2020, 9, 580–595. [Google Scholar] [CrossRef]
- Gevaux, D. The equation to bridge worlds. Nature 2014, 511, 6–7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Yao, L.; Zhu, M.; Liu, Y.; Jian, Z. Structural Evolution and Mechanical and Magnetic Properties of Nonequiatomic CoFe2NiMn0.3Alx (0.25 ≤ x ≤ 1.00) High-Entropy Alloys. J. Mater. Eng. Perform. 2021, 30, 1472–1478. [Google Scholar] [CrossRef]
- Han, J.; Su, B.; Lu, J.; Meng, J.; Zhang, A.; Wu, Y. Preparation of MoNbTaW refractory high entropy alloy powders by pressureless spark plasma sintering: Crystal structure and phase evolution. Intermetallics 2020, 123, 106832. [Google Scholar] [CrossRef]
- Laurent-Brocq, M.; Perrière, L.; Pirès, R.; Prima, F.; Vermaut, P.; Champion, Y. From diluted solid solutions to high entropy alloys: On the evolution of properties with composition of multi-components alloys. Mater. Sci. Eng. A 2017, 696, 228–235. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef]
- Chizmeshya, A.V.G.; Bauer, M.R.; Kouvetakis, J. Experimental and Theoretical Study of Deviations from Vegard’s Law in the SnxGe1-x System. Chem. Mater. 2003, 15, 2511–2519. [Google Scholar] [CrossRef]
- Alonso, J.C.; Alonso, O.C. Derivation of unit cell volume, and lattice parameter of cubic high entropy alloys from volume size factors. Intermetallics 2021, 137, 107299. [Google Scholar] [CrossRef]
- Pulikkotil, J.J.; Chroneos, A.; Schwingenschlögl, U. Structure of Sn1−xGex random alloys as obtained from the coherent potential approximation. J. Appl. Phys. 2011, 110, 036105. [Google Scholar] [CrossRef] [Green Version]
- Risti, R.; Figueroa, I.A.; Feti, A.S.; Zadro, K.; Babi, E. Transition from high-entropy to conventional (TiZrNbCu)1−xCox metallic glasses. J. Appl. Phys. 2021, 130, 195102. [Google Scholar] [CrossRef]
- Kuo, Y.-K.; Liou, B.-T.; Yen, S.-H.; Chu, H.-Y. Vegard’s law deviation in lattice constant and band gap bowing parameter of zincblende InxGa1−xN. Opt. Commun. 2004, 237, 363–369. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Jin, K.; Bei, H.; Chen, S.; Cao, W.; Zhu, J.; Lv, D. Understanding of the Elemental Diffusion Behavior in Concentrated Solid Solution Alloys. J. Phase Equilibria Diffus. 2017, 38, 434–444. [Google Scholar] [CrossRef] [Green Version]
- Ye, F.; Jiao, Z.; Yan, S.; Guo, L.; Feng, L.; Yu, J. Microbeam plasma arc remanufacturing: Effects of Al on microstructure, wear resistance, corrosion resistance and high temperature oxidation resistance of AlxCoCrFeMnNi high-entropy alloy cladding layer. Vacuum 2020, 174, 109178. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, Z.; Han, P.; Dan, Z.; Qin, F.; Chang, H. MnO2-decorated metallic framework supercapacitors fabricated from duplex-phase FeCrCoMnNiAl0.75 Cantor high entropy alloy precursors through selective phase dissolution. J. Alloy. Compd. 2021, 870, 159523. [Google Scholar] [CrossRef]
- Wu, M.; Chen, K.; Xu, Z.; Li, D.Y. Effect of Ti addition on the sliding wear behavior of AlCrFeCoNi high-entropy alloy. Wear 2020, 462, 203493. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Mondal, S.; van Smaalen, S.; Völkl, R.; Glatzel, U.; Wanderka, N. Investigation of phases in Al23Co15Cr23Cu8Fe15Ni16 and Al8Co17Cr17Cu8Fe17Ni33 high entropy alloys and comparison with equilibrium phases predicted by Thermo-Calc. J. Alloy. Compd. 2013, 552, 430–436. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, Y.; Geng, C.; Xu, J.; Wang, Y. Microstructural evolution, mechanical and corrosion behaviors of as-annealed CoCrFeNiMox (x = 0, 0.2, 0.5, 0.8, 1) high entropy alloys. J. Alloy. Compd. 2020, 820, 153273. [Google Scholar] [CrossRef]
- Qin, G.; Chen, R.; Zheng, H.; Fang, H.; Wang, L.; Su, Y.; Guo, J.; Fu, H. Strengthening FCC-CoCrFeMnNi high entropy alloys by Mo addition. J. Mater. Sci. Technol. 2019, 35, 578–583. [Google Scholar] [CrossRef]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Microstructures and compressive properties of multicomponent AlCoCrFeNiMox alloys. Mater. Sci. Eng. A 2010, 527, 6975–6979. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Kim, T.N.; Chen, G.L. Microstructure characterizations and strengthening mechanism of multi-principal component AlCoCrFeNiTi0.5 solid solution alloy with excellent mechanical properties. Mater. Lett. 2008, 62, 2673–2676. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Wang, S. Paracrystalline property of high-entropy alloys. AIP Adv. 2013, 3, 102105. [Google Scholar] [CrossRef]
- Tong, Z.; Ren, X.; Jiao, J.; Zhou, W.; Ren, Y.; Ye, Y.; Larson, E.A.; Gu, J. Laser additive manufacturing of FeCrCoMnNi high-entropy alloy: Effect of heat treatment on microstructure, residual stress and mechanical property. J. Alloy. Compd. 2019, 785, 1144–1159. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Lee, C.-F.; Shun, T.-T. Effect of Fe content on microstructure and mechanical properties of Al0·5CoCrFexNiTi0.5 high-entropy alloys. Mater. Charact. 2016, 114, 179–184. [Google Scholar] [CrossRef]
- Zhang, T.; Xin, L.; Wu, F.; Zhao, R.; Xiang, J.; Chen, M.; Jiang, S.; Huang, Y.; Chen, S. Microstructure and mechanical properties of FexCoCrNiMn high-entropy alloys. J. Mater. Sci. Technol. 2019, 35, 2331–2335. [Google Scholar] [CrossRef]
- Qiu, H.; Zhu, H.; Zhang, J.; Xie, Z. Effect of Fe content upon the microstructures and mechanical properties of FexCoNiCu high entropy alloys. Mater. Sci. Eng. A 2020, 769, 138514. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Juan, C.-C.; Chen, S.-T.; Sheu, T.-S.; Yeh, J.-W.; Chen, S.-K. Phase Diagrams of High-Entropy Alloy System Al-Co-Cr-Fe-Mo-Ni. JOM 2013, 65, 1829–1839. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Ng, C.; Lu, J.; Liu, C. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, Q.; Han, B.; Song, L.; Li, J.; Yang, J. Investigation on microstructure and properties of AlxCoCrFeMnNi high entropy alloys by ultrasonic impact treatment. J. Alloy. Compd. 2020, 816, 152626. [Google Scholar] [CrossRef]
- Gu, J.; Ni, S.; Liu, Y.; Song, M. Regulating the strength and ductility of a cold rolled FeCrCoMnNi high-entropy alloy via annealing treatment. Mater. Sci. Eng. A 2019, 755, 289–294. [Google Scholar] [CrossRef]
- Bracq, G.; Laurent-Brocq, M.; Varvenne, C.; Perrière, L.; Curtin, W.A.; Joubert, J.M.; Guillot, I. Combining experiments and modeling to explore the solid solution strengthening of high and medium entropy alloys. Acta Mater. 2019, 177, 266–279. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.D.; Xu, D.K.; Chen, X.B.; Han, E.H.; Dong, C. Effect of heat treatment on the corrosion resistance and mechanical properties of an as-forged Mg-Zn-Y-Zr alloy. Corros. Sci. 2015, 92, 228–236. [Google Scholar] [CrossRef]
- Wang, S.D.; Xu, D.K.; Wang, B.J.; Sheng, L.Y.; Qiao, Y.X.; Han, E.H.; Dong, C. Influence of phase dissolution and hydrogen absorption on the stress corrosion cracking behavior of Mg-7%Gd-5%Y-1%Nd-0.5%Zr alloy in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 142, 185–200. [Google Scholar] [CrossRef]
- Huo, W.; Zhou, H.; Fang, F.; Hu, X.; Xie, Z.; Jiang, J. Strain-rate effect upon the tensile behavior of CoCrFeNi high-entropy alloys. Mater. Sci. Eng. A 2017, 689, 366–369. [Google Scholar] [CrossRef]
- Wang, S.D.; Xu, D.K.; Wang, B.J.; Han, E.H.; Dong, C. Effect of solution treatment on the fatigue behavior of an as-forged Mg-Zn-Y-Zr alloy. Sci. Rep. 2016, 6, 23955. [Google Scholar] [CrossRef] [Green Version]
- Choi, N.; Lim, K.R.; Na, Y.S.; Glatzel, U.; Park, J.H. Characterization of non-metallic inclusions and their influence on the mechanical properties of a FCC single-phase high-entropy alloy. J. Alloy. Compd. 2018, 763, 546–557. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lim, K.R.; Won, J.W.; Na, Y.S.; Kim, H.-S. Mechanical properties and deformation twinning behavior of as-cast CoCrFeMnNi high-entropy alloy at low and high temperatures. Mater. Sci. Eng. A 2018, 712, 108–113. [Google Scholar] [CrossRef]
- Liu, K.; Komarasamy, M.; Gwalani, B.; Shukla, S.; Mishra, R.S. Fatigue behavior of ultrafine grained triplex Al0.3CoCrFeNi high entropy alloy. Scr. Mater. 2019, 158, 116–120. [Google Scholar] [CrossRef]
- Wang, S.D.; Xu, D.K.; Wang, B.J.; Han, E.H.; Dong, C. Effect of corrosion attack on the fatigue behavior of an as-cast Mg-7%Gd-5%Y-1%Nd-0.5%Zr alloy. Mater. Des. 2015, 84, 185–193. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H.; Pharr, G.M.; George, E.P. Temperature dependence of the mechanical properties of equiatomic solid solution alloys with face-centered cubic crystal structures. Acta Mater. 2014, 81, 428–441. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C. Effect of aluminum on the microstructure and properties of two refractory high-entropy alloys. Acta Mater. 2014, 68, 214–228. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, Y.; Zhang, L.; Du, X.; Li, B. A novel Fe 20 Co 20 Ni 41 Al 19 eutectic high entropy alloy with excellent tensile properties. Mater. Lett. 2018, 216, 144–146. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Jiang, L.; Wardini, J.L.; MacDonald, B.E.; Wen, H.M.; Xiong, W.; Zhang, D.L.; Zhou, Y.Z.; Rupert, T.J.; Chen, W.P.; et al. A high-entropy alloy with hierarchical nanoprecipitates and ultrahigh strength. Sci. Adv. 2018, 4, eaat8712. [Google Scholar] [CrossRef] [Green Version]







| Element (at.%) | Fe | Cr | Co | Mn | Ni | Al | Mo |
|---|---|---|---|---|---|---|---|
| Fe40Cr15Co15Mn10Ni20 | 39.2 | 16.4 | 15.4 | 9.7 | 19.3 | / | / |
| Fe40Cr15Co10Mn4Ni20Al11 | 39.0 | 16.7 | 10.1 | 4.1 | 20.0 | 10.1 | / |
| Fe40Cr15Co10Mn5Ni20Mo10 | 40.0 | 15.8 | 9.9 | 5.0 | 19.7 | / | 9.6 |
| Region | Chemical Compositions (at.%) | ||||
|---|---|---|---|---|---|
| Fe | Cr | Co | Mn | Ni | |
| 3 | 32.4 | 16.0 | 13.8 | 15.2 | 22.6 |
| A | 37.7 | 16.6 | 15.0 | 10.4 | 20.3 |
| Region | Phase | Chemical Compositions (at.%) | |||||
|---|---|---|---|---|---|---|---|
| Fe | Cr | Co | Mn | Ni | Al | ||
| B | FCC | 39.1 | 16.8 | 10.3 | 4.3 | 19.7 | 9.8 |
| 4 | BCC | 29.0 | 15.2 | 8.9 | 5.0 | 23.4 | 18.5 |
| 5 | BCC | 29.0 | 14.9 | 8.8 | 4.6 | 22.5 | 20.2 |
| The overall surface of the alloy | / | 35.0 | 15.6 | 9.8 | 4.6 | 22.1 | 12.9 |
| Region | Phase | Chemical Compositions (at.%) | |||||
|---|---|---|---|---|---|---|---|
| Fe | Cr | Co | Mn | Ni | Mo | ||
| D | FCC | 42.8 | 15.2 | 10.4 | 4.5 | 20.1 | 7.0 |
| E | μ | 30.5 | 19.5 | 8.1 | 4.0 | 10.2 | 27.7 |
| F | FCC | 40.3 | 15.7 | 9.8 | 4.9 | 20.2 | 9.1 |
| G | μ | 31.0 | 19.4 | 7.7 | 3.7 | 10.2 | 28.0 |
| The overall surface of the alloy | / | 41.3 | 15.4 | 10.0 | 4.6 | 19.9 | 8.8 |
| Region | Phase | Chemical Compositions (at.%) | |||||
|---|---|---|---|---|---|---|---|
| Fe | Cr | Co | Mn | Ni | Mo | ||
| H | σ | 38.4 | 17.1 | 9.6 | 5.4 | 18.2 | 11.3 |
| I | σ | 37.7 | 16.2 | 9.5 | 5.8 | 19.0 | 11.8 |
| Region | Chemical Compositions (at.%) | |||||
|---|---|---|---|---|---|---|
| Fe | Cr | Co | Mn | Ni | Al | |
| J | 22.2 | 11.4 | 2.1 | 3.8 | 5.5 | 55.0 |
| Region | Chemical Compositions (at.%) | |||||
|---|---|---|---|---|---|---|
| Fe | Cr | Co | Mn | Ni | Mo | |
| μ | 31.4 | 19.6 | 9.1 | 3.4 | 12.4 | 24.1 |
| σ | 36.8 | 17.0 | 9.1 | 5.8 | 19.5 | 11.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Chen, L.; Li, Q.; Wang, S.; Wu, M.; Yang, S.; Xiang, D. Effects of Al or Mo Addition on Microstructure and Mechanical Properties of Fe-Rich Nonequiatomic FeCrCoMnNi High-Entropy Alloy. Metals 2022, 12, 191. https://doi.org/10.3390/met12020191
Wang S, Chen L, Li Q, Wang S, Wu M, Yang S, Xiang D. Effects of Al or Mo Addition on Microstructure and Mechanical Properties of Fe-Rich Nonequiatomic FeCrCoMnNi High-Entropy Alloy. Metals. 2022; 12(2):191. https://doi.org/10.3390/met12020191
Chicago/Turabian StyleWang, Shuliang, Luyu Chen, Qilin Li, Shidong Wang, Mingyu Wu, Shuiyuan Yang, and Dinghan Xiang. 2022. "Effects of Al or Mo Addition on Microstructure and Mechanical Properties of Fe-Rich Nonequiatomic FeCrCoMnNi High-Entropy Alloy" Metals 12, no. 2: 191. https://doi.org/10.3390/met12020191
APA StyleWang, S., Chen, L., Li, Q., Wang, S., Wu, M., Yang, S., & Xiang, D. (2022). Effects of Al or Mo Addition on Microstructure and Mechanical Properties of Fe-Rich Nonequiatomic FeCrCoMnNi High-Entropy Alloy. Metals, 12(2), 191. https://doi.org/10.3390/met12020191






