Cytokine Profiling and Intra-Articular Injection of Autologous Platelet-Rich Plasma in Knee Osteoarthritis
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
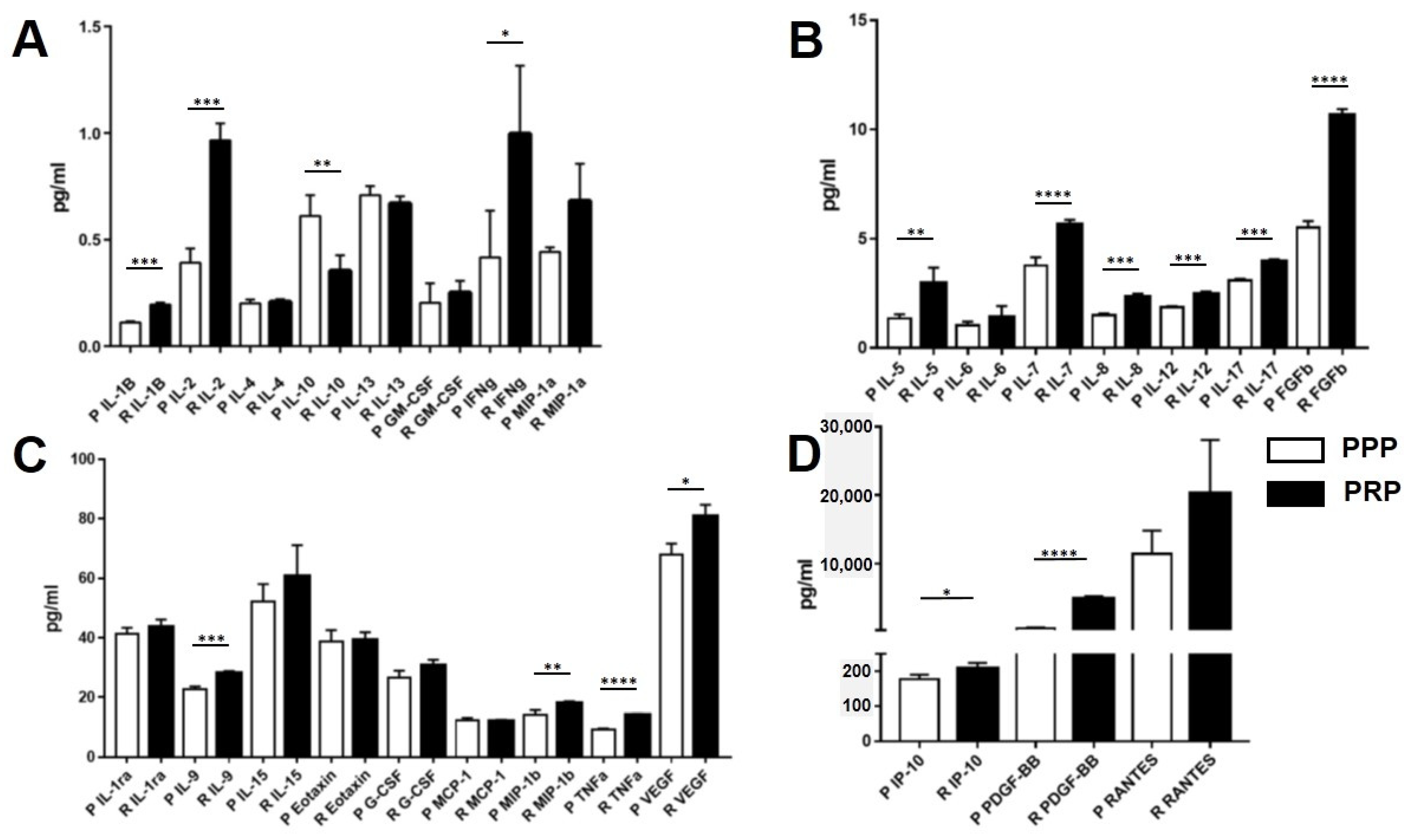
2.2. Growth Factor, Chemokine, and Cytokine Concentrations in PRP and PPP
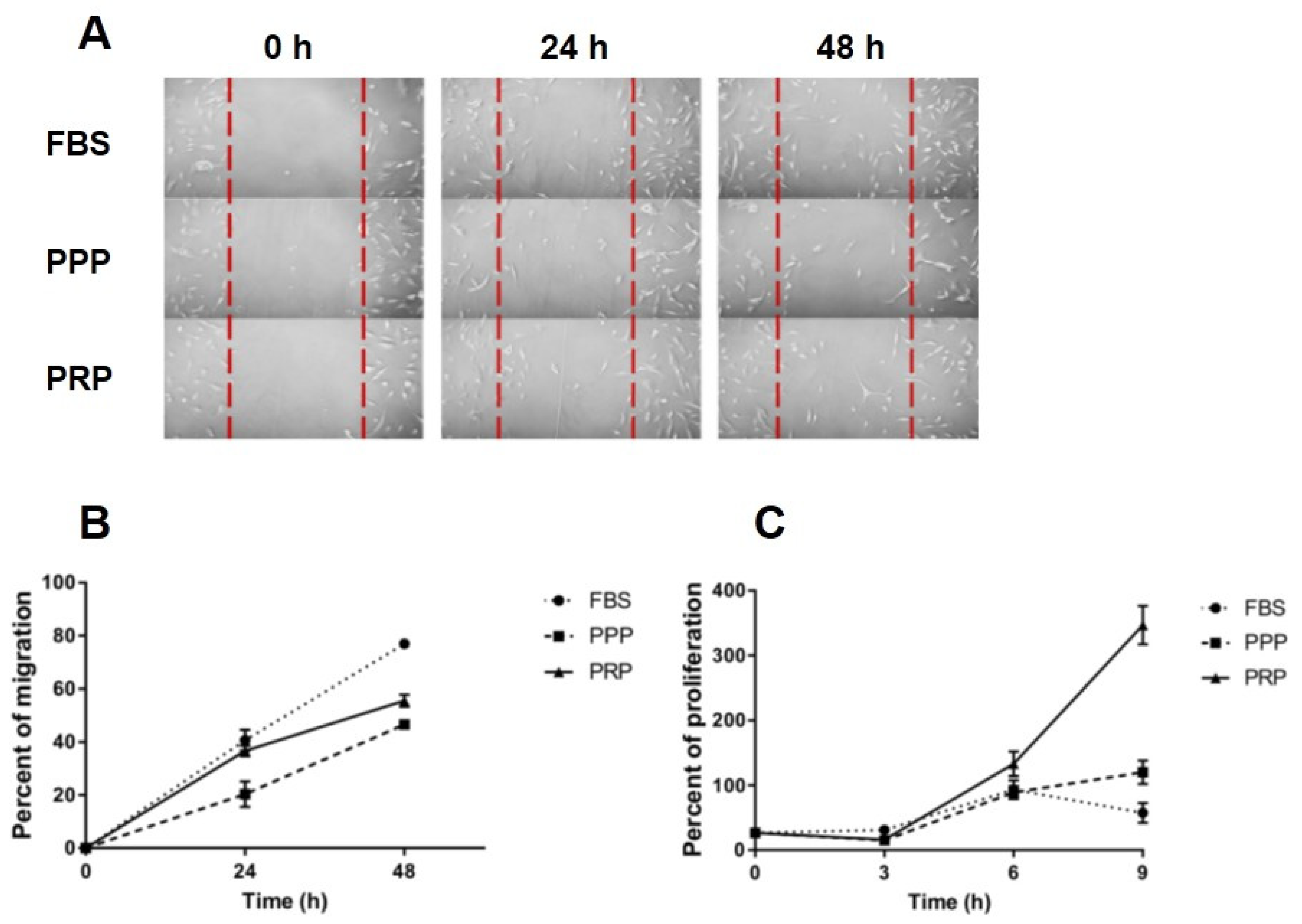
2.3. Effect of PRP on Migration of OA Chondrocytes
2.4. Effect of PRP on Proliferation of OA Chondrocytes
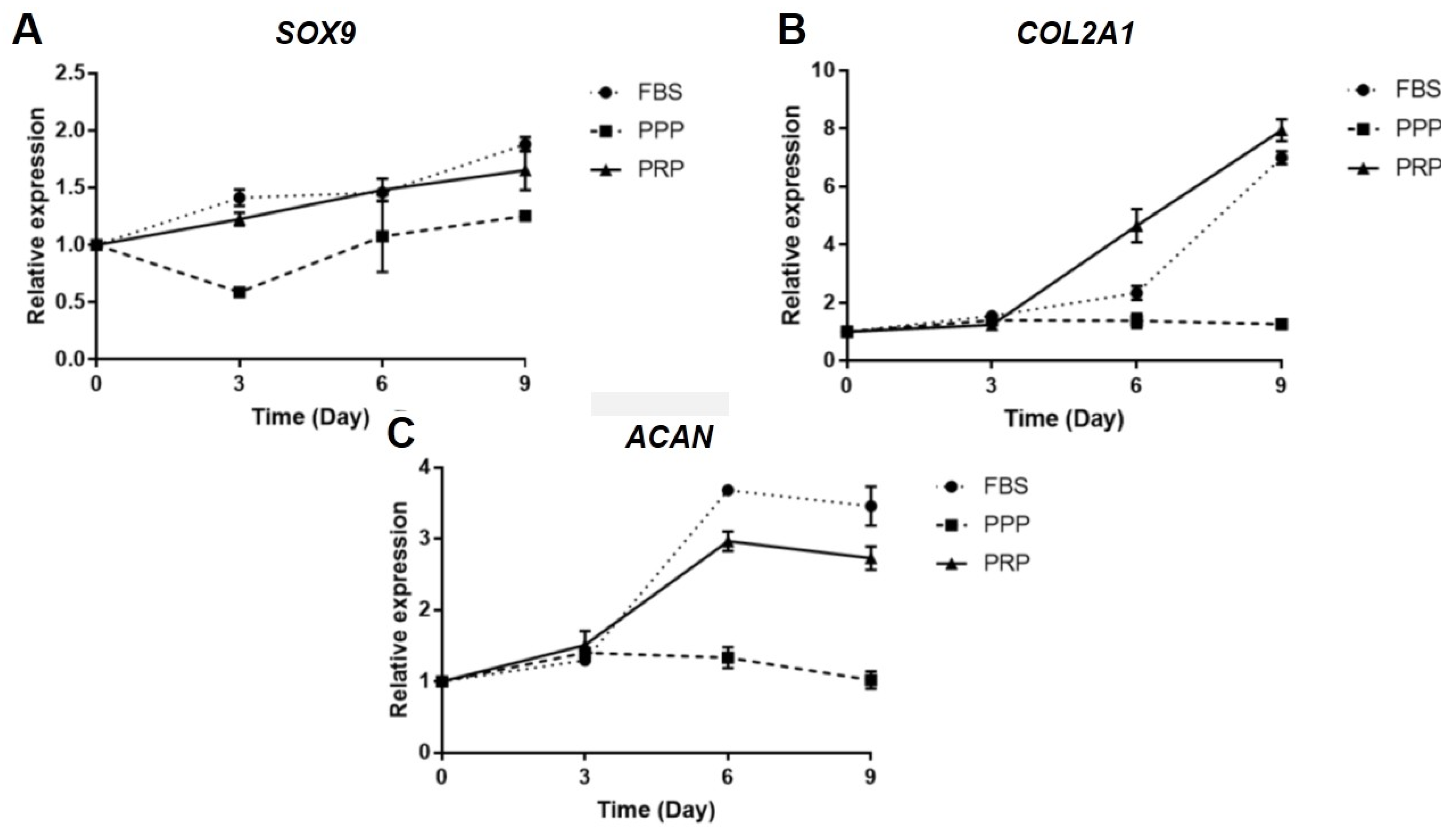
2.5. Effect of PRP on Gene Expression of OA Chondrocytes
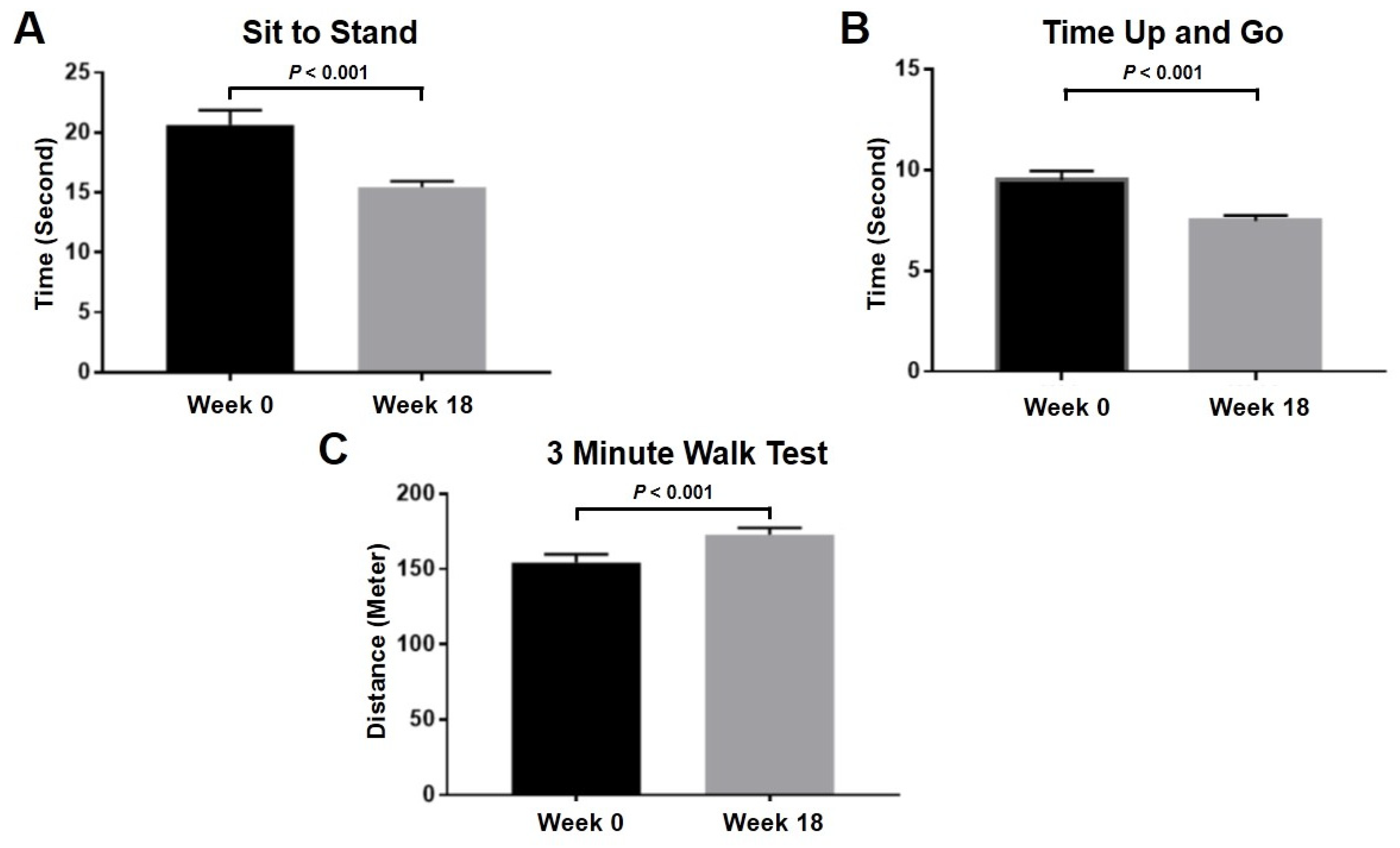
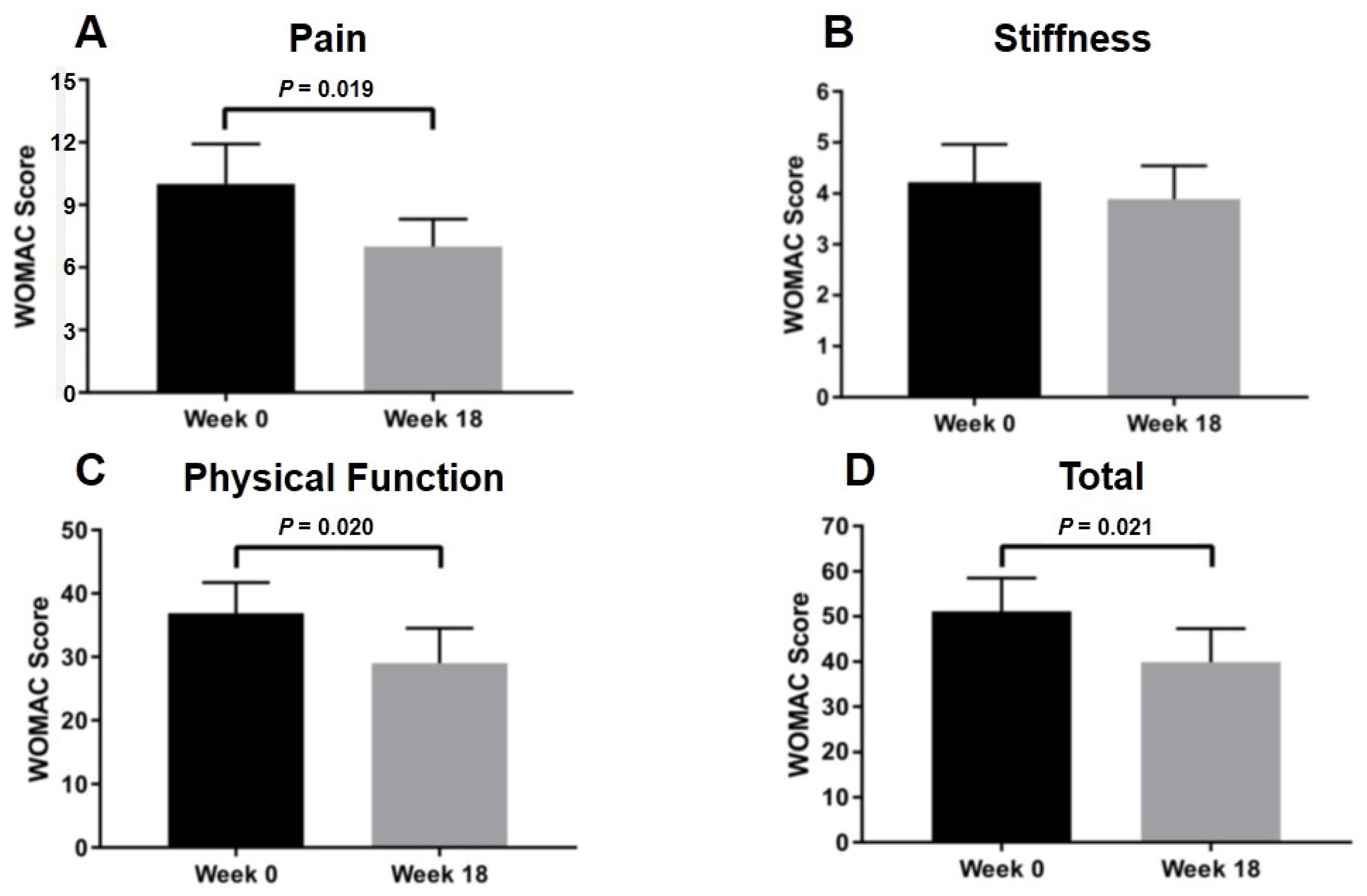
2.6. Clinical Evaluation after Intra-Articular PRP Injection in Knee OA Patients
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. PRP Preparation
4.3. Bio-Plex Pro Human Cytokine 27-Plex Assay
4.4. Chondrocyte Isolation and Culture
4.5. Effect of PRP on OA Chondrocyte Migration
4.6. Effect of PRP on OA Chondrocyte Proliferation
4.7. Effect of PRP on Gene Expression of OA Chondrocytes
4.8. Clinical Evaluation after Intra-Articular PRP Injection in Knee OA Patients
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACAN | Aggrecan |
| ACD-A | Acid-citrate dextrose anticoagulant |
| ACR | American College of Rheumatology |
| bFGF | Basic fibroblast growth factor |
| BMI | Body mass index |
| BMP | Bone morphogenetic protein |
| CaCl2 | Calcium chloride |
| COL2A1 | Collagen type II alpha 1 |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal bovine serum |
| GADPH | Glyceraldehyde 3-phosphate dehydrogenase |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| IL-1RA | IL-1 receptor antagonist |
| IP-10 | Interferon γ-induced protein-10 |
| KL | Kellgren and Lawrence |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MIP-1α | Macrophage inflammatory protein-1α |
| MMPs | Matrix metalloproteases |
| MTT | Methyl thiazolyl tetrazolium |
| NF-kB | Nuclear factor kappa b |
| OA | Osteoarthritis |
| PBS | Phosphate buffered saline |
| PCR | Polymerase chain reaction |
| PDGF | Platelet derived growth factor |
| PPP | Platelet-poor plasma |
| PRP | Platelet-rich plasma |
| RANTES | Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted |
| SOX9 | SRY-box transcription factor |
| TGF-β | Transforming growth factor-β |
| TKA | Total knee arthroplasty |
| TNF-α | Tumor necrosis factor-α |
| VAS | Visual analog scale |
| VEGF | Vascular endothelial growth factor |
| WOMAC | Western Ontario and McMaster Universities Arthritis Index |
References
- O’Brien, P.; Bunzli, S.; Lin, I.; Gunatillake, T.; Bessarab, D.; Coffin, J.; Garvey, G.; Dowsey, M.; Choong, P. Tackling the Burden of Osteoarthritis as a Health Care Opportunity in Indigenous Communities-A Call to Action. J. Clin. Med. 2020, 9, 2393. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990-2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef]
- Rai, D.; Singh, J.; Somashekharappa, T.; Singh, A. Platelet-rich plasma as an effective biological therapy in early-stage knee osteoarthritis: One year follow up. SICOT J. 2021, 7, 6. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.K.; Perry, T.A.; Bannuru, R.R.; Bruyère, O.; Cooper, C.; Haugen, I.K.; Hochberg, M.C.; McAlindon, T.E.; Mobasheri, A.; Reginster, J.Y. Non-surgical management of knee osteoarthritis: Comparison of ESCEO and OARSI 2019 guidelines. Nat. Rev. Rheumatol. 2021, 17, 59–66. [Google Scholar] [CrossRef]
- Colletti, A.; Cicero, A.F.G. Nutraceutical Approach to Chronic Osteoarthritis: From Molecular Research to Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 12920. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Matišić, V.; Hudetz, D.; Jeleč, Ž.; Rod, E.; Čukelj, F.; Vidović, D.; Vrdoljak, T.; Dobričić, B.; et al. Comprehensive Review of Knee Osteoarthritis Pharmacological Treatment and the Latest Professional Societies’ Guidelines. Pharmaceuticals 2021, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Cook, C.S.; Smith, P.A. Clinical Update: Why PRP Should Be Your First Choice for Injection Therapy in Treating Osteoarthritis of the Knee. Curr. Rev. Musculoskelet. Med. 2018, 11, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Le, A.D.K.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr. Rev. Musculoskelet. Med. 2018, 11, 624–634. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Huang, L.; Ma, Y.; Zhang, D.; Zhang, X.; Zhou, J.; Ruan, A.; Wang, Q. Intra-articular platelet-rich plasma injection for knee osteoarthritis: A summary of meta-analyses. J. Orthop. Surg. Res. 2019, 14, 385. [Google Scholar] [CrossRef]
- Jevsevar, D.S.; Brown, G.A.; Jones, D.L.; Matzkin, E.G.; Manner, P.A.; Mooar, P.; Schousboe, J.T.; Stovitz, S.; Sanders, J.; Bozic, K.J.; et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: Treatment of osteoarthritis of the knee, 2nd edition. J. Bone Jt. Surg. Am. 2013, 95, 1885–1886. [Google Scholar] [CrossRef]
- Hohmann, E.; Tetsworth, K.; Glatt, V. Is platelet-rich plasma effective for the treatment of knee osteoarthritis? A systematic review and meta-analysis of level 1 and 2 randomized controlled trials. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Rezuş, E.; Burlui, A.; Cardoneanu, A.; Macovei, L.A.; Tamba, B.I.; Rezuş, C. From Pathogenesis to Therapy in Knee Osteoarthritis: Bench-to-Bedside. Int. J. Mol. Sci. 2021, 22, 2697. [Google Scholar] [CrossRef]
- Filardo, G.; Previtali, D.; Napoli, F.; Candrian, C.; Zaffagnini, S.; Grassi, A. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage 2020, 13, 364S–375S. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Roffi, A.; Di Matteo, B.; Merli, M.L.; Marcacci, M. Platelet-rich plasma: Why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2459–2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.L.; Zhou, A.G.; Zhang, H.; Zhang, J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy 2017, 33, 659–670.e1. [Google Scholar] [CrossRef]
- Laudy, A.B.; Bakker, E.W.; Rekers, M.; Moen, M.H. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 657–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; Chen, H.; Zhao, L.; Huang, W. Platelet-Rich Plasma Versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Meta-analysis of 26 Randomized Controlled Trials. Arthroscopy 2021, 37, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Luo, X.; Xiong, Y.; Liu, G.; Wang, J.; Chen, X.; Mi, B. Platelet-rich plasma versus hyaluronic acid in knee osteoarthritis: A meta-analysis with the consistent ratio of injection. J. Orthop. Surg. 2020, 28, 2309499019887660. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, H.; Kantarci, A.; Deady, J.; Hasturk, H.; Liu, H.; Alshahat, M.; Van Dyke, T.E. Platelet-rich plasma: Growth factors and pro- and anti-inflammatory properties. J. Periodontol. 2007, 78, 661–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaseri, A.; Busch, F.; Mobasheri, A.; Buhrmann, C.; Aldinger, C.; Rad, J.S.; Shakibaei, M. IGF-1 and PDGF-bb suppress IL-1β-induced cartilage degradation through down-regulation of NF-κB signaling: Involvement of Src/PI-3K/AKT pathway. PLoS ONE 2011, 6, e28663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Su, G.; Wang, J.; Zhou, Z.; Li, L.; Liu, L.; Guan, M.; Zhang, Q.; Wang, H. Exogenous bFGF promotes articular cartilage repair via up-regulation of multiple growth factors. Osteoarthr. Cartil. 2013, 21, 1567–1575. [Google Scholar] [CrossRef] [Green Version]
- Woodell-May, J.E.; Sommerfeld, S.D. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. 2020, 38, 253–257. [Google Scholar] [CrossRef]
- Pereira, R.C.; Scaranari, M.; Benelli, R.; Strada, P.; Reis, R.L.; Cancedda, R.; Gentili, C. Dual effect of platelet lysate on human articular cartilage: A maintenance of chondrogenic potential and a transient proinflammatory activity followed by an inflammation resolution. Tissue Eng. Part A 2013, 19, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.C.; Morris, G.M.; Sokoloff, L. Effect of platelet lysate on growth and sulfated glycosaminoglycan synthesis in articular chondrocyte cultures. Arthritis Rheum. 1980, 23, 220–224. [Google Scholar] [CrossRef]
- Kaps, C.; Loch, A.; Haisch, A.; Smolian, H.; Burmester, G.R.; Häupl, T.; Sittinger, M. Human platelet supernatant promotes proliferation but not differentiation of articular chondrocytes. Med. Biol. Eng. Comput. 2002, 40, 485–490. [Google Scholar] [CrossRef]
- Kreuz, P.C.; Krüger, J.P.; Metzlaff, S.; Freymann, U.; Endres, M.; Pruss, A.; Petersen, W.; Kaps, C. Platelet-Rich Plasma Preparation Types Show Impact on Chondrogenic Differentiation, Migration, and Proliferation of Human Subchondral Mesenchymal Progenitor Cells. Arthroscopy 2015, 31, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Fearon, U.; Billinghurst, R.C.; Ionescu, M.; Reece, R.; Barwick, T.; Emery, P.; Poole, A.R.; Veale, D.J. Turnover of type II collagen and aggrecan in cartilage matrix at the onset of inflammatory arthritis in humans: Relationship to mediators of systemic and local inflammation. Arthritis Rheum. 2003, 48, 3085–3095. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Lefebvre, V. Unraveling the transcriptional regulatory machinery in chondrogenesis. J. Bone Miner. Metab. 2011, 29, 390–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, D.M.; Leung, K.K.; Wheatley, S.C.; Ng, L.J.; Zhou, S.; Ling, K.W.; Sham, M.H.; Koopman, P.; Tam, P.P.; Cheah, K.S. SOX9 directly regulates the type-II collagen gene. Nat. Genet. 1997, 16, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, V.; Niculescu-Morzsa, E.; Bauer, C.; Lacza, Z.; Nehrer, S. Platelet-Rich Plasma Supports Proliferation and Redifferentiation of Chondrocytes during In Vitro Expansion. Front. Bioeng. Biotechnol. 2017, 5, 75. [Google Scholar] [CrossRef]
- Lee, H.R.; Shon, O.J.; Park, S.I.; Kim, H.J.; Kim, S.; Ahn, M.W.; Do, S.H. Platelet-Rich Plasma Increases the Levels of Catabolic Molecules and Cellular Dedifferentiation in the Meniscus of a Rabbit Model. Int. J. Mol. Sci. 2016, 17, 120. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Yuan, T.; Chen, S.; Xie, X.; Zhang, C. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2017, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Luo, J.; Huang, X.; Wang, B.; Zhang, J.; Zhou, A. Efficacy of Platelet-Rich Plasma in Pain and Self-Report Function in Knee Osteoarthritis: A Best-Evidence Synthesis. Am. J. Phys. Med. Rehabil. 2017, 96, 793–800. [Google Scholar] [CrossRef]
- Park, M.S.; Moon, S.H.; Kim, T.H.; Oh, J.K.; Yoon, W.Y.; Chang, H.G. Platelet-rich plasma for the spinal fusion. J. Orthop. Surg. (Hong Kong) 2018, 26, 2309499018755772. [Google Scholar] [CrossRef] [Green Version]
- Nasirzade, J.; Kargarpour, Z.; Hasannia, S.; Strauss, F.J.; Gruber, R. Platelet-rich fibrin elicits an anti-inflammatory response in macrophages in vitro. J. Periodontol. 2020, 91, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Filardo, G.; Mariani, E.; Kon, E.; Marcacci, M.; Pereira Ruiz, M.T.; Facchini, A.; Grigolo, B. Comparison of platelet-rich plasma formulations for cartilage healing: An in vitro study. J. Bone Jt. Surg. Am. 2014, 96, 423–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudva, A.K.; Luyten, F.P.; Patterson, J. Initiating human articular chondrocyte re-differentiation in a 3D system after 2D expansion. J. Mater. Sci. Mater. Med. 2017, 28, 156. [Google Scholar] [CrossRef] [Green Version]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Olmos Calvo, I.; Fodor, E.; Kardos, D.; Hornyák, I.; Hinsenkamp, A.; Kuten-Pella, O.; Gyevnár, Z.; Erdélyi, G.; Bárdos, T.; Paukovits, T.M.; et al. A Pilot Clinical Study of Hyperacute Serum Treatment in Osteoarthritic Knee Joint: Cytokine Changes and Clinical Effects. Curr. Issues Mol. Biol. 2021, 43, 637–649. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riewruja, K.; Phakham, S.; Sompolpong, P.; Reantragoon, R.; Tanavalee, A.; Ngarmukos, S.; Udomsinprasert, W.; Suantawee, T.; Dechsupa, S.; Honsawek, S. Cytokine Profiling and Intra-Articular Injection of Autologous Platelet-Rich Plasma in Knee Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 890. https://doi.org/10.3390/ijms23020890
Riewruja K, Phakham S, Sompolpong P, Reantragoon R, Tanavalee A, Ngarmukos S, Udomsinprasert W, Suantawee T, Dechsupa S, Honsawek S. Cytokine Profiling and Intra-Articular Injection of Autologous Platelet-Rich Plasma in Knee Osteoarthritis. International Journal of Molecular Sciences. 2022; 23(2):890. https://doi.org/10.3390/ijms23020890
Chicago/Turabian StyleRiewruja, Kanyakorn, Suphattra Phakham, Patlapa Sompolpong, Rangsima Reantragoon, Aree Tanavalee, Srihatach Ngarmukos, Wanvisa Udomsinprasert, Tanyawan Suantawee, Sinsuda Dechsupa, and Sittisak Honsawek. 2022. "Cytokine Profiling and Intra-Articular Injection of Autologous Platelet-Rich Plasma in Knee Osteoarthritis" International Journal of Molecular Sciences 23, no. 2: 890. https://doi.org/10.3390/ijms23020890
APA StyleRiewruja, K., Phakham, S., Sompolpong, P., Reantragoon, R., Tanavalee, A., Ngarmukos, S., Udomsinprasert, W., Suantawee, T., Dechsupa, S., & Honsawek, S. (2022). Cytokine Profiling and Intra-Articular Injection of Autologous Platelet-Rich Plasma in Knee Osteoarthritis. International Journal of Molecular Sciences, 23(2), 890. https://doi.org/10.3390/ijms23020890





