Benefits on Hematological and Biochemical Parameters of a High-Intensity Interval Training Program for a Half-Marathon in Recreational Middle-Aged Women Runners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Training Programs
2.3. Blood Sampling and Preparations
2.4. Biochemical Assays
2.5. Statistical Analysis
3. Results
3.1. Hematological Parameters
3.2. Plasma Solute Concentrations
3.3. Liver and Lipid panel
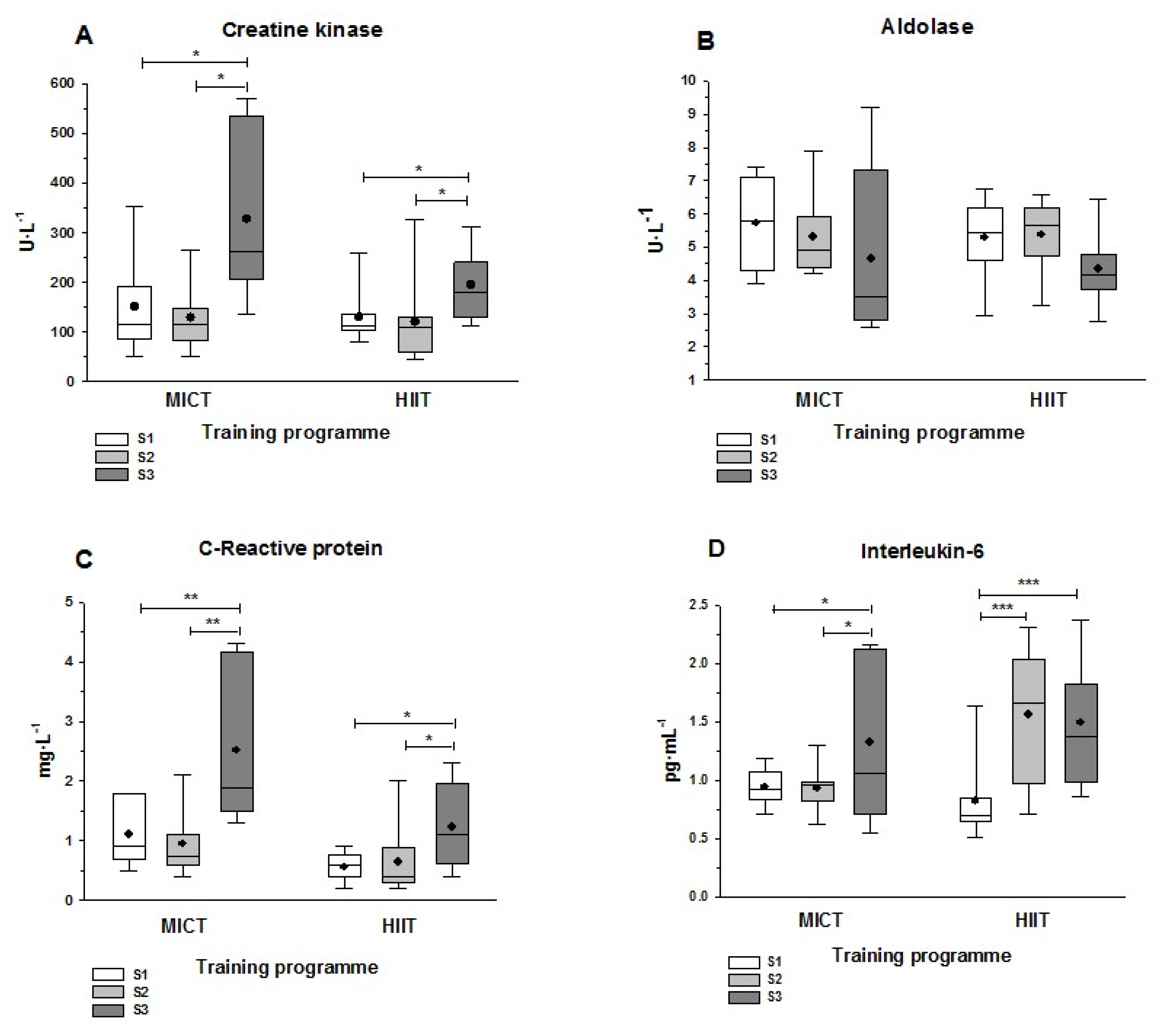
3.4. Muscle Damage and Inflammatory Markers
3.5. Oxidative Stress-Related Parameters
4. Discussion
4.1. Hematological Parameters
4.2. Plasma Solute Concentrations
4.3. Liver and Lipid Panel
4.4. Muscle Damage and Inflammation Markers
4.5. Markers of Oxidative Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Knechtle, B.; Nikolaidis, P.T.; Zingg, M.A.; Rosemann, T.; Christoph, A.; Rüst, C.A. Half-marathoners are younger and slower than marathoners. SpringerPlus 2016, 5, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Running USA. 2020 U.S. Running Trends Report. Royal Oak, MI. Available online: https://www.wpr.org/sites/default/files/running_usa_trends_report_2019-r4.pdf (accessed on 4 November 2021).
- Joyner, M.J. Physiological limits to endurance exercise performance: Influence of sex. J. Physiol. 2017, 595, 2949–2954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, W.; Prommer, N. Impact of Alterations in Total Hemoglobin Mass on O2max. Exerc. Sport Sci. Rev. 2010, 38, 68–75. [Google Scholar] [CrossRef]
- Pate, R.R.; O’Neill, J.R. American Women in the Marathon. Sports Med. 2007, 37, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Boccia, G.; Dardanello, D.; Tarperi, C.; Festa, L.; La Torre, A.; Pellegrini, B.; Schena, F.; Rainoldi, A. Women show similar central and peripheral fatigue to men after half-marathon. Eur. J. Sport Sci. 2018, 18, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Deaner, R.O.; Carter, R.E.; Joyner, M.J.; Hunter, S.K. Men Are More Likely than Women to Slow in the Marathon. Med. Sci. Sports Exerc. 2015, 47, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roepstorff, C.; Steffensen, C.H.; Madsen, M.; Stallknecht, B.M.; Kanstrup, I.-L.; Richter, E.A.; Kiens, B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am. J. Physiol. Metab. 2002, 282, E435–E447. [Google Scholar] [CrossRef] [Green Version]
- Devries, M.C. Sex-based differences in endurance exercise muscle metabolism: Impact on exercise and nutritional strategies to optimize health and performance in women. Exp. Physiol. 2015, 101, 243–249. [Google Scholar] [CrossRef]
- Kyröläinen, H.; Hackney, A.C.; Salminen, R.; Repola, J.; Häkkinen, K.; Haimi, J. Effects of Combined Strength and Endurance Training on Physical Performance and Biomarkers of Healthy Young Women. J. Strength Cond. Res. 2018, 32, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Reihmane, D.; Jurka, A.; Tretjakovs, P.; Dela, F. Increase in IL-6, TNF-α, and MMP-9, but not sICAM-1, concentrations depends on exercise duration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 113, 851–858. [Google Scholar] [CrossRef]
- Lippi, G.; Schena, F.; Dipalo, M.; Montagnana, M.; Salvagno, G.L.; Aloe, R.; Guidi, G.C. Troponin I measured with a high sensitivity immunoassay is significantly increased after a half marathon run. Scand. J. Clin. Lab. Investig. 2012, 72, 467–470. [Google Scholar] [CrossRef]
- Jassal, D.S.; Moffat, D.; Krahn, J.; Ahmadie, R.; Fang, T.; Eschun, G.; Sharma, S. Cardiac Injury Markers in Non-elite Marathon Runners. Int. J. Sports Med. 2009, 30, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Vieira, R.D.P.; Bischof, F.; Walter, M.; Movassaghi, M.; Berchtold, N.C.; Niess, A.M.; Cotman, C.W.; Northoff, H. Sex-specific variation in signaling pathways and gene expression patterns in human leukocytes in response to endotoxin and exercise. J. Neuroinflammation 2016, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippi, G.; Schena, F.; Salvagno, G.L.; Montagnana, M.; Gelati, M.; Tarperi, C.; Banfi, G.; Guidi, G.C. Acute variation of biochemical markers of muscle damage following a 21-km, half-marathon run. Scand. J. Clin. Lab. Investig. 2008, 68, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Banfi, G.; Montagnana, M.; Salvagno, G.L.; Schena, F.; Guidi, G.C. Acute variation of leucocytes counts following a half-marathon run. Int. J. Lab. Hematol. 2010, 32, 117–121. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Danese, E.; Tarperi, C.; Guidi, G.C.; Schena, F. Variation of Red Blood Cell Distribution Width and Mean Platelet Volume after Moderate Endurance Exercise. Adv. Hematol. 2014, 2014, 192173. [Google Scholar] [CrossRef] [Green Version]
- Bonet, J.; Magalhães, J.; Viscor, G.; Pagès, T.; Javierre, C.; Torrella, J.R. High-intensity interval versus moderate-intensity continuous half-marathon training programme for middle-aged women. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 120, 1083–1096. [Google Scholar] [CrossRef]
- Sellami, M.; Al-Muraikhy, S.; Al-Jaber, H.; Al-Amri, H.; Al-Mansoori, L.; Mazloum, N.A.; Donati, F.; Botre, F.; Elrayess, M.A. Age and Sport Intensity-Dependent Changes in Cytokines and Telomere Length in Elite Athletes. Antioxidants 2021, 10, 1035. [Google Scholar] [CrossRef] [PubMed]
- Minuzzi, L.G.; Rama, L.; Bishop, N.C.; Rosado, F.; Martinho, A.; Paiva, A.; Teixeira, A.M. Lifelong training improves anti-inflammatory environment and maintains the number of regulatory T cells in masters athletes. Eur. J. Appl. Physiol. Occup. Physiol. 2017, 37, 1131–1140. [Google Scholar] [CrossRef] [Green Version]
- Galloway, J.; Galloway, B. Half-Marathon: A Complete Guide for Women, 1st ed.; Meyer and Meyer Sport: Maidenhead, UK, 2012. [Google Scholar]
- Duca, L.; Da Ponte, A.; Cozzi, M.; Carbone, A.; Pomati, M.; Nava, I.; Cappellini, M.D.; Fiorelli, G. Changes in erythropoiesis, iron metabolism and oxidative stress after half-marathon. Intern. Emerg. Med. 2006, 1, 30–34. [Google Scholar] [CrossRef]
- Niemelä, M.; Kangastupa, P.; Niemelä, O.; Bloigu, R.; Juvonen, T. Acute Changes in Inflammatory Biomarker Levels in Recreational Runners Participating in a Marathon or Half-Marathon. Sports Med.-Open 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Withee, E.D.; Tippens, K.M.; Dehen, R.; Tibbitts, D.; Hanes, D.; Zwickey, H. Effects of Methylsulfonylmethane (MSM) on exercise-induced oxidative stress, muscle damage, and pain following a half-marathon: A double-blind, randomized, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2017, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lynn, A.; Garner, S.; Nelson, N.; Simper, T.N.; Hall, A.C.; Ranchordas, M.K. Effect of bilberry juice on indices of muscle damage and inflammation in runners completing a half-marathon: A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiewelhove, T.; Schneider, C.; Döweling, A.; Hanakam, F.; Rasche, C.; Meyer, T.; Kellmann, M.; Pfeiffer, M.; Ferrauti, A. Effects of different recovery strategies following a half-marathon on fatigue markers in recreational runners. PLOS ONE 2018, 13, e0207313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telford, R.; Sly, G.J.; Hahn, A.G.; Cunningham, R.B.; Bryant, C.; Smith, J.A. Footstrike is the major cause of hemolysis during running. J. Appl. Physiol. 2003, 94, 38–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.A. Exercise, Training and Red Blood Cell Turnover. Sports Med. 1995, 19, 9–31. [Google Scholar] [CrossRef]
- Schmidt, W.; Maassen, N.; Tegtbur, U.; Braumann, K.M. Changes in plasma volume and red cell formation after a marathon competition. Graefe’s Arch. Clin. Exp. Ophthalmol. 1989, 58, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Röcker, L.; Kirsch, K.A.; Heyduck, B.; Altenkirch, H.-U. Influence of Prolonged Physical Exercise on Plasma Volume, Plasma Proteins, Electrolytes, and Fluid-Regulating Hormones*. Endosc. 1989, 10, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Fellmann, N. Hormonal and Plasma Volume Alterations Following Endurance Exercise. Sports Med. 1992, 13, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.L.; McCammon, M.; Smith, S.; Chamness, M.; Israel, R.G.; O’Brien, K.F. White blood cell response to uphill walking and downhill jogging at similar metabolic loads. Graefe’s Arch. Clin. Exp. Ophthalmol. 1989, 58, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Pizza, F.; Mitchell, J.B.; Davis, B.H.; Starling, R.D.; Holtz, R.W.; Bigelow, N. Exercise-induced muscle damage: Effect on circulating leukocyte and lymphocyte subsets. Med. Sci. Sports Exerc. 1995, 27, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Traiperm, N.; Gatterer, H.; Burtscher, M. Plasma electrolyte and hematological changes after marathon running in adolescents. Med. Sci. Sports Exerc. 2013, 45, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, G.L.; Tarperi, C.; Gelati, M.; Montagnana, M.; Danese, E.; Festa, L.; Sanchis-Gomar, F.; Favaloro, E.J.; Schena, F.; Lippi, G. Prothrombotic State Induced by Middle-Distance Endurance Exercise in Middle-Aged Athletes. Semin. Thromb. Hemost. 2018, 44, 747–755. [Google Scholar] [CrossRef]
- Neergaard-Petersen, S.; Hvas, A.-M. Influence of Exercise on Platelet Function in Patients with Cardiovascular Disease. Semin. Thromb. Hemost. 2018, 44, 802–812. [Google Scholar] [CrossRef]
- Montero, D.; Lundby, C. Regulation of Red Blood Cell Volume with Exercise Training. Compr. Physiol. 2018, 9, 149–164. [Google Scholar] [CrossRef]
- Rhibi, F.; Prioux, J.; Ben Attia, M.; Hackney, A.C.; Zouhal, H.; Ben Abderrahman, A. Increase interval training intensity improves plasma volume variations and aerobic performances in response to intermittent exercise. Physiol. Behav. 2018, 199, 137–145. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Haykowsky, M.J.; Quinney, H.A.; Blackmore, D.; Teo, K.K.; Taylor, D.A.; McGavock, J.; Humen, D.P. Blood Volume Expansion and Cardiorespiratory Function: Effects of Training Modality. Med. Sci. Sports Exerc. 2004, 36, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Vincent, S.; Moussa, E.; Jacob, C.; Groussard, C.; Ben Abderrahaman, A.; Prioux, J. Influence of training status on plasma volume variations and plasma lactate concentrations in response to supramaximal exercise. Biol. Sport 2007, 24, 339–356. [Google Scholar]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2018, 43, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergström, J.P.; Larsson, J.; Nordström, H.; Vinnars, E.; Askanazi, J.; Elwyn, D.H.; Kinney, J.M.; Fürst, P.J. Influence of injury and nutrition on muscle water and electrolytes: Effect of severe injury, burns and sepsis. Acta Chir. Scand. 1987, 153, 261–266. [Google Scholar] [PubMed]
- Hoppel, F.; Calabria, E.; Pesta, D.; Kantner-Rumplmair, W.; Gnaiger, E.; Burtscher, M. Physiological and Pathophysiological Responses to Ultramarathon Running in Non-elite Runners. Front. Physiol. 2019, 10, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosner, M.H.; Kirven, J. Exercise-Associated Hyponatremia. Clin. J. Am. Soc. Nephrol. 2006, 2, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-J.; Chen, K.-T.; Shee, B.-W.; Chang, H.-C.; Huang, Y.-J.; Yang, R.-S. Effects of 24 h ultra-marathon on biochemical and hematological parameters. World J. Gastroenterol. 2004, 10, 2711–2714. [Google Scholar] [CrossRef]
- Välimäki, I.A.; Vuorimaa, T.; Ahotupa, M.; Vasankari, T. Effect of Continuous and Intermittent Exercises on Oxidised HDL and LDL Lipids in Runners. Int. J. Sports Med. 2016, 37, 1103–1109. [Google Scholar] [CrossRef]
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Eccentric Exercise: Physiological Characteristics and Acute Responses. Sports Med. 2016, 47, 663–675. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Hyldahl, R.D.; Chen, T.C.; Nosaka, K. Mechanisms and Mediators of the Skeletal Muscle Repeated Bout Effect. Exerc. Sport Sci. Rev. 2017, 45, 24–33. [Google Scholar] [CrossRef]
- Bonet, J.B.; Magalhães, J.; Viscor, G.; Pagès, T.; Ventura, J.L.; Torrella, J.R.; Javierre, C. Inter-Individual Different Responses to Continuous and Interval Training in Recreational Middle-Aged Women Runners. Front. Physiol. 2020, 11, 579835. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Pinho, R. Effects of reactive oxygen species and interplay of antioxidants during physical exercise in skeletal muscles. J. Physiol. Biochem. 2018, 74, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Di Penta, A.; Moreno, B.; Reix, S.; Fernandez-Diez, B.; Villanueva, M.; Errea, O.; Escala, N.; Vandenbroeck, K.; Comella, J.; Villoslada, P. Oxidative Stress and Proinflammatory Cytokines Contribute to Demyelination and Axonal Damage in a Cerebellar Culture Model of Neuroinflammation. PLoS ONE 2013, 8, e54722. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| MICT (n = 10) | HIIT (n = 10) | |||
|---|---|---|---|---|
| r | p | r | p | |
| Creatine kinase | −0.567 | 0.240 | −0.263 | 0.434 |
| Aldolase | −0.533 | 0.173 | 0.439 | 0.177 |
| C-Reactive protein | 0.047 | 0.929 | −0.635 | 0.091 |
| Interleukin-6 | −0.045 | 0.896 | −0.263 | 0.434 |
| TAS | −0.264 | 0.567 | 0.099 | 0.773 |
| SOD | 0.426 | 0.341 | −0.128 | 0.724 |
| TGSH | −0.571 | 0.236 | −0.431 | 0.394 |
| MICT (n = 10) | HIIT (n = 10) | |||||
|---|---|---|---|---|---|---|
| Hematimetric Indices | S1 | S2 | S3 | S1 | S2 | S3 |
| MCV (fL) | 85.6 ± 6.1 | 86.2 ± 5.9 | 86.4 ± 5.4 | 84.8 ± 3.6 | 85.1 ± 3.3 | 85.5 ± 3.4 * |
| MCH (pg) | 28.4 ± 2.3 | 27.2 ± 2.5 *** | 27.4 ± 2.2 *** | 28.5 ± 1.6 | 28.2 ± 1.7 | 27.2 ± 1.6 ***### |
| MCHC (g·dL−1) | 33.2 ± 0.8 | 31.5 ± 0.9 *** | 31.7 ± 1.1 *** | 33.8 ± 0.9 | 33.2 ± 1.3 | 31.9 ± 0.8 ***### |
| Formed elements (103·µL−1) | ||||||
| WBC | 5.95 ± 1.16 | 5.83 ± 1.12 | 6.10 ± 0.96 | 5.92 ± 1.45 | 5.43 ± 1.07 | 6.82 ± 1.54 # |
| PLT | 218 ± 45 | 218 ± 61 | 190 ± 36 *# | 209 ± 53 | 217 ± 42 | 181 ± 40 **## |
| MICT (n = 10) | HIIT (n = 10) | |||||
|---|---|---|---|---|---|---|
| Liver Panel | S1 | S2 | S3 | S1 | S2 | S3 |
| Total protein (g·L−1) | 67.2 ± 7.8 | 63.7 ± 8.5 | 48.6 ± 17.4 ***## | 72.0 ± 4.2 | 68.7 ± 4.3 | 60.2 ± 15.3 * |
| Albumin (g·L−1) | 40.9 ± 3.5 | 38.1 ± 4.9 | 28.8 ± 9.6 ***## | 42.2 ± 3.1 | 40.0 ± 2.6 | 35.6 ± 9.5 * |
| Bilirubin (mg·L−1) | 0.59 ± 0.15 | 0.47 ± 0.28 | 0.58 ± 0.41 | 0.49 ± 0.22 | 0.45 ± 0.13 | 0.56 ± 0.27 |
| AST (U·L−1) | 23.7 ± 5.2 | 24.4 ± 4.6 | 24.8 ± 11.0 | 22.1 ± 3.7 | 22.4 ± 4.4 | 23.5 ± 4.6 |
| ALT (U·L−1) | 12.9 ± 7.7 | 12.4 ± 3.6 | 12.7 ± 6.7 | 11.1 ± 2.5 | 10.3 ± 4.6 | 9.8 ± 2.4 |
| GGT (U·L−1) | 14.9 ± 5.4 | 17.1 ± 8.2 | 12.4 ± 7.9 | 12.9 ± 3.6 | 13.4 ± 4.5 | 12.0 ± 3.3 |
| Lipid panel (mg·L−1) | ||||||
| Total cholesterol | 193 ± 34 | 187 ± 32 | 131 ± 33 ***## | 188 ± 25 | 187 ± 32 | 157 ± 33 ***## |
| HDL | 63.3 ± 8.8 | 57.9 ± 9.5 | 51.9 ± 18.9 * | 61.9 ± 11.6 | 63.2 ± 9.3 | 61.9 ± 15.5 |
| LDL | 118 ± 29 | 113 ± 28 | 72 ± 14 ***## | 113 ± 25 | 114 ± 27 | 86 ± 21 ***### |
| TAG | 62.0 ± 17.2 | 74.1 ± 16.7 | 44.3 ± 13.0 ***## | 66.7 ± 20.2 | 58.5 ± 18.5 | 54.1 ± 15.4 |
| MICT (n = 10) | HIIT (n = 10) | |||||
|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S1 | S2 | S3 | |
| TAS (mmol·L−1) | 1.29 ± 0.11 | 1.35 ± 0.19 | 1.27 ± 0.15 | 1.29 ± 0.14 | 1.40 ± 0.18* | 1.27 ±0.14 # |
| Enzymes (U·L−1) | ||||||
| SOD | 15.4 ± 0.7 | 15.1 ± 0.9 | 16.4 ± 0.8 *# | 15.6 ± 1.8 | 16.2 ± 1.5 | 16.0 ± 1.5 |
| GPx | 986 ± 78 | 953 ± 73 | 935 ± 84 | 846 ± 55 | 849 ± 76 | 839 ± 64 |
| GR | 58.1 ± 6.8 | 59.7 ± 5.7 | 60.0 ± 7.3 | 59.2 ± 5.2 | 56.7 ± 4.6 | 59.6 ± 5.0 |
| Glutathione (nmol·mL−1) | ||||||
| TGSH | 4.85 ± 0.85 | 6.05 ± 1.42 | 8.85 ± 1.25 ***## | 5.78 ± 0.99 | 5.06 ± 1.01 | 8.59 ± 1.25 ***## |
| GSH | 4.60 ± 0.44 | 5.53 ± 1.59 | 8.11 ± 1.44 ***## | 5.42 ± 0.28 | 5.09 ± 1.06 | 8.26 ± 1.25 ***### |
| GSSG | 0.305 ± 1.21 | 0.200 ± 0.092 | 0.453 ± 0.12 ** | 0.385 ± 0.152 | 0.116 ± 0.05 ** | 0.318 ± 0.181 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonet, J.B.; Javierre, C.; Guimarães, J.T.; Martins, S.; Rizo-Roca, D.; Beleza, J.; Viscor, G.; Pagès, T.; Magalhães, J.; Torrella, J.R. Benefits on Hematological and Biochemical Parameters of a High-Intensity Interval Training Program for a Half-Marathon in Recreational Middle-Aged Women Runners. Int. J. Environ. Res. Public Health 2022, 19, 498. https://doi.org/10.3390/ijerph19010498
Bonet JB, Javierre C, Guimarães JT, Martins S, Rizo-Roca D, Beleza J, Viscor G, Pagès T, Magalhães J, Torrella JR. Benefits on Hematological and Biochemical Parameters of a High-Intensity Interval Training Program for a Half-Marathon in Recreational Middle-Aged Women Runners. International Journal of Environmental Research and Public Health. 2022; 19(1):498. https://doi.org/10.3390/ijerph19010498
Chicago/Turabian StyleBonet, Jèssica B., Casimiro Javierre, João Tiago Guimarães, Sandra Martins, David Rizo-Roca, Jorge Beleza, Ginés Viscor, Teresa Pagès, José Magalhães, and Joan R. Torrella. 2022. "Benefits on Hematological and Biochemical Parameters of a High-Intensity Interval Training Program for a Half-Marathon in Recreational Middle-Aged Women Runners" International Journal of Environmental Research and Public Health 19, no. 1: 498. https://doi.org/10.3390/ijerph19010498
APA StyleBonet, J. B., Javierre, C., Guimarães, J. T., Martins, S., Rizo-Roca, D., Beleza, J., Viscor, G., Pagès, T., Magalhães, J., & Torrella, J. R. (2022). Benefits on Hematological and Biochemical Parameters of a High-Intensity Interval Training Program for a Half-Marathon in Recreational Middle-Aged Women Runners. International Journal of Environmental Research and Public Health, 19(1), 498. https://doi.org/10.3390/ijerph19010498








