Insight on Polyunsaturated Fatty Acids in Endometrial Receptivity
Abstract
:1. Introduction
2. Materials and Methods
3. Endometrial Receptivity in Different Mammals
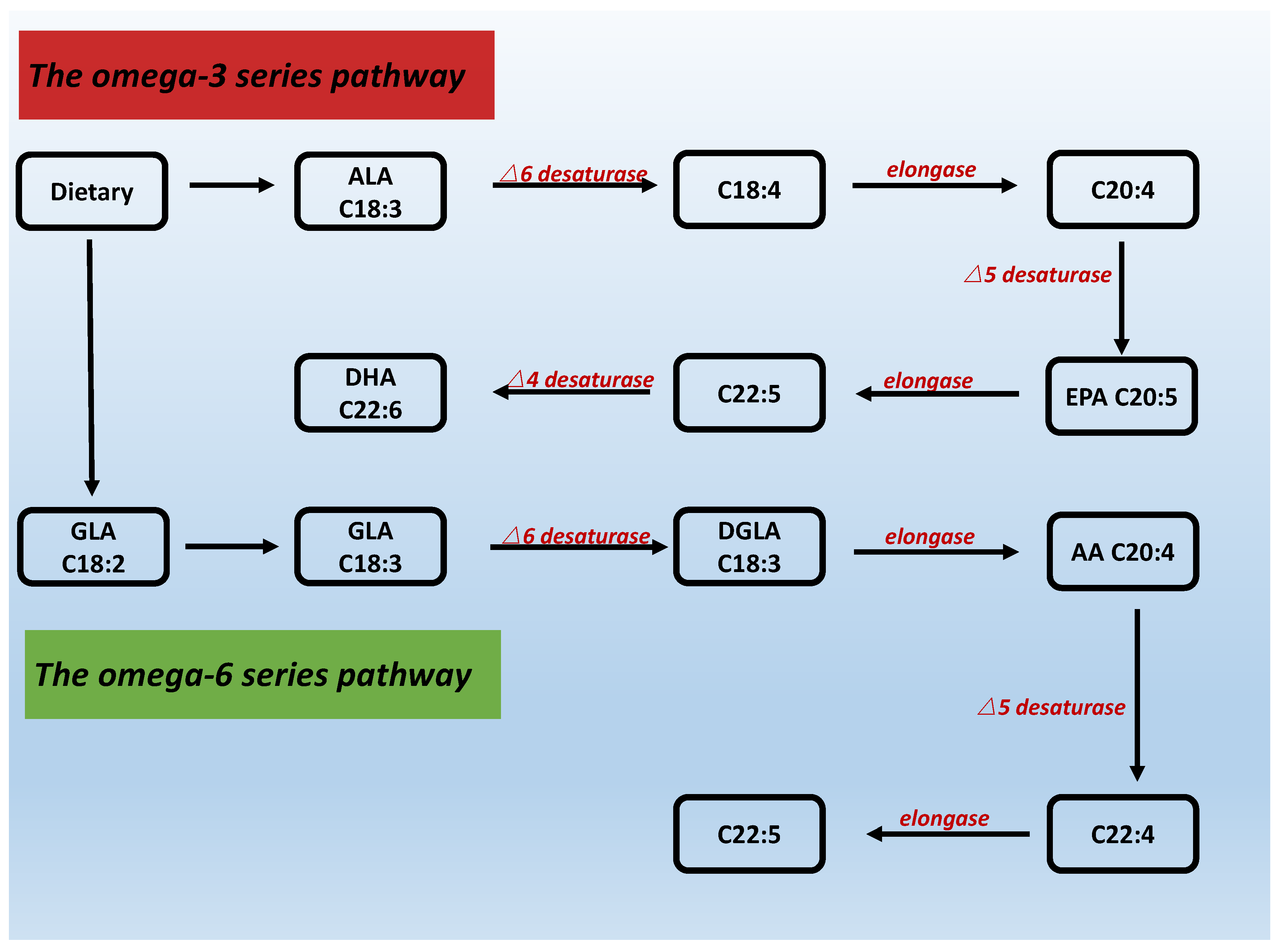
4. Structure, Synthesis and Classification of PUFAs
5. PUFAs, PGs, COX-2, COX-1 and Endometrial Receptivity
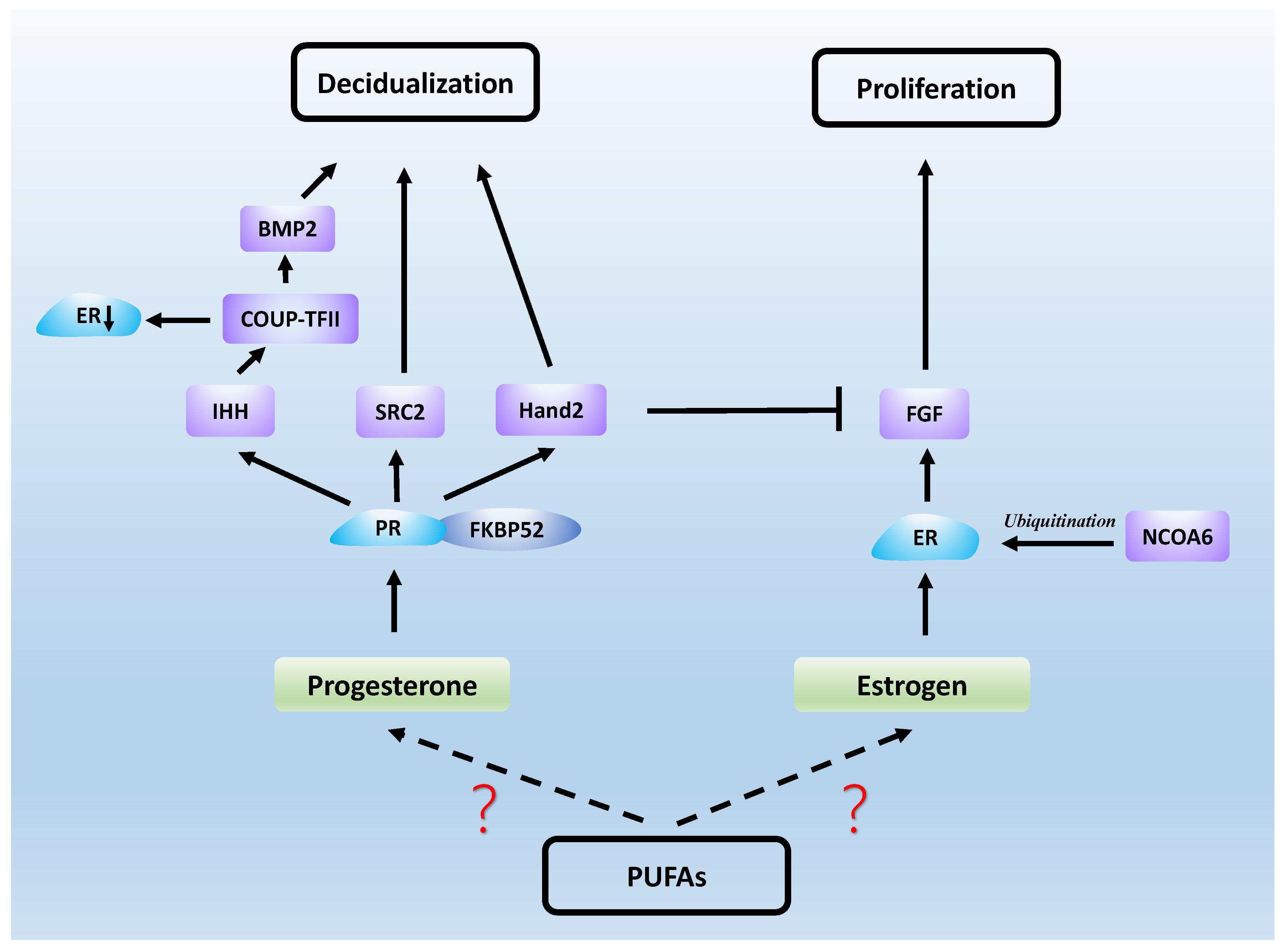
6. PUFAs, Sex Hormones and Endometrial Receptivity
6.1. Estrogen
6.2. Progesterone
6.3. Estrogen and Progesterone Signaling in Endometrial Receptivity
6.4. Androgen
7. PUFAs and Decidualization
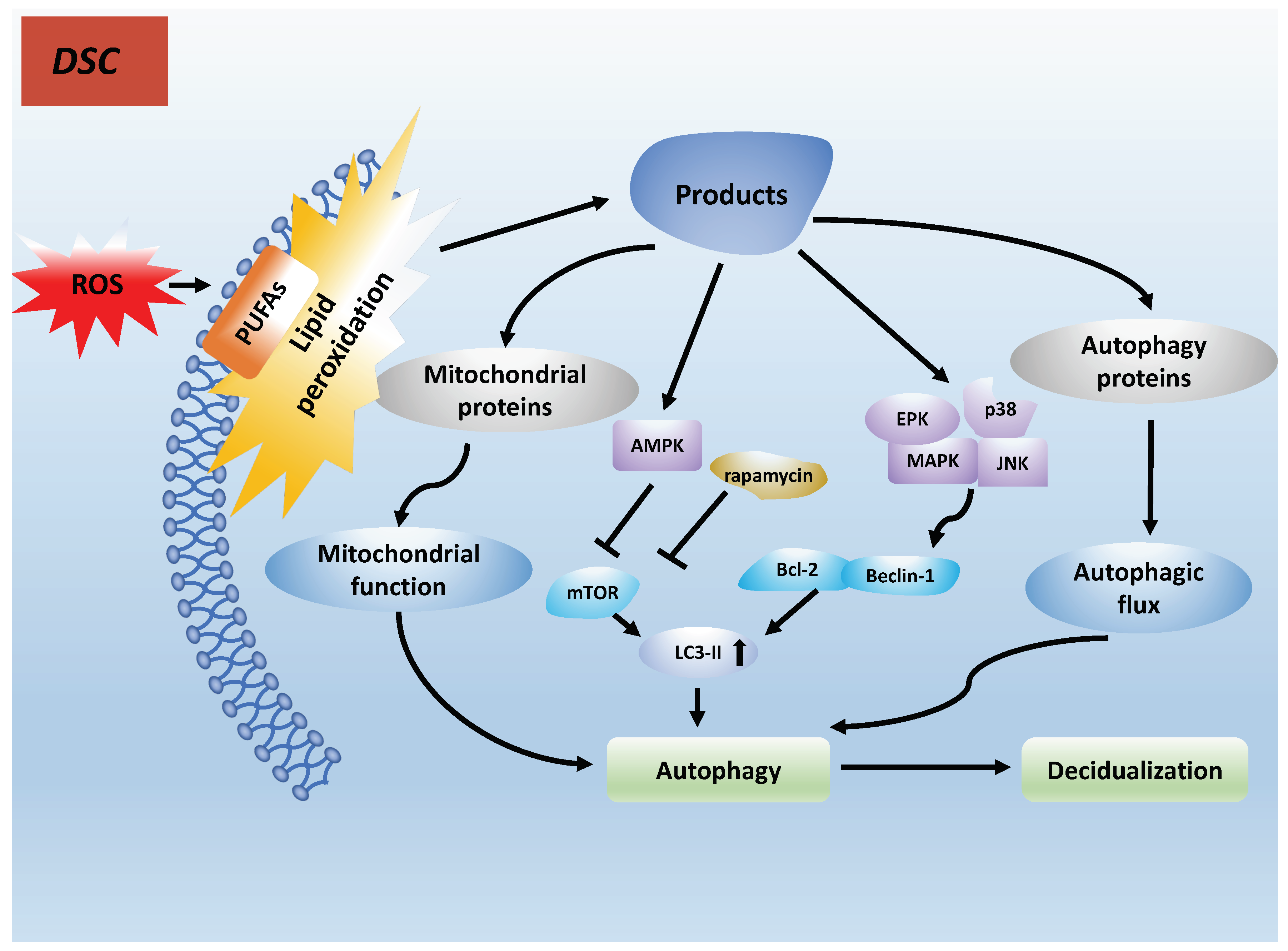
7.1. PUFAs Induce Autophagy in Decidualization by Lipid Peroxidation
7.2. PUFAs Acting on the Receptor GPR120 Result in Decidualization
7.3. PUFAs, COX-2 and Decidualization
8. LPA and S1P Can Influence the Endometrial Receptivity
8.1. LPA
8.2. SIP
9. PUFAs in Immune Regulation
9.1. Immune Cells
9.2. Inflammation and Cytokines
9.3. Adhesion Molecules
10. Hypothalamic-Pituitary-Adrenal(HPA)axis, PUFAs and Endometrial Receptivity
11. PUFAs and IVF Treatment
12. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ART | Assisted reproductive technology; |
| AA | Arachidonic acid; |
| ALA | Alpha-linolenic acid; |
| AR | Androgen receptor; |
| BMP2 | Bone morphogenetic protein 2; |
| CAM | Cell adhesion molecule; |
| COUP-TFII | Chicken ovalbumin upstream promoter transcription factor-2; |
| COX | Cyclooxygenase; |
| cPLA2 | cytoplasmic phospholipase A2; |
| DCs | Dendritic cells; |
| DHA | Docosahexaenoic acid; |
| DHEA | Dehydroepiandrosterone; |
| dNK | Decidual natural killer; |
| DSCs | Decidual stromal cells; |
| EPA | Eicosapentaenoic acid; |
| ER | Estrogen receptor; |
| ESCs | Endometrial stromal cells; |
| FKBP52 | FK506-binding protein; |
| FOXO1 | Forkhead box-O1; |
| GLUT1 | Glucose transporter-1; |
| GnRH | Gonadotropin-releasing hormone; |
| Hand2 | Heart and neural crest derivatives-expressed protein 2; |
| HB-EGF | Heparin-binding EGF-like growth factor; |
| HPA | Hypothalamic-pituitary-adrenal; |
| HPG | Hypothalamic-pituitary-gonadal; |
| ICAM-1 | Intercellular adhesion molecule-1; |
| ICM | Inner cell mass; |
| IHH | Indian hedgehog; |
| LA | Linoleic acid; |
| LPA | Lysophosphatidic acid; |
| LPA3 | Lysophosphatidic acid receptor-3; |
| NCOA6 | Nuclear receptor coactivator-6; |
| NK | Natural Killer; |
| PCOS | Polycystic ovarian syndrome; |
| PGE2 | Prostaglandin E2; |
| PGF2 | Prostaglandin F2; |
| PGs | Prostaglandins; |
| PPAR- | Peroxisome proliferator-activated receptor-; |
| PPAR | peroxisome-activated receptor-alpha; |
| PRs | Progesterone receptors; |
| ROS | Reactive oxygen species; |
| RXR | Retinoic acid X receptor; |
| S1P | Sphingosine 1-phosphate; |
| Sphk | Sphingosine kinase; |
| SPP1 | Secreted phosphoprotein 1; |
| SRC2 | Steroid receptor coactivator2; |
| Tregs | Regulatory T cells; |
| uDCs | uterine Dendritic cells; |
| VCAM-1 | Vascular cell adhesion molecule-1. |
References
- Teklenburg, G.; Salker, M.; Molokhia, M.; Lavery, S.; Trew, G.; Aojanepong, T.; Mardon, H.J.; Lokugamage, A.U.; Rai, R.; Landles, C.; et al. Natural selection of human embryos: Decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS ONE 2010, 5, e10258. [Google Scholar] [CrossRef] [PubMed]
- El-Mahdy Abdel-Moneim, M. Ectopic Pregnancy in Cases of Recurrent Implantation Failure and Cases of Recurrent Early Pregnancy Loss. Open J. Obstet. Gynecol. 2017, 7, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Margalioth, E.J.; Ben-Chetrit, A.; Gal, M.; Eldar-Geva, T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum. Reprod. 2006, 21, 3036–3043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egashira, M.; Hirota, Y. Uterine receptivity and embryo-uterine interactions in embryo implantation: Lessons from mice. Reprod. Med. Biol. 2013, 12, 127–132. [Google Scholar] [CrossRef]
- Kupka, M.S.; D’Hooghe, T.; Ferraretti, A.P.; de Mouzon, J.; Erb, K.; Castilla, J.A.; Calhaz-Jorge, C.; De Geyter, C.; Goossens, V. Assisted reproductive technology in Europe, 2011: Results generated from European registers by ESHRE. Hum. Reprod. 2016, 31, 233–248. [Google Scholar]
- Ye, X.; Hama, K.; Contos, J.J.A.; Anliker, B.; Inoue, A.; Skinner, M.K.; Suzuki, H.; Amano, T.; Kennedy, G.; Arai, H.; et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 2005, 435, 104–108. [Google Scholar] [CrossRef]
- Paria, B.C.; Ma, W.; Tan, J.; Raja, S.; Das, S.K.; Dey, S.K.; Hogan, B.L. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc. Natl. Acad. Sci. USA 2001, 98, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Mizugishi, K.; Li, C.; Olivera, A.; Bielawski, J.; Bielawska, A.; Deng, C.X.; Proia, R.L. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J. Clin. Investig. 2007, 117, 2993–3006. [Google Scholar] [CrossRef] [Green Version]
- Tapiero, H.; Ba, G.N.; Couvreur, P.; Tew, K.D. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Wang, D.D. Dietary n-6 polyunsaturated fatty acids and cardiovascular disease: Epidemiologic evidence. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 5–9. [Google Scholar] [CrossRef]
- Ross, B.M.; Malik, I.; Babay, S. Dietary omega-3 polyunsaturated fatty acid supplementation in an animal model of anxiety. Prostaglandins Leukot. Essent. Fat. Acids 2016, 114, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.A.; Marnett, L.J. Introduction to lipid biochemistry, metabolism, and signaling. Chem. Rev. 2011, 111, 5817–5820. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, E. Magnesium supplementation for premenstrual syndrome and premenstrual dysphoric disorder. Int. J. Pharm. Res. 2020, 13, 486–490. [Google Scholar]
- Surlis, C.; Cormican, P.; Waters, S.M.; Lonergan, P.; Keogh, K.; Doyle, D.N.; Kenny, D.A. Effects of dietary n-3-PUFA supplementation, post-insemination plane of nutrition and pregnancy status on the endometrial transcriptome of beef heifers. Sci. Rep. 2020, 10, 20798. [Google Scholar] [CrossRef]
- Vilella, F.; Ramirez, L.B.; Simón, C. Lipidomics as an emerging tool to predict endometrial receptivity. Fertil. Steril. 2013, 99, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Lim, H.; Das, S.K.; Reese, J.; Paria, B.C.; Daikoku, T.; Wang, H. Molecular Cues to Implantation. Endocr. Rev. 2004, 25, 341–373. [Google Scholar] [CrossRef]
- Zhou, Y.; Genbacev, O.; Fisher, S.J. The human placenta remodels the uterus by using a combination of molecules that govern vasculogenesis or leukocyte extravasation. Ann. N. Y. Acad. Sci. 2003, 995, 73–83. [Google Scholar] [CrossRef]
- Lim, H.; Song, H.; Paria, B.C.; Reese, J.; Das, S.K.; Dey, S.K. Molecules in blastocyst implantation: Uterine and embryonic perspectives. Vitam. Horm. 2002, 64, 43–76. [Google Scholar]
- Edwards, R.G. Human implantation: The last barrier in assisted reproduction technologies? Reprod. Biomed. Online 2006, 13, 887–904. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic Decidualization of the Human Endometrium in Reproductive Health and Failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.E.; Casper, R.F. Pinopodes: A questionable role in endometrial receptivity. Hum. Reprod. Update 2009, 15, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Qin, H.; Yang, Y.; Chen, X.; Zhang, J.; Laird, S.; Wang, C.C.; Chan, T.F.; Li, T.C. A comparison of transcriptomic profiles in endometrium during window of implantation between women with unexplained recurrent implantation failure and recurrent miscarriage. Reproduction 2017, 153, 749–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavatte-Palmer, P.; Tarrade, A. Placentation in different mammalian species. Ann. D’endocrinol. 2016, 77, 67–74. [Google Scholar] [CrossRef]
- Niakan, K.K.; Han, J.; Pedersen, R.A.; Simon, C.; Pera, R.A.R. Human pre-implantation embryo development. Development 2012, 139, 829–841. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, A.J.; Baird, D.D.; Weinberg, C.R. Time of implantation of the conceptus and loss of pregnancy. N. Engl. J. Med. 1999, 340, 1796–1799. [Google Scholar] [CrossRef]
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Comparative aspects of implantation. Reproduction 2009, 138, 195–209. [Google Scholar] [CrossRef] [Green Version]
- Yoshinaga, K. A sequence of events in the uterus prior to implantation in the mouse. J. Assist. Reprod. Genet. 2013, 30, 1017–1022. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.C. Development of bovine blastocyst with a note on implantation. Anat. Rec. 1952, 113, 143–161. [Google Scholar] [CrossRef]
- Ziecik, A.J.; Waclawik, A.; Kaczmarek, M.M.; Blitek, A.; Jalali, B.M.; Andronowska, A. Mechanisms for the establishment of pregnancy in the pig. Reprod. Domest. Anim. 2011, 46 (Suppl. 3), 31–41. [Google Scholar] [CrossRef]
- Geisert, R.D.; Yelich, J.V. Regulation of conceptus development and attachment in pigs. J. Reprod. Fertility. Suppl. 1997, 52, 133–149. [Google Scholar] [CrossRef]
- Denker, H.W.; Eng, L.A.; Hamner, C.E. Studies on the early development and implantation in the cat. Anat. Embryol. 1978, 154, 39–54. [Google Scholar] [CrossRef]
- Shimizu, T.; Tsutsui, T.; Murao, I.; Orima, H. Incidence for transuterine migration of embryos in the dog. Nihon Juigaku Zasshi. Jpn. J. Vet. Sci. 1990, 52, 1273–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verstegen-Onclin, K.; Verstegen, J. Endocrinology of pregnancy in the dog: A review. Theriogenology 2008, 70, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Byron, M.J.; Koester, D.C.; Edwards, K.L.; Mozdziak, P.E.; Farin, C.E.; Crosier, A.E. Immunoglobulin J chain as a non-invasive indicator of pregnancy in the cheetah (Acinonyx jubatus). PLoS ONE 2020, 15, e0225354. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; DeMayo, F.J. Animal models of implantation. Reproduction 2004, 128, 679–695. [Google Scholar] [CrossRef] [Green Version]
- Reese, J.; Wang, H.; Ding, T.; Paria, B.C. The hamster as a model for embryo implantation: Insights into a multifaceted process. Semin. Cell Dev. Biol. 2008, 19, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Orsini, M.W.; Donovan, B.T. Implantation and induced decidualization of the uterus in the guinea pig, as indicated by Pontamine blue. Biol. Reprod. 1971, 5, 270–281. [Google Scholar] [CrossRef] [Green Version]
- Meesapyodsuk, D.; Qiu, X. The front-end desaturase: Structure, function, evolution and biotechnological use. Lipids 2012, 47, 227–237. [Google Scholar] [CrossRef]
- Reddy, A.S.; Nuccio, M.L.; Gross, L.M.; Thomas, T.L. Isolation of a delta 6-desaturase gene from the cyanobacterium Synechocystis sp. strain PCC 6803 by gain-of-function expression in Anabaena sp. strain PCC 7120. Plant Mol. Biol. 1993, 22, 293–300. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Mattioli, S.; Signorini, C.; Cotozzolo, E.; Noto, D.; Moretti, E.; Brecchia, G.; Dal Bosco, A.; Belmonte, G.; Durand, T.; et al. Effect of Dietary n-3 Source on Rabbit Male Reproduction. Oxidative Med. Cell. Longev. 2019, 2019, 3279670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wathes, D.C.; Abayasekara, D.R.E.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J. Regulation of Neutrophil Function by Marine n-3 Fatty Acids-A Mini Review. Cell Biochem. Biophys. 2021, 79, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Mayoral Andrade, G.; Vásquez Martínez, G.; Pérez-Campos Mayoral, L.; Hernández-Huerta, M.T.; Zenteno, E.; Pérez-Campos Mayoral, E.; Martínez Cruz, M.; Martínez Cruz, R.; Matias-Cervantes, C.A.; Meraz Cruz, N.; et al. Molecules and Prostaglandins Related to Embryo Tolerance. Front. Immunol. 2020, 11, 555414. [Google Scholar] [CrossRef]
- Chakraborty, I.; Das, S.K.; Wang, J.; Dey, S.K. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J. Mol. Endocrinol. 1996, 16, 107–122. [Google Scholar] [CrossRef] [PubMed]
- St-Louis, I.; Singh, M.; Brasseur, K.; Leblanc, V.; Parent, S.; Asselin, E. Expression of COX-1 and COX-2 in the endometrium of cyclic, pregnant and in a model of pseudopregnant rats and their regulation by sex steroids. Reprod. Biol. Endocrinol. 2010, 8, 103. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Lim, H.; Paria, B.C.; Matsumoto, H.; Swift, L.L.; Morrow, J.; Bonventre, J.V.; Dey, S.K. Cytosolic phospholipase A2alpha is crucial [correction of A2alpha deficiency is crucial] for ’on-time’ embryo implantation that directs subsequent development. Development 2002, 129, 2879–2889. [Google Scholar] [CrossRef] [PubMed]
- Gokuldas, P.P.; Singh, S.K.; Tamuli, M.K.; Naskar, S.; Vashi, Y.; Thomas, R.; Barman, K.; Pegu, S.R.; Chethan, S.G.; Agarwal, S.K. Dietary supplementation of n-3 polyunsaturated fatty acid alters endometrial expression of genes involved in prostaglandin biosynthetic pathway in breeding sows (Sus scrofa). Theriogenology 2018, 110, 201–208. [Google Scholar] [CrossRef]
- Yi, D.; Zeng, S.; Guo, Y. A diet rich in n-3 polyunsaturated fatty acids reduced prostaglandin biosynthesis, ovulation rate, and litter size in mice. Theriogenology 2012, 78, 28–38. [Google Scholar] [CrossRef]
- Rosa Velazquez, M.; Batistel, F.; Pinos Rodriguez, J.M.; Relling, A.E. Effects of maternal dietary omega-3 polyunsaturated fatty acids and methionine during late gestation on fetal growth, DNA methylation, and mRNA relative expression of genes associated with the inflammatory response, lipid metabolism and DNA methylation in placenta and offspring’s liver in sheep. J. Anim. Sci. Biotechnol. 2020, 11, 111. [Google Scholar] [PubMed]
- Niringiyumukiza, J.D.; Cai, H.; Xiang, W. Prostaglandin E2 involvement in mammalian female fertility: Ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 2018, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Waclawik, A.; Kaczmarek, M.M.; Blitek, A.; Kaczynski, P.; Ziecik, A.J. Embryo-maternal dialogue during pregnancy establishment and implantation in the pig. Mol. Reprod. Dev. 2017, 84, 842–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczynski, P.; Kowalewski, M.P.; Waclawik, A. Prostaglandin F2α promotes angiogenesis and embryo-maternal interactions during implantation. Reproduction 2016, 151, 539–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waclawik, A.; Rivero-Muller, A.; Blitek, A.; Kaczmarek, M.M.; Brokken, L.J.S.; Watanabe, K.; Rahman, N.A.; Ziecik, A.J. Molecular Cloning and Spatiotemporal Expression of Prostaglandin F Synthase and Microsomal Prostaglandin E Synthase-1 in Porcine Endometrium. Endocrinology 2006, 147, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Waclawik, A.; Jabbour, H.N.; Blitek, A.; Ziecik, A.J. Estradiol-17beta, prostaglandin E2 (PGE2), and the PGE2 receptor are involved in PGE2 positive feedback loop in the porcine endometrium. Endocrinology 2009, 150, 3823–3832. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.H.; Catt, K.J. Roles of LPA3 and COX-2 in implantation. Trends Endocrinol. Metab. TEM 2005, 16, 397–399. [Google Scholar] [CrossRef]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Zhou, W.J.; Zhu, X.Y.; Lu, H.; Wu, K.; Yang, H.L.; Fu, Q.; Wei, C.Y.; Chang, K.K.; Jin, L.P.; et al. Suppression of autophagy and HCK signaling promotes PTGS2(high) FCGR3(-) NK cell differentiation triggered by ectopic endometrial stromal cells. Autophagy 2018, 14, 1376–1397. [Google Scholar] [CrossRef] [Green Version]
- Hua, F.; Li, C.H.; Wang, H.; Xu, H.G. Relationship between expression of COX-2, TNF-α, IL-6 and autoimmune-type recurrent miscarriage. Asian Pac. J. Trop. Med. 2013, 6, 990–994. [Google Scholar] [CrossRef] [Green Version]
- Souidan, I.I.; Salama, K.M. IS Three D power Doppler of the endometrial and sub endometrial regions effective in predicting endometrial implantation? Prospective cohort study. EGYFS 2020, 24, 41–50. [Google Scholar]
- Cheng, J.G.; Stewart, C.L. Loss of cyclooxygenase-2 retards decidual growth but does not inhibit embryo implantation or development to term. Biol. Reprod. 2003, 68, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blitek, A.; Waclawik, A.; Kaczmarek, M.M.; Stadejek, T.; Pejsak, Z.; Ziecik, A.J. Expression of cyclooxygenase-1 and -2 in the porcine endometrium during the oestrous cycle and early pregnancy. Reprod. Domest. Anim. 2006, 41, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Charpigny, G.; Reinaud, P.; Tamby, J.P.; Créminon, C.; Guillomot, M. Cyclooxygenase-2 unlike cyclooxygenase-1 is highly expressed in ovine embryos during the implantation period. Biol. Reprod. 1997, 57, 1032–1040. [Google Scholar] [CrossRef] [Green Version]
- Charpigny, G.; Reinaud, P.; Tamby, J.P.; Créminon, C.; Martal, J.; Maclouf, J.; Guillomot, M. Expression of cyclooxygenase-1 and -2 in ovine endometrium during the estrous cycle and early pregnancy. Endocrinology 1997, 138, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Reese, J.; Brown, N.; Paria, B.C.; Morrow, J.; Dey, S.K. COX-2 compensation in the uterus of COX-1 deficient mice during the pre-implantation period. Mol. Cell. Endocrinol. 1999, 150, 23–31. [Google Scholar] [CrossRef]
- Lim, H.; Paria, B.C.; Das, S.K.; Dinchuk, J.E.; Langenbach, R.; Trzaskos, J.M.; Dey, S.K. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 1997, 91, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.; Morin, S.; Jeong, J.W.; Scott, R.T.J.; Lessey, B.A. Local and systemic factors and implantation: What is the evidence? Fertil. Steril. 2016, 105, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.g.; Song, H.; Das, S.K.; Paria, B.C.; Dey, S.K. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc. Natl. Acad. Sci. USA 2003, 100, 2963–2968. [Google Scholar] [CrossRef] [Green Version]
- Kliman, H.J.; Honig, S.; Walls, D.; Luna, M.; McSweet, J.C.; Copperman, A.B. Optimization of endometrial preparation results in a normal endometrial function test (EFT) and good reproductive outcome in donor ovum recipients. J. Assist. Reprod. Genet. 2006, 23, 299–303. [Google Scholar] [CrossRef]
- Paramonova, N.B.; Kogan, E.A.; Kolotovkina, A.V.; Burmenskaya, O.V. [The morphological and molecular biological signs of impaired endometrial receptivity in infertility in women suffering from external genital endometriosis]. Arkhiv Patol. 2018, 80, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Alonso, M.; Blesa, D.; Díaz-Gimeno, P.; Gómez, E.; Fernández-Sánchez, M.; Carranza, F.; Carrera, J.; Vilella, F.; Pellicer, A.; Simón, C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil. Steril. 2013, 100, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Tucker, D.E.; Burchett, S.A.; Leslie, C.C. Properties of the Group IV phospholipase A2 family. Prog. Lipid Res. 2006, 45, 487–510. [Google Scholar] [CrossRef]
- Holinka, C.F.; Diczfalusy, E.; Coelingh Bennink, H.J.T. Estetrol: A unique steroid in human pregnancy. J. Steroid Biochem. Mol. Biol. 2008, 110, 138–143. [Google Scholar] [CrossRef]
- Tuckey, R.C. Progesterone synthesis by the human placenta. Placenta 2005, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, K.; Hennes, A.; Vriens, J. Isolation of Mouse Endometrial Epithelial and Stromal Cells for In Vitro Decidualization. J. Vis. Exp. JoVE 2017, 121, 55168. [Google Scholar] [CrossRef] [Green Version]
- Cooke, P.S.; Buchanan, D.L.; Young, P.; Setiawan, T.; Brody, J.; Korach, K.S.; Taylor, J.; Lubahn, D.B.; Cunha, G.R. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc. Natl. Acad. Sci. USA 1997, 94, 6535–6540. [Google Scholar] [CrossRef] [Green Version]
- Paria, B.C.; Das, S.K.; Andrews, G.K.; Dey, S.K. Expression of the epidermal growth factor receptor gene is regulated in mouse blastocysts during delayed implantation. Proc. Natl. Acad. Sci. USA 1993, 90, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Tranguch, S.; Cheung-Flynn, J.; Daikoku, T.; Prapapanich, V.; Cox, M.B.; Xie, H.; Wang, H.; Das, S.K.; Smith, D.F.; Dey, S.K. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. USA 2005, 102, 14326–14331. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Soyal, S.M.; Fernandez-Valdivia, R.; Gehin, M.; Chambon, P.; Demayo, F.J.; Lydon, J.P.; O’Malley, B.W. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol. Cell. Biol. 2006, 26, 6571–6583. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Valdivia, R.; Mukherjee, A.; Amato, P.; Allred, D.C.; Nguyen, J.; DeMayo, F.J.; Lydon, J.P. Progesterone-action in the murine uterus and mammary gland requires steroid receptor coactivator 2: Relevance to the human. Front. Biosci. J. Virtual Libr. 2007, 12, 3640–3647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawagoe, J.; Li, Q.; Mussi, P.; Liao, L.; Lydon, J.P.; DeMayo, F.J.; Xu, J. Nuclear receptor coactivator-6 attenuates uterine estrogen sensitivity to permit embryo implantation. Dev. Cell 2012, 23, 858–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Kannan, A.; DeMayo, F.J.; Lydon, J.P.; Cooke, P.S.; Yamagishi, H.; Srivastava, D.; Bagchi, M.K.; Bagchi, I.C. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 2011, 331, 912–916. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, I.; Lee, D.K.; Petit, F.G.; Jeong, J.; Lee, K.; Lydon, J.P.; DeMayo, F.J.; Tsai, M.J.; Tsai, S.Y. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007, 3, e102. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.K.; Kurihara, I.; Jeong, J.W.; Lydon, J.; Demayo, F.; Tsai, M.J.; Tsai, S. Suppression of ER alpha Activity by COUP-TFII Is Essential for Successful Implantation and Decidualization. Mol. Endocrinol. 2010, 24, 930–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhurke, A.S.; Bagchi, I.C.; Bagchi, M.K. Progesterone-Regulated Endometrial Factors Controlling Implantation. Am. J. Reprod. Immunol. 2016, 75, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Abbott, K.; Burrows, T.L.; Acharya, S.; Thota, R.N.; Garg, M.L. Dietary supplementation with docosahexaenoic acid rich fish oil increases circulating levels of testosterone in overweight and obese men. Prostaglandins Leukot. Essent. Fat. Acids 2020, 163, 102204. [Google Scholar] [CrossRef]
- Hajishafiee, M.; Askari, G.; Iranj, B.; Ghiasvand, R.; Bellissimo, N.; Totosy de Zepetnek, J.; Salehi-Abargouei, A. The Effect of n-3 Polyunsaturated Fatty Acid Supplementation on Androgen Status in Patients with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Clinical Trials. Horm. Metab. Res. 2016, 48, 281–289. [Google Scholar] [CrossRef]
- Tran, L.V.; Malla, B.A.; Sharma, A.N.; Kumar, S.; Tyagi, N.; Tyagi, A.K. Effect of omega-3 and omega-6 polyunsaturated fatty acid enriched diet on plasma IGF-1 and testosterone concentration, puberty and semen quality in male buffalo. Anim. Reprod. Sci. 2016, 173, 63–72. [Google Scholar] [CrossRef]
- Rahman, T.U.; Ullah, K.; Guo, M.X.; Pan, H.T.; Liu, J.; Ren, J.; Jin, L.Y.; Zhou, Y.Z.; Cheng, Y.; Sheng, J.Z.; et al. Androgen-induced alterations in endometrial proteins crucial in recurrent miscarriages. Oncotarget 2018, 9, 24627–24641. [Google Scholar] [CrossRef] [Green Version]
- Mokhtar, H.M.; Giribabu, N.; Muniandy, S.; Salleh, N. Testosterone decreases the expression of endometrial pinopode and L-selectin ligand (MECA-79) in adult female rats during uterine receptivity period. Int. J. Clin. Exp. Pathol. 2014, 7, 1967–1976. [Google Scholar] [PubMed]
- Mokhtar, M.H.; Giribabu, N.; Salleh, N. Testosterone Reduces Tight Junction Complexity and Down-regulates Expression of Claudin-4 and Occludin in the Endometrium in Ovariectomized, Sex-steroid Replacement Rats. In Vivo 2020, 34, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.A.; Simitsidellis, I.; Cousins, F.L.; Critchley, H.O.D.; Saunders, P.T.K. Intracrine Androgens Enhance Decidualization and Modulate Expression of Human Endometrial Receptivity Genes. Sci. Rep. 2016, 6, 19970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, H.; Wu, W.; Xu, J.; Yu, D.; Qiao, B.; Liu, H.; Yang, B.; Li, Y.; Ling, Y.; Kuang, H. Flutamide ameliorates uterine decidualization and angiogenesis in the mouse hyperandrogenemia model during mid-pregnancy. PLoS ONE 2019, 14, e0217095. [Google Scholar] [CrossRef]
- Qin, A.; Qin, J.; Jin, Y.; Xie, W.; Fan, L.; Jiang, L.; Mo, F. DHEA improves the antioxidant capacity of endometrial stromal cells and improves endometrium receptivity via androgen receptor. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 198, 120–126. [Google Scholar] [CrossRef]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef] [Green Version]
- Phang, M.; Skilton, M.R. Marine Omega-3 Fatty Acids, Complications of Pregnancy and Maternal Risk Factors for Offspring Cardio-Metabolic Disease. Mar. Drugs 2018, 16, 138. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.; Zhong, H.; Xia, L.; Tao, Y.; Yin, H. Pathophysiology of mitochondrial lipid oxidation: Role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria. Free Radic. Biol. Med. 2017, 111, 316–327. [Google Scholar] [CrossRef]
- Dodson, M.; Wani, W.Y.; Redmann, M.; Benavides, G.A.; Johnson, M.S.; Ouyang, X.; Cofield, S.S.; Mitra, K.; Darley-Usmar, V.; Zhang, J. Regulation of autophagy, mitochondrial dynamics, and cellular bioenergetics by 4-hydroxynonenal in primary neurons. Autophagy 2017, 13, 1828–1840. [Google Scholar] [CrossRef] [Green Version]
- Khatif, H.; Drexler, I. Autophagy and its implication in antiviral immunity. SOJ Immunol. 2014, 2, 1–8. [Google Scholar]
- Dunlop, E.A.; Tee, A.R. mTOR and autophagy: A dynamic relationship governed by nutrients and energy. Semin. Cell Dev. Biol. 2014, 36, 121–129. [Google Scholar] [CrossRef]
- Yan, D.; An, G.; Kuo, M.T. C-Jun N-terminal kinase signalling pathway in response to cisplatin. J. Cell. Mol. Med. 2016, 20, 2013–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, A.; Ariyoshi, W.; Yoshioka, Y.; Hikiji, H.; Nishihara, T.; Okinaga, T. Docosahexaenoic acid enhances M2 macrophage polarization via the p38 signaling pathway and autophagy. J. Cell. Biochem. 2019, 120, 12604–12617. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yurube, T.; Kakutani, K.; Maeno, K.; Takada, T.; Terashima, Y.; Kakiuchi, Y.; Takeoka, Y.; Miyazaki, S.; Kuroda, R.; et al. Selective interference of mTORC1/RAPTOR protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism with Akt and autophagy induction. Osteoarthr. Cartil. 2017, 25, 2134–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Lin, G.; Song, C.; Wu, Y.; Feng, N.; Chen, W.; He, Z.; Chen, Y.Q. RA and ω-3 PUFA co-treatment activates autophagy in cancer cells. Oncotarget 2017, 8, 109135–109150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.J.; Zhang, H.; Chen, Z.Q.; Zhang, W.; Liu, X.M.; Fang, J.Y.; Liu, F.J.; Kwak-Kim, J. Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod. Biol. Endocrinol. 2019, 17, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestre Citrinovitz, A.C.; Strowitzki, T.; Germeyer, A. Decreased Autophagy Impairs Decidualization of Human Endometrial Stromal Cells: A Role for ATG Proteins in Endometrial Physiology. Int. J. Mol. Sci. 2019, 20, 3066. [Google Scholar] [CrossRef] [Green Version]
- Avagliano, L.; Terraneo, L.; Virgili, E.; Martinelli, C.; Doi, P.; Samaja, M.; Bulfamante, G.P.; Marconi, A.M. Autophagy in Normal and Abnormal Early Human Pregnancies. Reprod. Sci. 2015, 22, 838–844. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Quesada-López, T.; Cereijo, R.; Turatsinze, J.V.; Planavila, A.; Cairó, M.; Gavaldà-Navarro, A.; Peyrou, M.; Moure, R.; Iglesias, R.; Giralt, M.; et al. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat. Commun. 2016, 7, 13479. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Xue, M.; Zhang, J.; Yu, H.; Gu, Y.; Du, M.; Ye, W.; Wan, B.; Jin, M.; Zhang, Y. Protective role of GPR120 in the maintenance of pregnancy by promoting decidualization via regulation of glucose metabolism. EBioMedicine 2019, 39, 540–551. [Google Scholar] [CrossRef] [Green Version]
- Milne, S.A.; Perchick, G.B.; Boddy, S.C.; Jabbour, H.N. Expression, Localization, and Signaling of PGE2 and EP2/EP4 Receptors in Human Nonpregnant Endometrium across the Menstrual Cycle. J. Clin. Endocrinol. Metab. 2001, 86, 4453–4459. [Google Scholar] [CrossRef]
- Lim, H.; Gupta, R.A.; Ma, W.G.; Paria, B.C.; Moller, D.E.; Morrow, J.D.; DuBois, R.N.; Trzaskos, J.M.; Dey, S.K. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999, 13, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Pakrasi, P.L.; Jain, A.K. Cyclooxygenase-2 derived PGE2 and PGI2 play an important role via EP2 and PPARdelta receptors in early steps of oil induced decidualization in mice. Placenta 2008, 29, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 2005, 25, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.W. Pregnancy maintenance and parturition: The role of prostaglandin in manipulating the immune and inflammatory response. Endocr. Rev. 1994, 15, 684–706. [Google Scholar] [CrossRef]
- Shireman, T.I.; Kerling, E.H.; Gajewski, B.J.; Colombo, J.; Carlson, S.E. Docosahexaenoic acid supplementation (DHA) and the return on investment for pregnancy outcomes. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Okudaira, M.; Inoue, A.; Shuto, A.; Nakanaga, K.; Kano, K.; Makide, K.; Saigusa, D.; Tomioka, Y.; Aoki, J. Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. J. Lipid Res. 2014, 55, 2178–2192. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Chun, J. Lysophosphatidic acid (LPA) signaling in vertebrate reproduction. Trends Endocrinol. Metab. TEM 2010, 21, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Hama, K.; Aoki, J.; Inoue, A.; Endo, T.; Amano, T.; Motoki, R.; Kanai, M.; Ye, X.; Chun, J.; Matsuki, N.; et al. Embryo spacing and implantation timing are differentially regulated by LPA3-mediated lysophosphatidic acid signaling in mice. Biol. Reprod. 2007, 77, 954–959. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Kohama, T.; Edsall, L.; Nava, V.; Cuvillier, O.; Poulton, S.; Spiegel, S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J. Cell Biol. 1999, 147, 545–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Sugiura, M.; Nava, V.E.; Edsall, L.C.; Kono, K.; Poulton, S.; Milstien, S.; Kohama, T.; Spiegel, S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 2000, 275, 19513–19520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlap, K.A.; Kwak, H.i.; Burghardt, R.C.; Bazer, F.W.; Magness, R.R.; Johnson, G.A.; Bayless, K.J. The sphingosine 1-phosphate (S1P) signaling pathway is regulated during pregnancy in sheep. Biol. Reprod. 2010, 82, 876–887. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Olson, D.M.; van Bennekom, M.; Brindley, D.N.; Hemmings, D.G. Increased expression of enzymes for sphingosine 1-phosphate turnover and signaling in human decidua during late pregnancy. Biol. Reprod. 2010, 82, 628–635. [Google Scholar] [CrossRef] [Green Version]
- Skaznik-Wikiel, M.E.; Kaneko-Tarui, T.; Kashiwagi, A.; Pru, J.K. Sphingosine-1-phosphate receptor expression and signaling correlate with uterine prostaglandin-endoperoxide synthase 2 expression and angiogenesis during early pregnancy. Biol. Reprod. 2006, 74, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Al-Khalaifah, H. Modulatory Effect of Dietary Polyunsaturated Fatty Acids on Immunity, Represented by Phagocytic Activity. Front. Vet. Sci. 2020, 7, 569939. [Google Scholar] [CrossRef]
- Gnainsky, Y.; Granot, I.; Aldo, P.B.; Barash, A.; Or, Y.; Schechtman, E.; Mor, G.; Dekel, N. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil. Steril. 2010, 94, 2030–2036. [Google Scholar] [CrossRef] [Green Version]
- Yaqoob, P.; Newsholme, E.A.; Calder, P.C. Influence of cell culture conditions on diet-induced changes in lymphocyte fatty acid composition. Biochim. Biophys. Acta 1995, 1255, 333–340. [Google Scholar] [CrossRef]
- Palombo, J.D.; Demichele, S.J.; Lydon, E.; Bistrian, B.R. Cyclic vs Continuous Enteral Feeding With ω-3 and γ-Linolenic Fatty Acids: Effects on Modulation of Phospholipid Fatty Acids in Rat Lung and Liver Immune Cells. J. Parenter. Enter. Nutr. 1997, 21, 123–132. [Google Scholar] [CrossRef]
- de La Puerta Vázquez, R.; Martínez-Domínguez, E.; Sánchez Perona, J.; Ruiz-Gutiérrez, V. Effects of different dietary oils on inflammatory mediator generation and fatty acid composition in rat neutrophils. Metab. Clin. Exp. 2004, 53, 59–65. [Google Scholar] [CrossRef]
- James, M.J.; Cleland, L.G.; Gibson, R.A.; Hawkes, J.S. Interaction between fish and vegetable oils in relation to rat leucocyte leukotriene production. J. Nutr. 1991, 121, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Careaga-Houck, M.; Sprecher, H. Effect of a fish oil diet on the composition of rat neutrophil lipids and the molecular species of choline and ethanolamine glycerophospholipids. J. Lipid Res. 1989, 30, 77–87. [Google Scholar] [CrossRef]
- Laskarin, G.; Kämmerer, U.; Rukavina, D.; Thomson, A.W.; Fernandez, N.; Blois, S.M. Antigen-presenting cells and materno-fetal tolerance: An emerging role for dendritic cells. Am. J. Reprod. Immunol. 2007, 58, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668 (Suppl. 1), S50–S58. [Google Scholar] [CrossRef] [PubMed]
- Roessler, C.; Kuhlmann, K.; Hellwing, C.; Leimert, A.; Schumann, J. Impact of Polyunsaturated Fatty Acids on miRNA Profiles of Monocytes/Macrophages and Endothelial Cells-A Pilot Study. Int. J. Mol. Sci. 2017, 18, 284. [Google Scholar] [CrossRef] [Green Version]
- Manaster, I.; Mizrahi, S.; Goldman-Wohl, D.; Sela, H.Y.; Stern-Ginossar, N.; Lankry, D.; Gruda, R.; Hurwitz, A.; Bdolah, Y.; Haimov-Kochman, R.; et al. Endometrial NK cells are special immature cells that await pregnancy. J. Immunol. 2008, 181, 1869–1876. [Google Scholar] [CrossRef] [Green Version]
- Schraml, B.U.; Reis e Sousa, C. Defining dendritic cells. Curr. Opin. Immunol. 2015, 32, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Bar-On, L.; Jung, S. Defining dendritic cells by conditional and constitutive cell ablation. Immunol. Rev. 2010, 234, 76–89. [Google Scholar] [CrossRef]
- Wang, H.; Hao, Q.; Li, Q.R.; Yan, X.W.; Ye, S.; Li, Y.S.; Li, N.; Li, J.S. Omega-3 polyunsaturated fatty acids affect lipopolysaccharide-induced maturation of dendritic cells through mitogen-activated protein kinases p38. Nutrition 2007, 23, 474–482. [Google Scholar] [CrossRef]
- Plaks, V.; Birnberg, T.; Berkutzki, T.; Sela, S.; BenYashar, A.; Kalchenko, V.; Mor, G.; Keshet, E.; Dekel, N.; Neeman, M.; et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J. Clin. Investig. 2008, 118, 3954–3965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blois, S.; Alba Soto, C.D.; Olmos, S.; Chuluyan, E.; Gentile, T.; Arck, P.C.; Margni, R.A. Therapy with dendritic cells influences the spontaneous resorption rate in the CBA/J x DBA/2J mouse model. Am. J. Reprod. Immunol. 2004, 51, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Tsuda, H.; Sakai, M.; Hori, S.; Sasaki, Y.; Futatani, T.; Miyawaki, T.; Saito, S. Predominance of Th2-promoting dendritic cells in early human pregnancy decidua. J. Leukoc. Biol. 2003, 74, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Houser, B.L.; Tilburgs, T.; Hill, J.; Nicotra, M.L.; Strominger, J.L. Two unique human decidual macrophage populations. J. Immunol. 2011, 186, 2633–2642. [Google Scholar] [CrossRef] [Green Version]
- Mor, G. Role of Inflammation during implantation. Placenta 2019, 83, e2. [Google Scholar] [CrossRef]
- Williams, P.J.; Searle, R.F.; Robson, S.C.; Innes, B.A.; Bulmer, J.N. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 2009, 82, 24–31. [Google Scholar] [CrossRef]
- Yaqoob, P.; Newsholme, E.A.; Calder, P.C. The effect of dietary lipid manipulation on rat lymphocyte subsets and proliferation. Immunology 1994, 82, 603–610. [Google Scholar]
- Endres, S.; Meydani, S.N.; Ghorbani, R.; Schindler, R.; Dinarello, C.A. Dietary supplementation with n-3 fatty acids suppresses interleukin-2 production and mononuclear cell proliferation. J. Leukoc. Biol. 1993, 54, 599–603. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Y.; Wang, S.; Zhou, J.; Zhou, J.; Lu, X.; Bai, X.; Wang, X.Y.; Chen, Z.; Zuo, D. Endogenous n-3 Polyunsaturated Fatty Acids Attenuate T Cell-Mediated Hepatitis via Autophagy Activation. Front. Immunol. 2016, 7, 350. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.H.; Hou, Y.C.; Pai, M.H.; Yeh, C.L.; Yeh, S.L. Dietary ω-6/ω-3 Polyunsaturated Fatty Acid Ratios Affect the Homeostasis of Th/Treg Cells in Mice With Dextran Sulfate Sodium-Induced Colitis. JPEN J. Parenter. Enter. Nutr. 2017, 41, 647–656. [Google Scholar] [CrossRef]
- Miles, E.A.; Banerjee, T.; Wells, S.J.; Calder, P.C. Limited effect of eicosapentaenoic acid on T-lymphocyte and natural killer cell numbers and functions in healthy young males. Nutrition 2006, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Mukaro, V.R.; Costabile, M.; Murphy, K.J.; Hii, C.S.; Howe, P.R.; Ferrante, A. Leukocyte numbers and function in subjects eating n-3 enriched foods: Selective depression of natural killer cell levels. Arthritis Res. Ther. 2008, 10, R57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Lei, H.; Tian, Z.; Wang, X.; Cheng, D.; Wang, C. The immunomodulatory activity and mechanism of docosahexenoic acid (DHA) on immunosuppressive mice models. Food Funct. 2018, 9, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Schwerbrock, N.M.J.; Karlsson, E.A.; Shi, Q.; Sheridan, P.A.; Beck, M.A. Fish oil-fed mice have impaired resistance to influenza infection. J. Nutr. 2009, 139, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Thies, F.; Nebe-von Caron, G.; Powell, J.R.; Yaqoob, P.; Newsholme, E.A.; Calder, P.C. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am. J. Clin. Nutr. 2001, 73, 539–548. [Google Scholar] [CrossRef] [Green Version]
- McMaster, M.T.; Dey, S.K.; Andrews, G.K. Association of monocytes and neutrophils with early events of blastocyst implantation in mice. J. Reprod. Fertil. 1993, 99, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauss-Etschmann, S.; Hartl, D.; Rzehak, P.; Heinrich, J.; Shadid, R.; Del Carmen Ramírez-Tortosa, M.; Campoy, C.; Pardillo, S.; Schendel, D.J.; Decsi, T.; et al. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J. Allergy Clin. Immunol. 2008, 121, 464–470.e6. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: A randomized, controlled trial. J. Allergy Clin. Immunol. 2003, 112, 1178–1184. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Maternal fish oil supplementation in pregnancy reduces interleukin-13 levels in cord blood of infants at high risk of atopy. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2003, 33, 442–448. [Google Scholar] [CrossRef]
- Olsen, S.F.; Østerdal, M.L.; Salvig, J.D.; Mortensen, L.M.; Rytter, D.; Secher, N.J.; Henriksen, T.B. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am. J. Clin. Nutr. 2008, 88, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sela, H.Y.; Goldman-Wohl, D.S.; Haimov-Kochman, R.; Greenfield, C.; Natanson-Yaron, S.; Hamani, Y.; Revel, A.; Lavy, Y.; Singer, O.; Yachimovich-Cohen, N.; et al. Human trophectoderm apposition is regulated by interferon γ-induced protein 10 (IP-10) during early implantation. Placenta 2013, 34, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Jones, R.L.; White, C.A.; Salamonsen, L.A. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol. Reprod. 2006, 74, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, F.; Martínez, S.; Quiñonero, A.; Loro, F.; Horcajadas, J.A.; Pellicer, A.; Simón, C. CXCL10 and IL-6 induce chemotaxis in human trophoblast cell lines. Mol. Hum. Reprod. 2008, 14, 423–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, S.; Schmidt, E.; Schlemmer, A.; Rasmussen, C.; Johansen, M.; Christensen, J. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: A randomized, double blind, placebo-controlled trial. Scand. J. Rheumatol. 2018, 47, 27–36. [Google Scholar] [CrossRef]
- Van Sinderen, M.; Menkhorst, E.; Winship, A.; Cuman, C.; Dimitriadis, E. Preimplantation human blastocyst-endometrial interactions: The role of inflammatory mediators. Am. J. Reprod. Immunol. 2013, 69, 427–440. [Google Scholar] [CrossRef]
- Granot, I.; Gnainsky, Y.; Dekel, N. Endometrial inflammation and effect on implantation improvement and pregnancy outcome. Reproduction 2012, 144, 661–668. [Google Scholar] [CrossRef]
- Liu, S.; Diao, L.; Huang, C.; Li, Y.; Zeng, Y.; Kwak-Kim, J.Y.H. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol. 2017, 124, 44–53. [Google Scholar] [CrossRef]
- Adam, O. Dietary fatty acids and immune reactions in synovial tissue. Eur. J. Med. Res. 2003, 8, 381–387. [Google Scholar]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Smalley, D.M.; Ley, K. L-selectin: Mechanisms and physiological significance of ectodomain cleavage. J. Cell. Mol. Med. 2005, 9, 255–266. [Google Scholar] [CrossRef]
- Genbacev, O.D.; Prakobphol, A.; Foulk, R.A.; Krtolica, A.R.; Ilic, D.; Singer, M.S.; Yang, Z.Q.; Kiessling, L.L.; Rosen, S.D.; Fisher, S.J. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science 2003, 299, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [PubMed]
- Joseph, D.N.; Whirledge, S. Stress and the HPA Axis: Balancing Homeostasis and Fertility. Int. J. Mol. Sci. 2017, 18, 2224. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.L.; Tan, X.W.; Cui, X.Z.; Yuan, H.J.; Li, H.; Jiao, G.Z.; Ji, C.L.; Tan, J.H. Preimplantation maternal stress impairs embryo development by inducing oviductal apoptosis with activation of the Fas system. Mol. Hum. Reprod 2016, 22, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Wiebold, J.L.; Stanfield, P.H.; Becker, W.C.; Hillers, J.K. The effect of restraint stress in early pregnancy in mice. J. Reprod. Fertil. 1986, 78, 185–192. [Google Scholar] [CrossRef]
- Liu, G.; Dong, Y.; Wang, Z.; Cao, J.; Chen, Y. Restraint stress delays endometrial adaptive remodeling during mouse embryo implantation. Stress 2015, 18, 699–709. [Google Scholar] [CrossRef]
- Jafari, Z.; Faraji, J.; Mirza Agha, B.; Metz, G.A.S.; Kolb, B.E.; Mohajerani, M.H. The Adverse Effects of Auditory Stress on Mouse Uterus Receptivity and Behaviour. Sci. Rep. 2017, 7, 4720. [Google Scholar] [CrossRef] [Green Version]
- Bremner, J.D.; Moazzami, K.; Wittbrodt, M.T.; Nye, J.A.; Lima, B.B.; Gillespie, C.F.; Rapaport, M.H.; Pearce, B.D.; Shah, A.J.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef]
- Kim, E.Y.; Choi, J.E.; Kim, M.; Hong, J.; Park, Y. N-3 PUFA Have Antidepressant-like Effects Via Improvement of the HPA-Axis and Neurotransmission in Rats Exposed to Combined Stress. Mol. Neurobiol. 2020, 57, 3860–3874. [Google Scholar] [CrossRef]
- Hammiche, F.; Vujkovic, M.; Wijburg, W.; de Vries, J.H.M.; Macklon, N.S.; Laven, J.S.E.; Steegers-Theunissen, R.P.M. Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology. Fertil. Steril. 2011, 95, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, E.S.; Macones, G.A.; Odem, R.R.; Patterson, B.W.; Moley, K.H. Elevated serum α-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil. Steril. 2011, 96, 880–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jungheim, E.S.; Frolova, A.I.; Jiang, H.; Riley, J.K. Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. J. Clin. Endocrinol. Metab. 2013, 98, E1364–E1368. [Google Scholar] [CrossRef] [Green Version]
- Eskew, A.M.; Wormer, K.C.; Matthews, M.L.; Norton, H.J.; Papadakis, M.A.; Hurst, B.S. The association between fatty acid index and in vitro fertilization outcomes. J. Assist. Reprod. Genet. 2017, 34, 1627–1632. [Google Scholar] [CrossRef]
- Mirabi, P.; Chaichi, M.J.; Esmaeilzadeh, S.; Ali Jorsaraei, S.G.; Bijani, A.; Ehsani, M.; Hashemi Karooee, S.F. The role of fatty acids on ICSI outcomes: A prospective cohort study. Lipids Health Dis. 2017, 16, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaskins, A.J.; Chavarro, J.E. Diet and fertility: A review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, S.L.; Lane, M.; Schulz, S.J.; Hebart, M.L.; Thompson, J.G.; Mitchell, M. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E425–E434. [Google Scholar] [CrossRef] [PubMed]
| Species | Development to Blastocyst | Apposition and Adhesion | Implantation | Type of Implantation | Relevant References |
|---|---|---|---|---|---|
| Human | Day 6 | Day 7/8 | Day 8 | Interstitial | [26] |
| Mouse | Day 4 | Day 4 | Day 4–5 | Eccentric | [28] |
| Cow | Day 7 | Days 19–22 | Day 30 | Centric | [29] |
| Pig | Day 6 | Days 16–18 | Days 20–22 | Centric | [30,31] |
| Domestic cat | Day 7 | Day 12 | Day 14 | Centric | [32] |
| Domestic dog | Day 7 | Day 20 | Day 20 | Centric | [33,34] |
| Cheetah | Day 7 | Day 19 | Day 21 | Centric | [35] |
| Rats | Day 5.5 | delay implantation | delayed implantation | eccentric | [36] |
| Hamsters | Day 3 | Day 4 | Day 4 | eccentric | [37] |
| Guinea pigs | Day 6 | Day 6/7 | Day 7 | Interstitial | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Zheng, Z.; Shi, J.; Shao, J. Insight on Polyunsaturated Fatty Acids in Endometrial Receptivity. Biomolecules 2022, 12, 36. https://doi.org/10.3390/biom12010036
Chen M, Zheng Z, Shi J, Shao J. Insight on Polyunsaturated Fatty Acids in Endometrial Receptivity. Biomolecules. 2022; 12(1):36. https://doi.org/10.3390/biom12010036
Chicago/Turabian StyleChen, Min, Zimeng Zheng, Jialu Shi, and Jun Shao. 2022. "Insight on Polyunsaturated Fatty Acids in Endometrial Receptivity" Biomolecules 12, no. 1: 36. https://doi.org/10.3390/biom12010036
APA StyleChen, M., Zheng, Z., Shi, J., & Shao, J. (2022). Insight on Polyunsaturated Fatty Acids in Endometrial Receptivity. Biomolecules, 12(1), 36. https://doi.org/10.3390/biom12010036





