How Efficient Is My (Medicinal) Chemistry?
Abstract
:1. Introduction
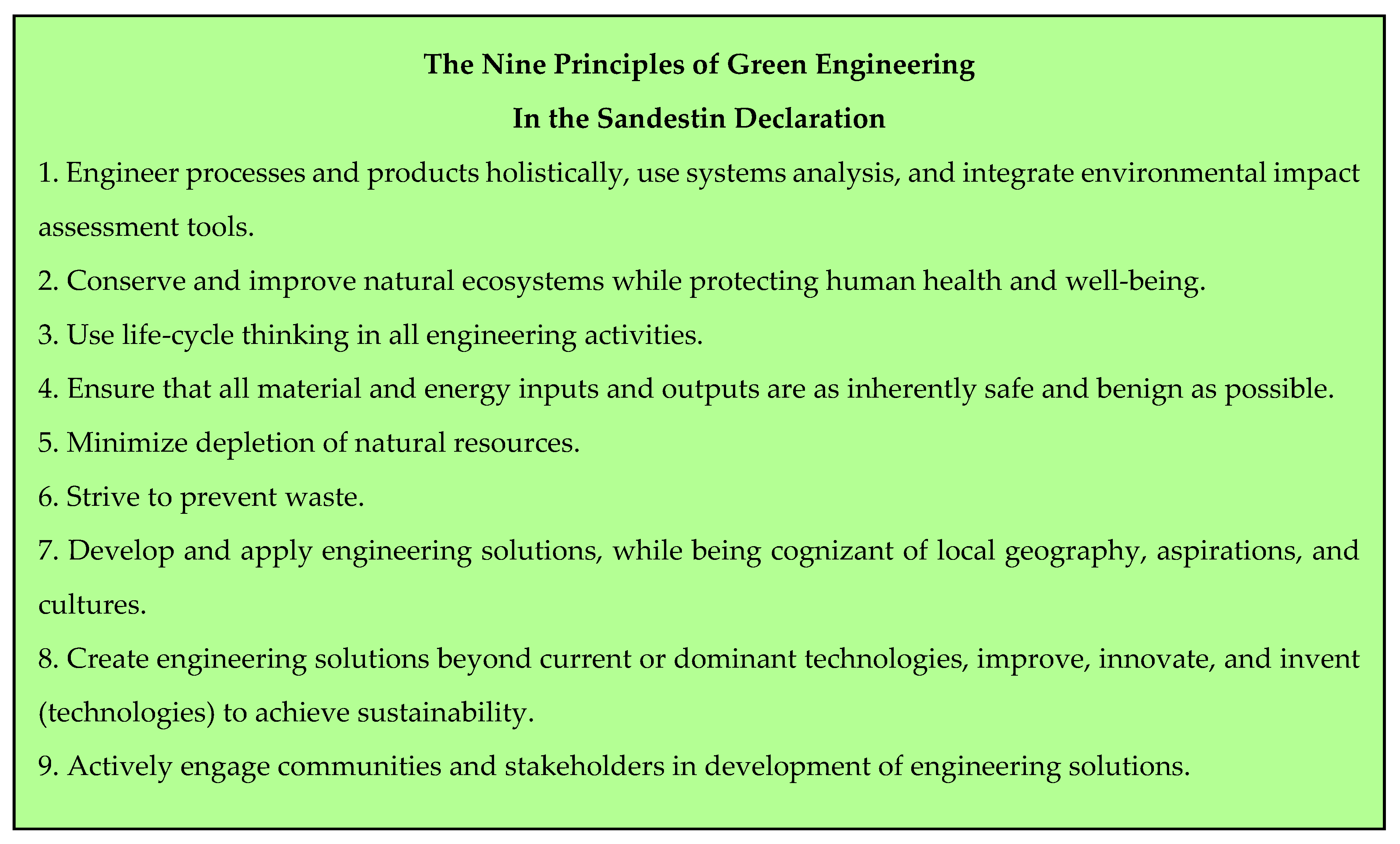
2. The Twelve Principles of Green Chemistry and the Principles of Green Engineering
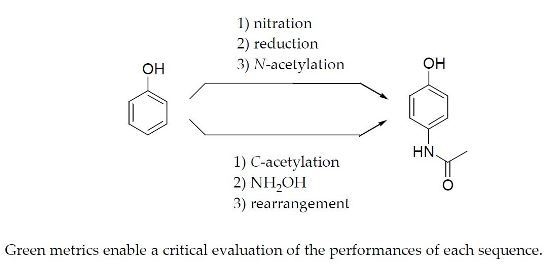
3. Some Synthetic Routes to Paracetamol
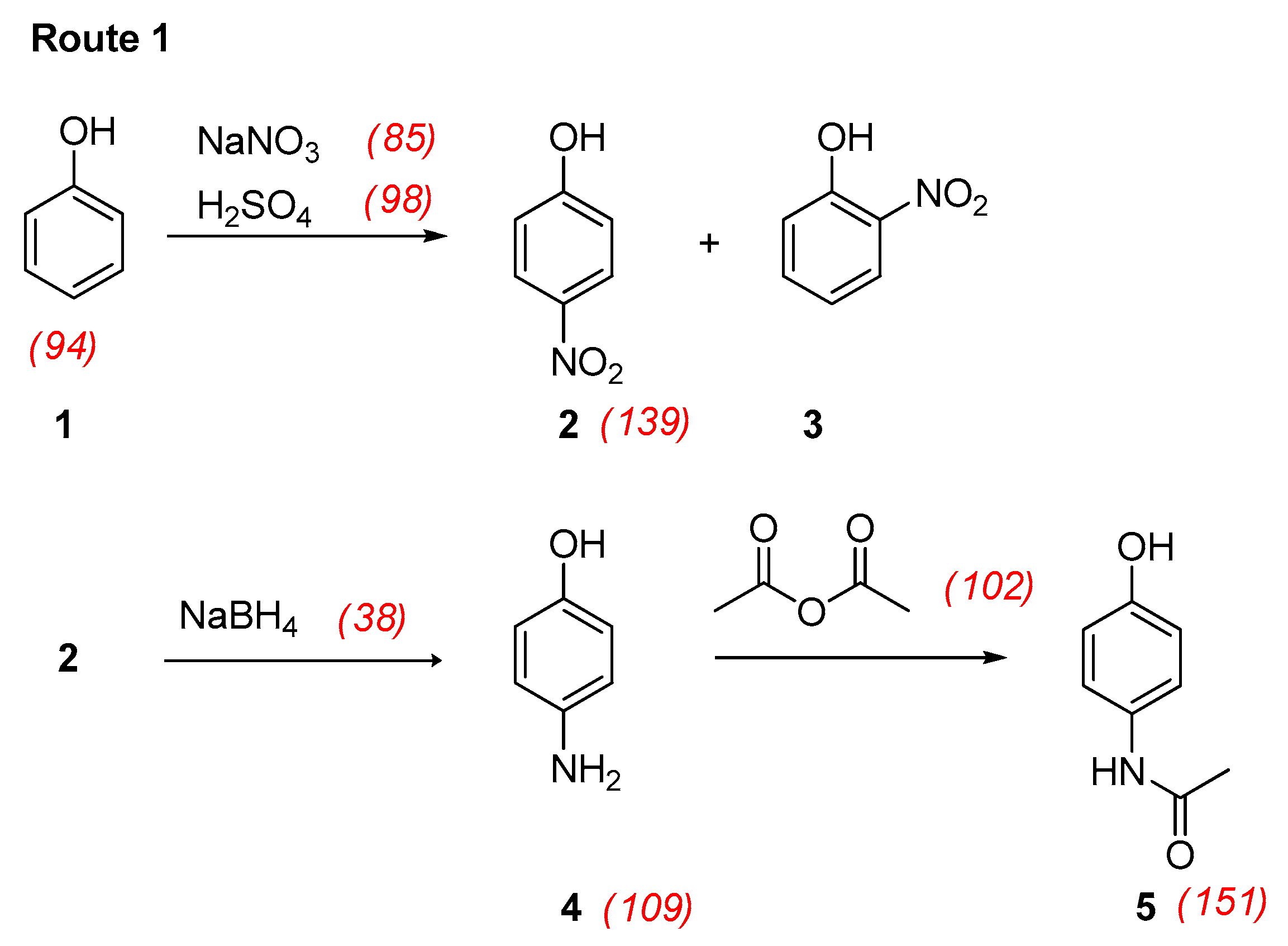
3.1. Nitration of Phenol
3.2. Reduction of 4-Nitrophenol
3.3. Acetylation of 4-Aminophenol
3.3.1. With Diluted Acetic Anhydride
3.3.2. With Pure Acetic Anhydride in the Presence of a Catalyst
3.3.3. With Acetic Anhydride under Solvent-Free and Catalyst-Free Conditions
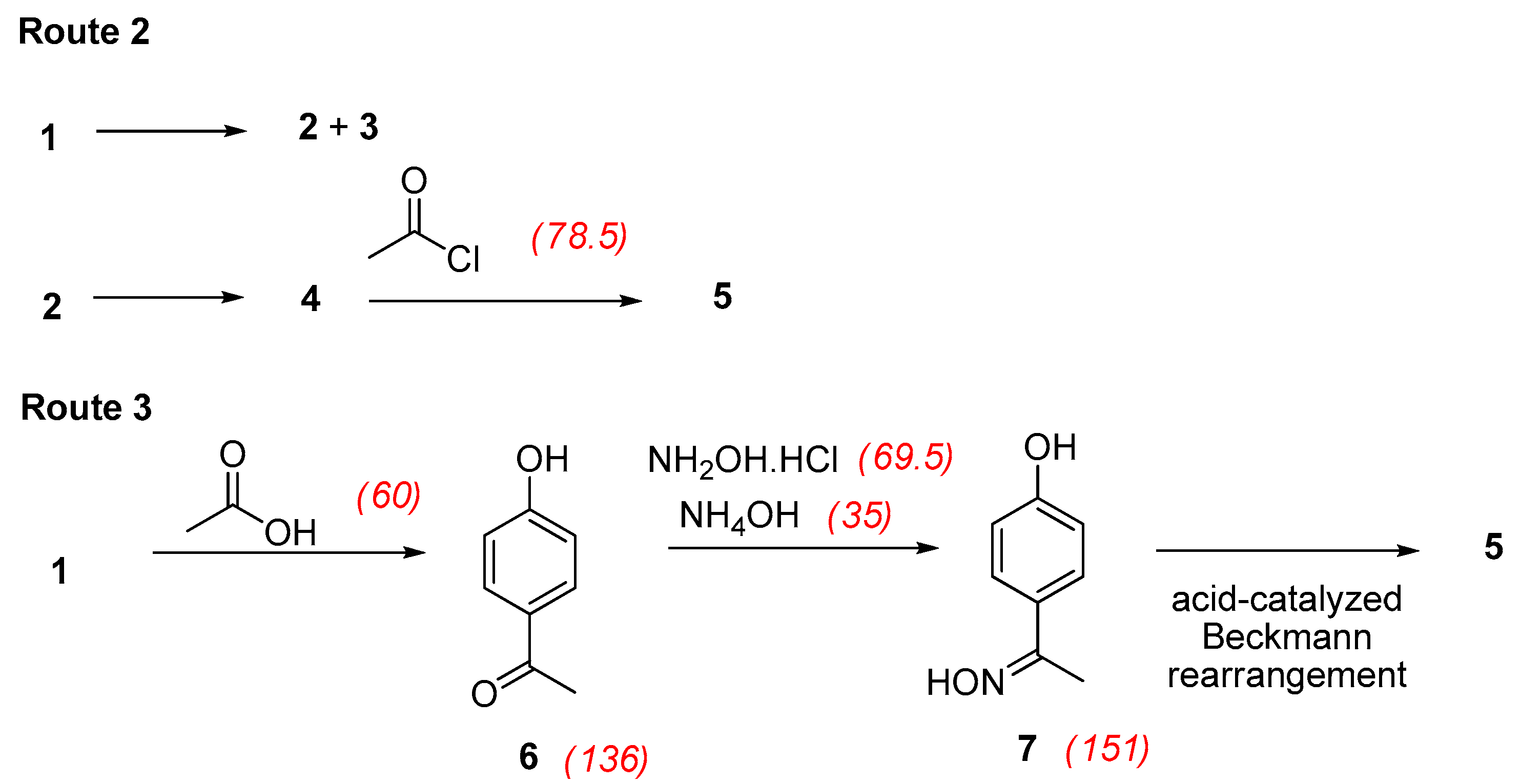
3.3.4. With Acetic Chloride under Solvent-Free and Catalyst-Free Conditions
3.4. Acetylation of Phenol
3.5. Formation of the Oxime of 4′-Hydroxyacetophenone
3.6. Formation of Paracetamol by an Acid-Catalyzed Beckmann Rearrangement
4. Mass Balances
4.1. When Reactants and Product(s) Only Are Considered
4.1.1. Atom Economy: A Theoretical Value
4.1.2. Yield: A Realistic Experimental Value
4.1.3. Reaction Mass Efficiency Following Curzons
- Following Route 1 and acetylation with diluted acetic anhydridewhere 2.47 is the weight (in g) of paracetamol and 9.40, 15, 25, 2.88, and 3.49 are the weights (in g) of phenol, sodium nitrate, sulfuric acid, sodium borohydride, and acetic anhydride, respectively;overall RMECurzons = [2.47/(9.40 + 15 + 25 + 2.88 + 3.49)] × 100 = 4%
- Following Route 2 (N-acetylation with acetyl chloride)overall RMECurzons = [4.10/(9.40 + 15 + 25 + 2.88 + 2.17)] × 100 = 8%
- Following Route 3 (Hoechst-Celanese process)overall RMECurzons = [10.33/(9.40 + 12.0 + 7.32 + 4.82)] × 100 = 31%
4.1.4. Stoichiometric Factor
4.2. When Reactants, Product(s), As Well As Solvents and Any Additional Substance Are Considered
4.2.1. Global Reaction Mass Efficiency and Effective Mass Yield
4.2.2. Process Mass Intensity and E Factor
5. The EcoScale
6. The Radial Polygon Representation
7. Conclusions
Conflicts of Interest
References
- Paris Agreement—European Commission. Available online: http://ec.europa.eu/clima/policies/international/negotiations/paris/index_en.htm (accessed on 1 May 2016).
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: London, UK, 1998. [Google Scholar]
- 20th Annual Green Chemistry and Engineering Conference. Available online: http://www.gcande.org/ (accessed on 1 May 2016).
- Anastas, P.T.; Zimmerman, J.B. Design through the 12 principles of engineering. Environ. Sci. Technol. 2003, 37, 94A–101A. [Google Scholar] [CrossRef]
- Abraham, M.A.; Nguyen, N. Green engineering: Defining the principles—Results from the Sandestin conference. Environ. Prog. 2003, 22, 233–206. [Google Scholar] [CrossRef]
- Winterton, N. Twelve more green chemistry principles. Green Chem. 2001, 3, G73–G81. [Google Scholar]
- Bogle, D.; Seaman, M. The six principles of sustainability. TCE Magazine, March 2010; 30–32. [Google Scholar]
- Ellis, F. Paracetamol—A Curriculum Resource; Osborne, C., Pack, M., Eds.; Royal Society of Chemistry: London, UK, 2002. [Google Scholar]
- Anantharaman, P.N. Paracetamol from phenol via para-nitrophenol. Bull. Electrochem. 1985, 1, 471. [Google Scholar]
- Gharib, A.; Scheeren, J.H.W.; Bamoharram, F.F.; Roshani, M.; Jahangir, M. Acetylation of p-aminophenol by Preyssler’s anion [NaP5W30O110]14−, [NaP5W29MoO110]14− with green condition at room temperature. Pol. J. Chem. Technol. 2009, 11, 31–35. [Google Scholar] [CrossRef]
- Amin, M.; Iqbal, M.S. Solvent-free Synthesis of Acetaminophen. US Patent 9006488, 14 Apirl 2015. [Google Scholar]
- Davenport, K.G.; Hilton, C.B. Process for Producing N-acyl-hydroxy Aromatic Amines. US Patent 4524217, 18 June 1985. [Google Scholar]
- Fritch, J.R.; Fruchey, S.O.; Horlenko, T.; Aguilar, D.A.; Hilton, C.B.; Snyder, P.S.; Seeliger, W.J. Production of Acetaminophen. US Patent 5155273, 13 October 1992. [Google Scholar]
- Trost, B.M. The atom economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Organic Synthesis—Past, present and future. Chemistry and Industry: London, UK, 1992; pp. 903–906. [Google Scholar]
- Curzons, A.D.; Constable, D.J.C.; Mortimer, D.N.; Cunningham, V.L. So you think your process is green, how do you know?—Using principles of sustainability to determine what is green—A corporate perspective. Green Chem. 2001, 3, 1–6. [Google Scholar] [CrossRef]
- Dicks, A.P.; Hent, A. Atom economy and reaction mass efficiency. In Green Chemistry Metrics. A Guide to Determining and Evaluating Process Greenness; Springer: Heidelberg, Germany, 2015. [Google Scholar]
- Andraos, J. Unification of reaction metrics for green chemistry: Application to reaction analysis. Org. Process Res. Dev. 2005, 9, 149–163. [Google Scholar] [CrossRef]
- Andraos, J.; Hent, A. Simplified application of material efficiency green metrics to synthesis plans: Pedagogical case studies selected from Organic Synthesis. J. Chem. Educ. 2015, 92, 1820–1830. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Curzons, A.D.; Cunningham, V.L. Metrics to “green” chemistry—Which are the best? Green Chem. 2002, 4, 521–527. [Google Scholar] [CrossRef]
- Dunn, P.J. The Importance of Green Chemistry in process Research & Development. In Pharmaceutical Process Development—Current Chemical and Engineering Challenges; Blacker, A.J., Williams, M.T., Eds.; RSC Publications: Cambrige, UK, 2011; pp. 117–137. [Google Scholar]
- Hudlicky, T.; Frey, D.A.; Koroniak, L.; Claeboe, C.D.; Brammer, L.E., Jr. Toward a reagent free synthesis. Green Chem. 1999, 1, 57–59. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Accounting for Chemical Sustainability. In Sustainable Industrial Chemistry: Principles, Tools and Industrial Examples; Cavani, F., Centi, G., Perathoner, S., Trifiro, G., Eds.; Wiley-VCH: Weinheim, Germany, 2009; pp. 279–318. [Google Scholar]
- Sheldon, R.A. The E factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Avres, R.U. Life cycle analysis: A critique. Resour. Conserv. Recyl. 1995, 14, 199–223. [Google Scholar] [CrossRef]
- Anastas, P.T.; Lankey, R.L. Life cycle assessment and green chemistry: The yin and yang of industrial ecology. Green Chem. 2000, 2, 289–295. [Google Scholar] [CrossRef]
- Cespi, D.; Beach, E.S.; Swarr, T.E.; Passarini, F.; Vassura, I.; Dunn, P.J.; Anastas, P.T. Life cycle inventory improvement in the pharmaceutical sector: Assessment of the sustainability combining PMI and LCA tools. Green Chem. 2015, 17, 3390–3400. [Google Scholar] [CrossRef]
- ISO 14040:2006(en). Environmental management—Life cycle assessment—Principles and framework. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14040:ed-2:v1:en (accessed on 1 May 2016).
- Van Aken, K.; Strekowski, L.; Patiny, L. EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein J. Org. Chem. 2006, 2. [Google Scholar] [CrossRef] [PubMed]
- Ecoscale.org. 2016. Available online: www.ecoscale.org (accessed on 1 May 2016).
- Dash, R.; Song, J.J.; Roschangar, F.; Samstag, W.; Senanayake, C.H. The eight criteria defining a good manufacturing process. Org. Process Res. Dev. 2012, 16, 1697–1706. [Google Scholar]
- Andraos, J.; Sayed, M. On the use of “green” metrics in the undergraduate organic chemistry lecture and lab to assess the mass efficiency of organic reactions. J. Chem. Educ. 2007, 84, 1004–1010. [Google Scholar] [CrossRef]
- Andraos, J. Inclusion of environmental impact parameters in radial pentagon material efficiency metrics analysis: Using benign indices as a step towards a complete assessment of “greenness” for chemical reactions and synthesis plans. Org. Process Res. Dev. 2012, 16, 1482–1506. [Google Scholar] [CrossRef]
- Lapkin, A.; Constable, D.C. Green Chemistry Metrics: Measuring and Monitoring Sustainable Processes; John Wiley & Sons, Ltd: Chichester, UK, 2008. [Google Scholar]
- Calvos-Flores, F.G. Sustainable chemistry metrics. ChemSusChem 2009, 2, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Dunn, P.J.; Wells, A.S.; William, M.T. Green Chemistry in the Pharmaceutical Industry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Lancaster, M. Green Chemistry: An Introductory Text, 2nd ed.; RSC Publishing: Cambridge, UK, 2010. [Google Scholar]
- Jimenez-Gonzalez, C.; Poechlauer, P.; Broxterman, Q.B.; Yang, B.-S.; Ende, D.A.; Baird, J.; Bertsch, C.; Hannah, R.E.; Dell’Orco, P.; Noorman, H.; et al. Key green engineering research areas for sustainable manufacturing: A perspective from pharmaceutical and fine chemicals manufacturers. Org. Process Res. Dev. 2011, 15, 900–911. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Ponder, C.S.; Broxterman, Q.B.; Manley, J.B. Using the right green yardstick: Why process mass intensity is used in the pharmaceutical industry do drive more sustainable processes. Org. Process Res. Dev. 2011, 15, 912–917. [Google Scholar] [CrossRef]
- Mercer, S.M.; Andraos, J.; Jessop, P.G. Choosing the greenest synthesis: A multivariate metric green chemistry exercise. J. Chem. Educ. 2012, 89, 215–220. [Google Scholar] [CrossRef]
- Andraos, J. The Algebra of Organic Synthesis: Green Metrics, Design Strategy, Route Selection, and Optimization; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Ribeiro, M.G.T.C.; Machado, A.A.S.C. Greenness of chemical reactions –limitations of mass metrics. Green Chem. Lett. Rev. 2013, 6, 1–18. [Google Scholar] [CrossRef]
- Baird, C.; Cann, M. Environmental Chemistry, 5th ed.; Edition, W.H., Ed.; Freeman and Co: New York, NY, USA, 2012. [Google Scholar]
- Anastas, P.T.; Zimmerman, J.B. Innovations in Green Chemistry and Green Engineering: Selected Entries from the Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2013. [Google Scholar]
- Koenig, S.G. Scalable Green Chemistry: Case Studies from the Pharmaceutical Industry; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Toniolo, S.; Arico, F.; Tundo, P. A comparative environmental assessment for the synthesis of 1,3-oxazin-2-one by metrics: greenness evaluation and blind spots. ACS Sustain. Chem. Eng. 2014, 2, 1056–1062. [Google Scholar] [CrossRef]
- Albini, A.; Protti, S. Paradigms in Green Chemistry and Technology; Springer Verlag GmbH: Berlin, Germany, 2016. [Google Scholar]


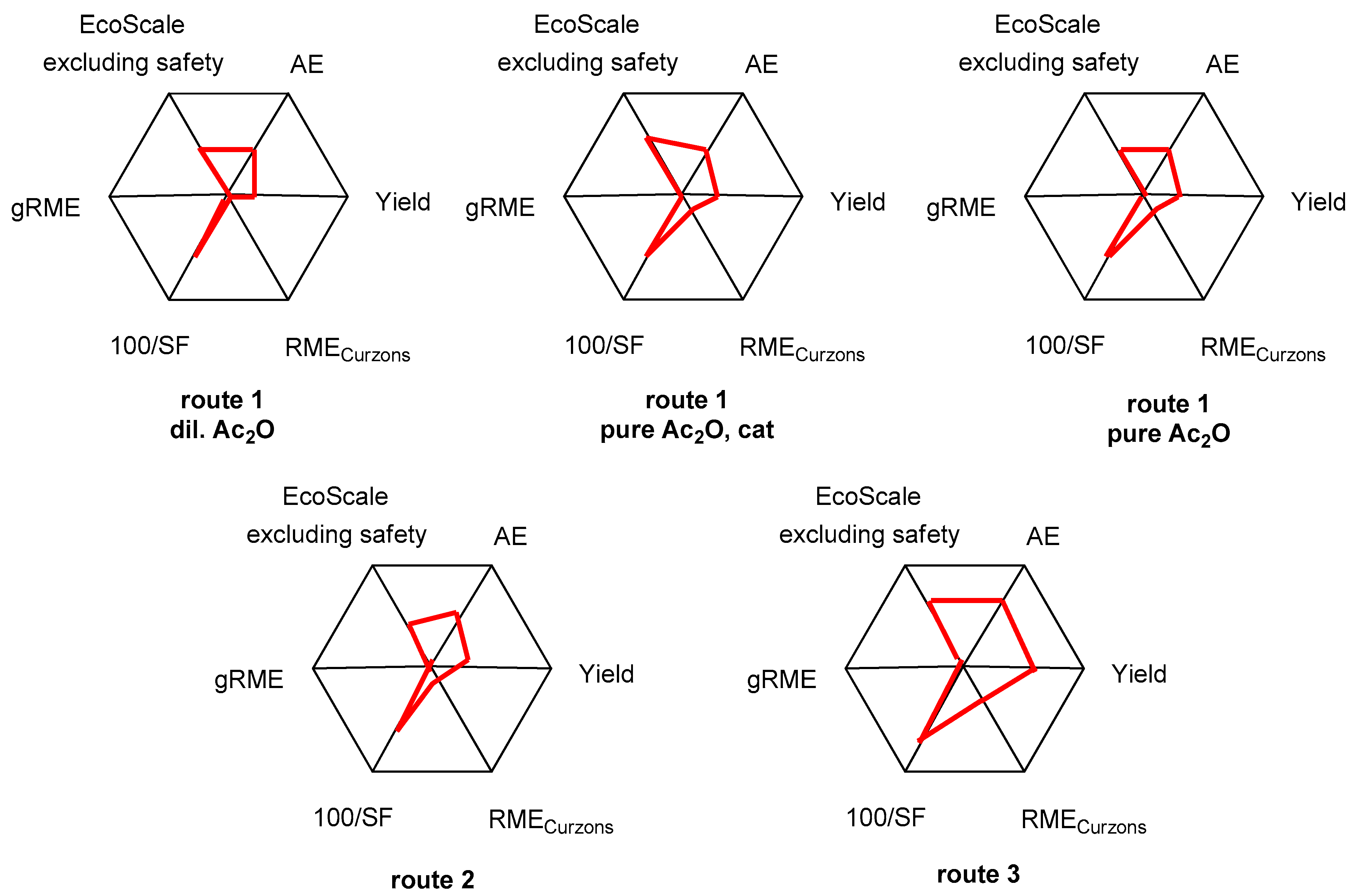
| AE (%) | Yield (%) | RMECurzons (%) | SF | gRME (%) | |
|---|---|---|---|---|---|
| Route 1 | |||||
| Nitration of phenol | 50 | 37 | 10 | 1.79 | 4 |
| Reduction | 62 | 74 | 37 | 1.22 | 2 |
| Acetylation (dil. Ac2O) | 72 | 60 | 38 | 1.12 | 7 |
| Overall (dil. Ac2O) | 36 | 16 | 4 | 1.67 | 1 |
| Acetylation (pure Ac2O, cat.) | 72 | 91 | 23 | 1.92 | 4 |
| Overall (pure Ac2O, cat.) | 36 | 25 | 6 | 2.08 | 1 |
| Acetylation (pure Ac2O) | 72 | 97 | 69 | 1.01 | 69 |
| Overall (pure Ac2O) | 36 | 27 | 7 | 1.61 | 2 |
| Route 2 | |||||
| Acetylation (AcCl) | 81 | 99 | 79 | 1.01 | 79 |
| Overall (AcCl) | 38 | 27 | 8 | 1.64 | 2 |
| Route 3 | |||||
| Acetylation of phenol | 88 | 96 | 61 | 1.39 | 6 |
| Oximation | 63 | 99 | 58 | 1.09 | 8 |
| Rearrangement | 100 | 71 | 71 | 1.00 | 5 |
| Overall | 58 | 68 | 31 | 1.32 | 2 |
| Parameters | Penalty Points |
|---|---|
| Yield | (100 − Effective Yield)/2 |
| Price of the reaction components (to obtain 10 mmol) | |
| Inexpensive (< 10 US$) | 0 |
| Expensive (between 10 and 50 US$) | 3 |
| Very expensive (> 50 US$) | 5 |
| Safety (adapted for the Globally Harmonized System of Classification and Labeling of Chemicals) | |
| GHS09 (dangerous for the environment) | 5 |
| GHS06 (toxic) | 5 |
| GHS02 (flammable) | 5 |
| GHS01 (explosive) | 10 |
| GHS07, GHS08 (extremely toxic) | 10 |
| Technical setup | |
| Common setup | 0 |
| Instruments for controlled addition (dropping funnel, etc.) | 1 |
| Unconventional activation technique (microwave, etc.) | 2 |
| Pressure equipment > 1 atm | 3 |
| Any additional special glassware | 1 |
| (Inert) gas atmosphere | 1 |
| Glove box | 3 |
| Temperature/Time | |
| Room temperature, < 1 h | 0 |
| Room temperature, < 24 h | 1 |
| Heating < 1 h | 2 |
| Heating > 1 h | 3 |
| Cooling to 0 °C | 4 |
| Cooling < 0 °C | 5 |
| Workup/Purification | |
| None | 0 |
| Cooling to room temperature | 0 |
| Adding solvent | 0 |
| Simple filtration | 0 |
| Removal of solvent with bp < 150 °C | 0 |
| Crystallization and filtration | 1 |
| Removal of solvent with bp > 150 °C | 2 |
| Solid phase extraction | 2 |
| Distillation | 3 |
| Sublimation | 3 |
| Liquid-liquid extraction | 3 |
| Classical chromatography | 10 |
| Parameters | Penalty Points for Route | ||||
|---|---|---|---|---|---|
| 1, Acetylation with dil. Ac2O | 1, Acetylation with Pure Ac2O and Cat. | 1, Acetylation with Pure Ac2O | 2 | 3 | |
| Yield | 42.0 (37%) | 37.5 (25%) | 36.5 (27%) | 36.5 (27%) | 16 (68%) |
| Safety | |||||
| Reagents a | 45 | 45 | 45 | 50 | 60 |
| Intermediates b | 50 | 50 | 50 | 50 | 20 |
| Solvents, auxiliaries c | 30 | 30 | 30 | 30 | 50 |
| Technical setup | 1 | 1 | 3 | 3 | 4 |
| Temperature/Time | 8 | 8 | 8 | 8 | 14 |
| Workup/Purification | 6 | 6 | 6 | 6 | 6 |
| Overall EcoScale scores (excluding safety) | 43.0 | 47.5 | 46.5 | 46.5 | 60.0 |
© 2016 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanden Eynde, J.J. How Efficient Is My (Medicinal) Chemistry? Pharmaceuticals 2016, 9, 26. https://doi.org/10.3390/ph9020026
Vanden Eynde JJ. How Efficient Is My (Medicinal) Chemistry? Pharmaceuticals. 2016; 9(2):26. https://doi.org/10.3390/ph9020026
Chicago/Turabian StyleVanden Eynde, Jean Jacques. 2016. "How Efficient Is My (Medicinal) Chemistry?" Pharmaceuticals 9, no. 2: 26. https://doi.org/10.3390/ph9020026
APA StyleVanden Eynde, J. J. (2016). How Efficient Is My (Medicinal) Chemistry? Pharmaceuticals, 9(2), 26. https://doi.org/10.3390/ph9020026