Cognitive Dysfunction in Major Depressive Disorder. A Translational Review in Animal Models of the Disease
Abstract
:1. Introduction
2. Cognition in Patients Suffering from MDD
2.1. Cognitive Performances in MDD through Different Ages
2.2. Cognitive Neuropsychological Assessments Instruments Used for MDD Patients
- Attention processing monitoring: the Digit Span test and the Continuous Performance test,
- Processing speed: the Trail Making Test-Part A, the Digit symbol test and the Finger Tapping test,
- Executive functions and verbal memory: the Stroop Color Word test, the Trail Making Test-Part B and the Wisconsin Card Sorting Test,
- Memory functions: the Rey Auditory Verbal Learning Test, the Wechsler Memory Scale and the California Verbal Learning task
2.3. Progression of Cognitive Symptoms along the Course of MMD
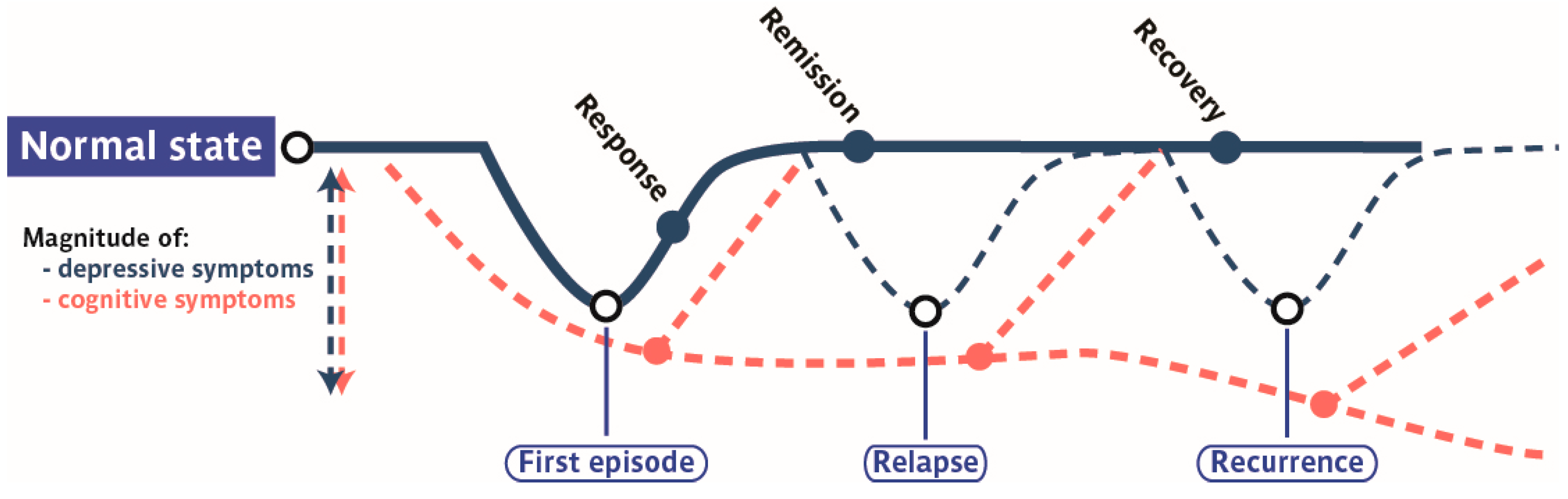
2.4. Cognitive Dysfunctions According to the MDD Subtypes
| MDD Type | Sex | Mean Age (Years) | Cognitive Domains Tested | Assessment Methods | Main Results | Reference |
|---|---|---|---|---|---|---|
| MEL (n = 20) Non-MEL (n = 18) CTRL (n = 38) | ♂/♀ | 39 | Psychomotor tasks | Fitt’s task Figure-copying task Symbol digit substitution task | MEL patients were slower performing all the tasks compared to non-MEL and CTRL patients. | [67] |
| MEL (n = 7) Non-MEL (n = 8) CTRL (n = 8) | ♂/♀ | 40 | Response selection Attention Executive function | Choice reaction task (T1) Spatial Stroop task SRC task Spatial Stroop + SRC task |
| [68] |
| MEL (n = 11-20) Non-MEL (n = 11–20) CTRL (n = 11–20) | ♀ | 50 | Executive function Memory | CANTAB battery: ID/ED set shifting task, SOC Spatial Recognition Memory, PAL |
| [69,70] |
| MEL (n = 26) Non-MEL (n = 9) CTRL (n = 26) | ♀ | 34 | Explicit episodic memory Implicit (procedural) learning | WMS-R SRTT |
| [71] |
| MEL (n = 17) Non-MEL (n = 17) | ♂/♀ | 41 | IQ Executive function Attention/working memory Learning/long-term verbal memory Prospective memory Attention, response inhibition Set shifting, feedback use Semantic memory/ verbal fluency Planning, self-monitoring, multi-tasking | NART Donders Simple Reaction Time Digit Span test CVLT Prospective memory task SCWT Shortened WCST COWAT SET | Baseline MDD: MEL patients showed deficits in memory tasks (CVLT trial 1, CVLT total trials) and prospective memory (delayed free recall). MEL group recalled fewer words overall; MEL patients performed more poorly than non-MEL group in executive functions tests: digit span backwards, SCWT interference score, WCST categories completed, WCST perseverative errors and modified Six Elements Test; Remitted MDD: Same cognitive impairments in MEL patients were observed. | [72] |
| MEL (n = 20) Non-MEL (n = 20) CTRL (n = 20) | ♂/♀ | 47 | Working memory Emotion classification Arousal and valence rating | Emotion face paradigms | MEL patients showed better memory for sad faces (sad benefit). | [73] |
| MEL (n = 65) Non-MEL (n = 59) CTRL (n = 124) | ♂/♀ | 39 | Memory recall Attention Short-term working memory Executive functioning Semantic knowledge, language Spatial visual memory Cognitive flexibility, selective attention | Verbal recall, recognition task Time estimation task Reverse digit span task Executive maze task Word Generation test Span of visual memory task Verbal Interference test Switching of attention task | MEL patients showed poorer performances in spatial visual memory and attention task compared to non-MEL group. | [74]) |
| MEL (n = 142) Atypical (n = 76) Undifferentiated (n = 91) CTRL (n = 200) | ♂/♀ | 34 | Processing speed Attention Shifting Planning Verbal fluency Visual spatial memory Verbal working memory | TMT-A, Digit symbol coding subtest Digit Span Forward of the WAIS-RC Modified WCST, TMT-BTower of Hanoi (TOH) Animal naming WMR-RC Digit span backward subtest of the WAIS-RC | MDD state: In the domains of processing speed (TMT-A and Digital Symbol Coding of WAIS-RC) and verbal fluency (animal naming), MEL patients performed significantly worse than atypical patients; Remitted state : MEL patients performed worse than controls in processing speed, shifting and verbal fluency. | [63] |
| MEL (n = 25) Non-MEL (n = 63) | ♂/♀ | 48 | Attention Information processing speed/Mental flexibility Psychomotor speed Response inhibition Planning Working memory Semantic verbal fluency | TMT A-B; WAIS-ITMT A-B ; SCWT I/IIFinger Tapping test SCWT III Tower of London WAIS-I/WAIS-IINaming | Baseline: MEL patients performed worse than non-MEL patients in most of neuropsychological tests (TMT-B; WAIS I/II, SCWT, TOL and in the Finger Tapping Test); Remitted state: Overall, MEL patients performed worse than non-MEL patients. Significant differences were found in the TMT-B and the semantic verbal fluency. | [62] |
| MEL (n = 279) Non-MEL (n = 544) CTRL (n = 247) | ♂/♀ | 37 | Motor coordination Response inhibition Sustained attention Decision speed Information processing Verbal memory Working memory Executive function Cognitive flexibility Explicit emotion identification Implicit emotion identification | Finger tapping Go-NoGo Continuous performance task Choice reaction time task Switching attention Memory recall and cognition Digit span task Maze task Verbal interference Identification accuracy/RTPriming RT | MEL patients performed worse at switching attention, decision speed and verbal interference compared to non-MEL patients; MEL patients were significantly slower than non-MEL patients to identify happy faces in explicit and implicit emotion identification. | [75] |
3. Cognitive Behavioral Paradigms Used to Assess Learning and Memory Performances in Rodent Models of MDD
| Cognitive Domains and Functions | Behavioral Paradigms in Rodents |
|---|---|
| Attention | 5-choice serial reaction time task (5-CSRTT) |
Executive function
| Attentional set-shifting task (ASST) Reversal Morris water maze Prepulse inhibition (PPI) |
| Learning and memory | |
| Working memory | Delayed alternation Y-maze Delayed alternation T-maze Delayed match-to-sample with odors, subjects Modified MWM, BM, RAWM, RAM |
| Episodic memory | Novel object recognition test Object location recognition Passive avoidance place Social discrimination procedure |
| Reference spatial memory | Morris water maze (MWM) Barnes maze (BM) Radial arm water maze (RAWM) Object location recognition/ Object-in-place |
| Associative memory | Contextual/cued fear conditioning Extinction fear conditioning Passive/active avoidance place |
4. Episodic Memory in Rodent Models of Anxiety/Depression
4.1. WHAT?
| Behavioral Test | Animal Model | Species | Sex | Age When Tested | Interval Intertrial | Effect on Discrimination Index (DI) | Reference |
|---|---|---|---|---|---|---|---|
| WHAT? | PNS | Mouse | ♂/♀ | Juvenile (PN23) | 4 h | No effect | [105] |
| ♂ | Adult (PN45) | No effect | |||||
| ♀ | Impairment | ||||||
| Rat | ♂/♀ | Juvenile (PN23) | 2 h | No competent for the task | [106] | ||
| ♂ | Adult (PN56) | Impairment | |||||
| ♀ | No effect | ||||||
| ♀ | Juvenile (PN28) | 1 h | No competent for the task | [107] | |||
| Adult (PND90) | 1 h | Impairment | |||||
| ♂ | Adult (PND80) | 1 h | Impairment | [108] | |||
| ♀ | 1 h | No effect | |||||
| ♂ | Adult (PND60) | 40 min | Improvement | [114] | |||
| ♀ | 40 min | No effect | |||||
| ♂/♀ | Adult (PND63) | 15 min | No effect | [109] | |||
| ♂ | 1 h | No effect | |||||
| ♀ | Impairment | ||||||
| ♂ | 3 h | Impairment | |||||
| ♀ | Impairment | ||||||
| ♂/♀ | Adult (PND63) | 24 h | Impairment | [110] | |||
| MS | Mouse | ♀ | Adult (PND85) | 6 h | Impairment | [111] | |
| ♂/♀ | Adult (PND60) | 24 h | Impairment | [112] | |||
| Rat | ♂ | Adult (PND60) | 1 h, 4 h | Impairment | [113] | ||
| ♂ | Adult (PND70) | 1 h | No effect | [116] | |||
| ♂ | Adult (PND75) | 1 h | No effect | [117] | |||
| 24 h | |||||||
| ♂ & ♀ | Adult (PND90) | 2 h | No effect | [118] | |||
| ♀ | Adult (PND55) | 1 h | Improvement | [115] | |||
| 24 h | |||||||
| MD | ♂ | Juvenile (PND35) | 1 h | No effect | [119] | ||
| ♀ | Impairment | ||||||
| ♂/♀ | Adult (PND60) | 4 h | Impairment | [120] | |||
| Early stress life | Mouse | ♂/♀ | Adult (PND > 90) | 24 h | Impairment | [121] | |
| Social defeat | Mouse | ♂ | Adult | 1 h | Impairment | [122] | |
| 24 h | Impairment | ||||||
| UCMS | Mouse | ♂ | 1 h, 2 h | Impairment | [123,124,125] | ||
| 24 h | Impairment | [126] | |||||
| Rat | ♂ | 1 h | Impairment | [125,127,128] | |||
| CORT | Mouse | ♂ | 5 min | Impairment | [102] | ||
| 1 h | Impairment | [129,130] | |||||
| 24 h | Impairment | ||||||
| Rat | ♂/♀ | 1 h | No effect | [118] | |||
| WHERE? | PNS | Rat | ♂ | Adult (PND80) | 1 h | Impairment | [108] |
| ♀ | 1 h | No effect | |||||
| Early stress life | Mouse | ♂ | Adult (PND > 90) | 24 h | Impairment | [121] | |
| ♀ | 24 h | No effect | |||||
| MS | Rat | ♂ | Adult (PND75) | 1 h | No effect | [117] | |
| 24 h | Improvement | ||||||
| UCMS | Mouse | ♂ | Adult | 24 h | Impairment | [126] | |
| Rat | ♂ | 4 h | Impairment | [127] | |||
| WHEN? | PNS | Rat | ♂ | Juvenile (PND30–40) | 1 h | No effect | [131] |
| MS | Adult (PND75) | 1 h, 3 h | Improvement | [117] | |||
| Adult (PND60) | 3 h | Impairment | [132] |
4.2. Where and When?
5. Working Memory Deficits in Rodent Models of Anxiety/Depression
| Cognitive Domain | Model | Species | Gender | Effect on Working Memory | Reference |
|---|---|---|---|---|---|
| Working alternation task: T-maze or Y-maze | PNS | Rat | ♂/♀ | ↓ alternation | [139,140,141] |
| MS | Rat | ♂/♀ | No effect | [118,119,142,143,144] | |
| Mouse | No effect | [112] | |||
| ♂ | Strain-specific effects | [145] | |||
| Social defeat | Mouse | ♂ | ↓ alternation | [146] | |
| Rat | ♂ | Delay-specific effects | [147] | ||
| ♂/♀ | No effect | [148] | |||
| CMS | Rat | ♂ | ↓ alternation | [149,150,151,152] | |
| Mouse | ↓ alternation | [153] | |||
| CORT | Rat | ♂/♀ | No effect | [118] | |
| ♂ | No effect | [118,142,144,154,155] | |||
| Working spatial memory: cued MWM/BM/RAW | PNS | Rat | ♂/♀ | No effect | [109] |
| Learning impairment | [140] | ||||
| MS | Rat | ♂/♀ | No effect | [156] | |
| ♀ | No effect in learning | [115] | |||
| Enhancement in retention | |||||
| Social defeat | Rat | ♂ | Delay-specific effects | [157,158] | |
| CMS | Rat | ♂ | Learning impairment | [159] | |
| Retention impairment | [160] | ||||
| CORT | Rat | ♂ | No effect | [161,162] |
6. Attention and Executive Functions in Rodent Models of Anxiety/Depression
| Executive Function | Task | Model | Species | Gender | Behavioral Effect | Reference |
|---|---|---|---|---|---|---|
| Attention/Impulsivity | 5-CSRTT | CRH-KO | Mouse | ♂ | Impairment | [167] |
| PNS | Rat | ♂/♀ | Impairment | [168] | ||
| CMS | ♂ | Impairment | [169] | |||
| CORT | ♂ | Bidirectional effects | [170] | |||
| Attentional set-shifting task | ASST | MS | Mouse | ♂/♀ | Strain-specific impairment | [145] |
| Rat | ♂ | Impairment | [132] | |||
| UCMS | Impairment | [175,176] | ||||
| CRS | Impairment | [177] | ||||
| CORT | Impairment | [178] | ||||
| Reversal learning | MWM | MS | Mouse | ♂ | Reversal learning impairment | [111] |
| ♀ | No effect | |||||
| Rat | ♂/♀ | Reversal learning impairment | [179] | |||
| Reversal learning enhancement | [180] | |||||
| Social defeat | Mouse | ♂ | Reversal learning impairment | [181] | ||
| ♀ | No effect | |||||
| UCMS | Mouse | ♂ | Reversal learning Impairment | [182] | ||
| Rat | ♂ | Reversal learning impairment | [183,184] | |||
| CORT | Mouse | ♂ | Reversal learning/retention impairment | [102] | ||
| Rat | ♂ | Reversal learning impairment | [161] |
7. Spatial Learning and Memory Deficits in Rodent Models of Anxiety/Depression
| Model | Test | Species | Gender | Age When Tested | Learning | Retention | Reference |
|---|---|---|---|---|---|---|---|
| PNS | MWM | Rat | ♂ | Juvenile | Impairment | Impairment | [141] |
| ♀ | No effect | No effect | |||||
| ♂/♀ | Adult | Impairment | Impairment | [106,194,195] | |||
| ♂ | No effect | Impairment | [195] | ||||
| ♂/♀ | No effect | Impairment | [196] | ||||
| No effect | Improvement | [109] | |||||
| No effect | NA | [197] | |||||
| Mouse | ♂/♀ | No effect | Impairment | [198] | |||
| BM | Mouse | ♂ | No effect | No effect a | [199] | ||
| Improvement a | |||||||
| MS | MWM | Rat | ♂/♀ | Juvenile | No effect | No effect | [180] |
| Adult | Impairment | Impairment | |||||
| No effect | No effect | [200] | |||||
| ♂ | No effect | No effect | [132,179] | ||||
| No effect | Impairment | [201,202] | |||||
| No effect | Improvement | [203] | |||||
| ♀ | No effect | Improvement | [115] | ||||
| Chronic early life stress | MWM | Mouse | ♂ | Adult | Impairment | Impairment | [121] |
| ♀ | No effect | No effect | |||||
| Social defeat | MWM | Mouse | ♂ | Adult | No effect | No effect | [122,146] |
| BM | Mouse | ♂ | No effect | NA | [181] | ||
| ♀ | No effect | NA | |||||
| RAWM | Rat | ♂ | NA | Impairment | [157] | ||
| Learned helplessness | MWM | Mouse | ♂ | Adult | Impairment | Impairment | [190] |
| UCMS | MWM | Mouse | ♂ | Adult | Impairment | Impairment | [123,182,190,204] |
| Rat | Impairment | Impairment | [128,159,205] | ||||
| CUR | RAWM | Rat | ♂ | Adult | No effect | Impairment b | [206] |
| No effect | Improvement b | ||||||
| Impairment | No effect b | [207] | |||||
| ♀ | No effect | No effect b | |||||
| CORT | BM | Mouse | ♂ | Adult | Impairment | Impairment | [102] |
| MWM | Mouse | Impairment | Impairment | ||||
| Rat | Impairment | Impairment | [208] | ||||
| RAM | Rat | Impairment | Impairment | [209] | |||
| BM | Rat | Impairment | Impairment | [154,160,209] | |||
| No effect | Improvement | [162] |
8. Associative Memory in Rodent Models of Anxiety/Depression
| Type of Task | Model | Species | Age When Tested | Sex | Fear Conditioning | Fear Extinction | Reference |
|---|---|---|---|---|---|---|---|
| Contextual/cued associative task | PNS | Mouse | Weaning | ♂/♀ | No effect | No effect | [105] |
| Juvenile | No effect | No effect | |||||
| Rat | Adult | - | Impairment | [208] | |||
| Impairment | No effect | [106] | |||||
| MS | Mouse | Adult | ♂/♀ | Impairment | - | [111,112] | |
| Rat | Juvenile | Impairment | No effect | [218] | |||
| Adult | Impairment | Impairment | |||||
| ♀ | No effect | No effect | [219] | ||||
| No effect | - | [220] | |||||
| Social defeat | Mouse | Adult | ♂ | Improvement | - | [146,221,227] | |
| Learned Helplessness | Rat | Improvement | Impairment | [222] | |||
| UCMS | Rat | Improvement | - | [149,223] | |||
| CORT | Mouse | Impairment | - | [102] | |||
| Rat | No effect | Impairment | [228] | ||||
| No effect | No effect | [155] | |||||
| Improvement | - | [224,225,226] |
| Type of Task | Model | Species | Gender | Latency to Enter into the Dark Compartment Compared to Controls | Reference |
|---|---|---|---|---|---|
| Passive avoidance task | PNS | Rat | ♂ | Decreased | [139,197] |
| ♂/♀ | Decreased | [140,196,233,234] | |||
| MS | Rat | ♂ | Decreased | [116,235] | |
| Social defeat | Mouse | ♂ | No effect | [238] | |
| CMS | Rat | ♂ | Decreased | [223,236] | |
| No effect | [149] | ||||
| CORT | ♂ | Decreased | [208,237] |
9. Cognitive and Emotional Deficits in Rodent Models of Anxiety/Depression and Their Relationship with Hippocampus Function
9.1. Hippocampal Formation and Its Role in Cognitive/Emotional Deficits
9.2. Impact of Neurogenesis on Cognitive and Emotional Function
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Porter, R.J.; Gallagher, P.; Thompson, J.M.; Young, A.H. Neurocognitive impairment in drug-free patients with major depressive disorder. Br. J. Psychiatry 2003, 182, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Hammar, A.; Ardal, G. Cognitive functioning in major depression—A summary. Front. Hum. Neurosci. 2009, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.H.; Greer, T.L. Cognitive dysfunction in unipolar depression: Implications for treatment. J. Affect. Disord. 2014, 152–154, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, B.J.; Knorr, U.; Kessing, L.V. Cognitive impairment in the remitted state of unipolar depressive disorder: A systematic review. J. Affect. Disord. 2011, 134, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Snyder, H.R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol. Bull. 2013, 139, 81–132. [Google Scholar] [CrossRef] [PubMed]
- Gonda, X.; Pompili, M.; Serafini, G.; Carvalho, A.F.; Rihmer, Z.; Dome, P. The role of cognitive dysfunction in the symptoms and remission from depression. Ann. Gen. Psychiatry 2015, 14, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershon, A.; Hayward, C.; Schraedley-Desmond, P.; Rudolph, K.D.; Booster, G.D.; Gotlib, I.H. Life stress and first onset of psychiatric disorders in daughters of depressed mothers. J. Psychiatr. Res. 2011, 45, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Hammen, C.; Hazel, N.A.; Brennan, P.A.; Najman, J. Intergenerational transmission and continuity of stress and depression: Depressed women and their offspring in 20 years of follow-up. Psychol. Med. 2012, 42, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Nishi, M.; Horii-Hayashi, N.; Sasagawa, T. Effects of early life adverse experiences on the brain: Implications from maternal separation models in rodents. Front. Neurosci. 2014, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Richman, C.L.; Dember, W.N.; Kim, P. Spontaneous alternation behavior in animals: A review. Curr. Psychol. 1986, 5, 358–391. [Google Scholar] [CrossRef]
- Blanchard, R.J.; McKittrick, C.R.; Blanchard, D.C. Animal models of social stress: Effects on behavior and brain neurochemical systems. Physiol. Behav. 2001, 73, 261–271. [Google Scholar] [CrossRef]
- Chourbaji, S.; Zacher, C.; Sanchis-Segura, C.; Dormann, C.; Vollmayr, B.; Gass, P. Learned helplessness: Validity and reliability of depressive-like states in mice. Brain Res. Brain Res. Protoc. 2005, 16, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005, 52, 90–110. [Google Scholar] [CrossRef] [PubMed]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.P.; et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Belzung, C.; Lemoine, M. Criteria of validity for animal models of psychiatric disorders: Focus on anxiety disorders and depression. Biol. Mood Anxiety Disord. 2011, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. The validity of animal models of depression. Psychopharmacology 1984, 83, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Consoli, G.; Picchetti, M.; Carlini, M.; Faravelli, L. Cognitive impairment in major depression. Eur. J. Pharmacol. 2010, 626, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Hermens, D.F.; Porter, M.A.; Redoblado-Hodge, M.A. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J. Affect. Disord. 2012, 140, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Iacoviello, B.; Neumeister, A.; Charney, D.S.; Iosifescu, D.V. Cognitive dysfunction in depression: Neurocircuitry and new therapeutic strategies. Neurobiol. Learn. Mem. 2011, 96, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J.; Agid, Y.; Brune, M.; Bullmore, E.T.; Carter, C.S.; Clayton, N.S.; Connor, R.; Davis, S.; Deakin, B.; DeRubeis, R.J.; et al. Cognitive dysfunction in psychiatric disorders: Characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012, 11, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J. Clin. Psychiatry 2014, 75, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.V.; Pardo, P.J.; Humes, S.W.; I Posner, M. Neurocognitive dysfunction in antidepressant-free, non-elderly patients with unipolar depression: Alerting and covert orienting of visuospatial attention. J. Affect. Disord. 2006, 92, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Godard, J.; Grondin, S.; Baruch, P.; Lafleur, M.F. Psychosocial and neurocognitive profiles in depressed patients with major depressive disorder and bipolar disorder. Psychiatry Res. 2011, 190, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, C.T. A practical approach to objective attention deficit/hyperactivity disorder diagnosis and management. Psychiatry 2005, 2, 16–25. [Google Scholar] [PubMed]
- Fossati, P.; Ergis, A.M.; Allilaire, J.F. Executive functioning in unipolar depression: A review. L'Encephale 2002, 28, 97–107. [Google Scholar] [PubMed]
- Gohier, B.; Ferracci, L.; Surguladze, S.A.; Lawrence, E.; El Hage, W.; Kefi, M.Z.; Allain, P.; Garre, J.B.; Le Gall, D. Cognitive inhibition and working memory in unipolar depression. J. Affect. Disord. 2009, 116, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Dymond, S.; Cooper, A. Impaired flexible decision-making in major depressive disorder. J. Affect. Disord. 2010, 124, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Gruber, O.; Zilles, D.; Kennel, J.; Gruber, E.; Falkai, P. A systematic experimental neuropsychological investigation of the functional integrity of working memory circuits in major depression. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Borbely-Ipkovich, E.; Janacsek, K.; Nemeth, D.; Gonda, X. The effect of negative mood and major depressive episode on working memory and implicit learning. Neuropsychopharmacol. Hung. 2014, 16, 29–42. [Google Scholar] [PubMed]
- Schmid, M.; Hammar, A. A follow-up study of first episode major depressive disorder. Impairment in inhibition and semantic fluency-potential predictors for relapse? Front. Psychol. 2013, 4, 633. [Google Scholar] [CrossRef] [PubMed]
- Foland-Ross, L.C.; Gotlib, I.H. Cognitive and neural aspects of information processing in major depressive disorder: An integrative perspective. Front. Psychol. 2012, 3, 489. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, A.E.; Tuulio-Henriksson, A.; Marttunen, M.; Suvisaari, J.; Lonnqvist, J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J. Affect. Disord. 2008, 106, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Best, J.R.; Miller, P.H.; Naglieri, J.A. Relations between executive function and academic achievement from ages 5 to 17 in a large, representative national sample. Learn. Individ. Differ. 2011, 21, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Vilgis, V.; Silk, T.J.; Vance, A. Executive function and attention in children and adolescents with depressive disorders: A systematic review. Eur. Child Adolesc. Psychiatry 2015, 24, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Baune, B.T.; Fuhr, M.; Air, T.; Hering, C. Neuropsychological functioning in adolescents and young adults with major depressive disorder—a review. Psychiatry Res. 2014, 218, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Nebes, R.D.; Butters, M.A.; Mulsant, B.H.; Pollock, B.G.; Zmuda, M.D.; Houck, P.R.; Reynolds, C.F., 3rd. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol. Med. 2000, 30, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Baudic, S.; Tzortzis, C.; Barba, G.D.; Traykov, L. Executive deficits in elderly patients with major unipolar depression. J. Geriatr. Psychiatry Neurol. 2004, 17, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Barch, D.M.; Garcia, K.; Gersing, K.; Pieper, C.; Welsh-Bohmer, K.; Steffens, D.C.; Doraiswamy, P.M. Cognitive function in late life depression: Relationships to depression severity, cerebrovascular risk factors and processing speed. Biol. Psychiatry 2006, 60, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.M.; Butters, M.A. Cognition in late life depression: Treatment considerations. Curr. Treat. Options Psychiatry 2014, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Mahon, K.; Burdick, K.E. Measuring cognitive function in MDD: Emerging assessment tools. Depression Anxiety 2015, 32, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I. Antidepressants and their effect on cognition in major depressive disorder. J. Clin. Psychiatry 2015, 76, e1046. [Google Scholar] [CrossRef] [PubMed]
- El Hammi, E.; Samp, J.; Remuzat, C.; Auray, J.P.; Lamure, M.; Aballea, S.; Kooli, A.; Akhras, K.; Toumi, M. Difference of perceptions and evaluation of cognitive dysfunction in major depressive disorder patients across psychiatrists internationally. Ther. Adv. Psychopharmacol. 2014, 4, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Bakkour, N.; Samp, J.; Akhras, K.; El Hammi, E.; Soussi, I.; Zahra, F.; Duru, G.; Kooli, A.; Toumi, M. Systematic review of appropriate cognitive assessment instruments used in clinical trials of schizophrenia, major depressive disorder and bipolar disorder. Psychiatry Res. 2014, 216, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Oh, I.K.; Han, C.; Huh, Y.J.; Jung, I.K.; Patkar, A.A.; Steffens, D.C.; Jang, B.H. Sensitivity of cognitive tests in four cognitive domains in discriminating mdd patients from healthy controls: A meta-analysis. Int. Psychogeriatr. 2013, 25, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Gallassi, R.; Di Sarro, R.; Morreale, A.; Amore, M. Memory impairment in patients with late-onset major depression: The effect of antidepressant therapy. J. Affect. Disord. 2006, 91, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Guzman, I.; Gudayol-Ferre, E.; Herrera-Abarca, J.E.; Herrera-Guzman, D.; Montelongo-Pedraza, P.; Padros Blazquez, F.; Pero-Cebollero, M.; Guardia-Olmos, J. Major depressive disorder in recovery and neuropsychological functioning: Effects of selective serotonin reuptake inhibitor and dual inhibitor depression treatments on residual cognitive deficits in patients with major depressive disorder in recovery. J. Affect. Disord. 2010, 123, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Diaconescu, A.O.; Kramer, E.; Hermann, C.; Ma, Y.; Dhawan, V.; Chaly, T.; Eidelberg, D.; McIntosh, A.R.; Smith, G.S. Distinct functional networks associated with improvement of affective symptoms and cognitive function during citalopram treatment in geriatric depression. Hum. Brain Mapp. 2011, 32, 1677–1691. [Google Scholar] [CrossRef] [PubMed]
- Culang, M.E.; Sneed, J.R.; Keilp, J.G.; Rutherford, B.R.; Pelton, G.H.; Devanand, D.P.; Roose, S.P. Change in cognitive functioning following acute antidepressant treatment in late-life depression. Am. J. Geriatr. Psychiatry 2009, 17, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.M.; Wesnes, K.A.; Schwartz, G.E. Reboxetine versus paroxetine versus placebo: Effects on cognitive functioning in depressed patients. Int. Clin. Psychopharmacol. 2003, 18, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.G.; Guimaraes, L.S.; Trentini, C.M. Neurocognitive changes in depressed patients in psychodynamic psychotherapy, therapy with fluoxetine and combination therapy. J. Affect. Disord. 2013, 151, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Gudayol-Ferre, E.; Guardia-Olmos, J.; Pero-Cebollero, M. Effects of remission speed and improvement of cognitive functions of depressed patients. Psychiatry Res. 2015, 226, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, S.U.; Demir, B.; Senturk, S.; Tatar, I.; Aldur, M.M.; Ulug, B. Hippocampus, glucocorticoids and neurocognitive functions in patients with first-episode major depressive disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, B.J. Cognition and neuroplasticity in the remitted state of unipolar depressive disorder. Dan. Med. J. 2015, 62, B5080. [Google Scholar] [PubMed]
- Burcusa, S.L.; Iacono, W.G. Risk for recurrence in depression. Clin. Psychol. Rev. 2007, 27, 959–985. [Google Scholar] [CrossRef] [PubMed]
- Galecki, P.; Talarowska, M.; Anderson, G.; Berk, M.; Maes, M. Mechanisms underlying neurocognitive dysfunctions in recurrent major depression. Med. Sci. Monit. 2015, 21, 1535–1547. [Google Scholar] [PubMed]
- Lampe, I.K.; Sitskoorn, M.M.; Heeren, T.J. Effects of recurrent major depressive disorder on behavior and cognitive function in female depressed patients. Psychiatry Res. 2004, 125, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Talarowska, M.; Zajaczkowska, M.; Galecki, P. Cognitive functions in first-episode depression and recurrent depressive disorder. Psychiatria Danubina 2015, 27, 38–43. [Google Scholar] [PubMed]
- Karabekiroglu, A.; Topcuoglu, V.; Gimzal Gonentur, A.; Karabekiroglu, K. Executive function differences between first episode and recurrent major depression patients. Turk Psikiyatri Derg. 2010, 21, 280–288. [Google Scholar] [PubMed]
- Chen, J.; Ma, W.; Zhang, Y.; Wu, X.; Wei, D.; Liu, G.; Deng, Z.; Zhang, Z.; Yang, L. Distinct facial processing related negative cognitive bias in first-episode and recurrent major depression: Evidence from the n170 erp component. PLoS ONE 2014, 9, e109176. [Google Scholar] [CrossRef] [PubMed]
- Caldieraro, M.A.; Baeza, F.L.; Pinheiro, D.O.; Ribeiro, M.R.; Parker, G.; Fleck, M.P. Clinical differences between melancholic and nonmelancholic depression as defined by the core system. Compr. Psychiatry 2013, 54, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.; Monzon, S.; Vives, M.; Lopez-Navarro, E.; Garcia-Toro, M.; Vicens, C.; Garcia-Campayo, J.; Harrison, J.; Gili, M. Cognitive function after clinical remission in patients with melancholic and non-melancholic depression: A 6 month follow-up study. J. Affect. Disord. 2015, 171, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Xu, G.; Lu, W.; Ouyang, H.; Dang, Y.; Lorenzo-Seva, U.; Guo, Y.; Bessonov, D.; Akiskal, H.S.; So, K.F.; et al. Neuropsychological performance in melancholic, atypical and undifferentiated major depression during depressed and remitted states: A prospective longitudinal study. J. Affect. Disord. 2014, 168, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Lamers, F.; Vogelzangs, N.; Merikangas, K.R.; de Jonge, P.; Beekman, A.T.; Penninx, B.W. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 2013, 18, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Antonijevic, I. HPA axis and sleep: Identifying subtypes of major depression. Stress 2008, 11, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Parker, G. Defining melancholia: The primacy of psychomotor disturbance. Acta Psychiatr. Scand. Suppl. 2007, 115, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Pier, M.P.; Hulstijn, W.; Sabbe, B.G. Differential patterns of psychomotor functioning in unmedicated melancholic and nonmelancholic depressed patients. J. Psychiatr. Res. 2004, 38, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Bellgrove, M.A.; Chiu, E.; Mileshkin, C.; Bradshaw, J.L. Response selection deficits in melancholic but not nonmelancholic unipolar major depression. J. Clin. Exp. Neuropsychol. 2004, 26, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, I.; Zervas, I.M.; Papakosta, V.M.; Tsaltas, E.; Papageorgiou, C.; Manessi, T.; Papakostas, Y.G.; Lykouras, L.; Soldatos, C.R. Set shifting deficits in melancholic vs. Non-melancholic depression: Preliminary findings. Eur. Psychiatry 2006, 21, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, I.; Zervas, I.M.; Pantelis, C.; Tsaltas, E.; Papakosta, V.M.; Boufidou, F.; Nikolaou, C.; Papageorgiou, C.; Soldatos, C.R.; Lykouras, L. Neuropsychological and hypothalamic-pituitary-axis function in female patients with melancholic and non-melancholic depression. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Exner, C.; Lange, C.; Irle, E. Impaired implicit learning and reduced pre-supplementary motor cortex size in early-onset major depression with melancholic features. J. Affect. Disord. 2009, 119, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Withall, A.; Harris, L.M.; Cumming, S.R. A longitudinal study of cognitive function in melancholic and non-melancholic subtypes of major depressive disorder. J. Affect. Disord. 2010, 123, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.C.; Jackson, M.C.; Subramanian, L.; Healy, D.; Linden, D.E. Sad benefit in face working memory: An emotional bias of melancholic depression. J. Affect. Disord. 2011, 135, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.R.; Harris, A.; Felmingham, K.; Boyce, P.; Kemp, A. The impact of depression heterogeneity on cognitive control in major depressive disorder. Aust. N. Z. J. Psychiatry 2012, 46, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Day, C.V.; Gatt, J.M.; Etkin, A.; DeBattista, C.; Schatzberg, A.F.; Williams, L.M. Cognitive and emotional biomarkers of melancholic depression: An ispot-d report. J. Affect. Disord. 2015, 176, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.P.; Mitchell, P.; Hadzi-Pavlovic, D.; Hickie, I.; Parker, G.; Chan, J.; Eyers, K. Effect of apomorphine on motor and cognitive function in melancholic patients: A preliminary report. Psychiatry Res. 2000, 97, 207–215. [Google Scholar] [CrossRef]
- Romero, N.; Sanchez, A.; Vazquez, C. Memory biases in remitted depression: The role of negative cognitions at explicit and automatic processing levels. J. Behav. Ther. Exp. Psychiatry 2014, 45, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Behnken, A.; Schoning, S.; Gerss, J.; Konrad, C.; de Jong-Meyer, R.; Zwanzger, P.; Arolt, V. Persistent non-verbal memory impairment in remitted major depression—Caused by encoding deficits? J. Affect. Disord. 2010, 122, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Munkler, P.; Rothkirch, M.; Dalati, Y.; Schmack, K.; Sterzer, P. Biased recognition of facial affect in patients with major depressive disorder reflects clinical state. PLoS ONE 2015, 10, e0129863. [Google Scholar] [CrossRef] [PubMed]
- Arnow, B.A.; Blasey, C.; Williams, L.M.; Palmer, D.M.; Rekshan, W.; Schatzberg, A.F.; Etkin, A.; Kulkarni, J.; Luther, J.F.; Rush, A.J. Depression subtypes in predicting antidepressant response: A report from the iSPOT-D trial. Am. J. Psychiatry 2015, 172, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.G. Toward a cognitive-behavioral classification system for mental disorders. Behav. Ther. 2014, 45, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Whitton, A.E.; Treadway, M.T.; Pizzagalli, D.A. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatry 2015, 28, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Eshel, N.; Roiser, J.P. Reward and punishment processing in depression. Biol. Psychiatry 2010, 68, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Wang, L.Z.; Shang, H.R.; Shen, Y.; Li, Z.; Cheung, E.F.; Chan, R.C. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia 2014, 53, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, E.; Pizzagalli, D.A.; Demyttenaere, K.; Hompes, T.; Sienaert, P.; de Boer, P.; Schmidt, M.; Claes, S. Reduced reward learning predicts outcome in major depressive disorder. Biol. Psychiatry 2013, 73, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Treadway, M.T.; Zald, D.H. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci. Biobehav. Rev. 2011, 35, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Olausson, P.; Kiraly, D.D.; Gourley, S.L.; Taylor, J.R. Persistent effects of prior chronic exposure to corticosterone on reward-related learning and motivation in rodents. Psychopharmacology 2013, 225, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.S.; Quinn, K.; Sanislow, C.; Wang, P. Research domain criteria (RDOC): Toward a new classification framework for research on mental disorders. Am. J. Psychiatry 2010, 167, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.; Geyer, M.A.; Carter, C.S.; Barch, D.M. Harnessing cognitive neuroscience to develop new treatments for improving cognition in schizophrenia: Cntrics selected cognitive paradigms for animal models. Neurosci. Biobehav. Rev. 2013, 37, 2087–2091. [Google Scholar] [CrossRef] [PubMed]
- Young, J.W.; Powell, S.B.; Risbrough, V.; Marston, H.M.; Geyer, M.A. Using the matrics to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol. Ther. 2009, 122, 150–202. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.L.; Ballard, T.M.; Glavis-Bloom, C. Animal paradigms to assess cognition with translation to humans. Handb. Exp. Pharmacol. 2015, 228, 27–57. [Google Scholar] [PubMed]
- Oomen, C.A.; Hvoslef-Eide, M.; Heath, C.J.; Mar, A.C.; Horner, A.E.; Bussey, T.J.; Saksida, L.M. The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat. Protoc. 2013, 8, 2006–2021. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Barnhofer, T.; Crane, C.; Herman, D.; Raes, F.; Watkins, E.; Dalgleish, T. Autobiographical memory specificity and emotional disorder. Psychol. Bull. 2007, 133, 122–148. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, H.; Moscovitch, M.; Kumar, N.; Daskalakis, Z.J.; Flint, A.; Herrmann, N.; Levine, B. Autobiographical episodic memory in major depressive disorder. J. Abnorm. Psychol. 2014, 123, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.A.; Griffith, J.W.; Mineka, S. Overgeneral autobiographical memory as a predictor of the course of depression: A meta-analysis. Behav. Res. Ther. 2010, 48, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Nandrino, J.L.; Pezard, L.; Poste, A.; Reveillere, C.; Beaune, D. Autobiographical memory in major depression: A comparison between first-episode and recurrent patients. Psychopathology 2002, 35, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D.; Bellgowan, P.S.; Bodurka, J.; Drevets, W.C. Neurophysiological correlates of autobiographical memory deficits in currently and formerly depressed subjects. Psychol. Med. 2014, 44, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Woody, M.L.; Burkhouse, K.L.; Gibb, B.E. Overgeneral autobiographical memory in children of depressed mothers. Cogn. Emot. 2015, 29, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Gronli, J.; Murison, R.; Fiske, E.; Bjorvatn, B.; Sorensen, E.; Portas, C.M.; Ursin, R. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol. Behav. 2005, 84, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Kleen, J.K.; Sitomer, M.T.; Killeen, P.R.; Conrad, C.D. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav. Neurosci. 2006, 120, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Darcet, F.; Mendez-David, I.; Tritschler, L.; Gardier, A.M.; Guilloux, J.P.; David, D.J. Learning and memory impairments in a neuroendocrine mouse model of anxiety/depression. Front. Behav. Neurosci. 2014, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Abdul Aziz, N.H.; Kendall, D.A.; Pardon, M.C. Prenatal exposure to chronic mild stress increases corticosterone levels in the amniotic fluid and induces cognitive deficits in female offspring, improved by treatment with the antidepressant drug amitriptyline. Behav. Brain Res. 2012, 231, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Taylor, A.R.; Taylor, S.B.; Bell, D.B.; Koenig, J.I. Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Front. Behav. Neurosci. 2010, 4, 173. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, D.E.; Neigh, G.N.; Bourke, C.H.; Nemeth, C.L.; Hazra, R.; Ryan, S.J.; Rowson, S.; Jairam, N.; Sholar, C.A.; Rainnie, D.G.; et al. Prenatal stress, regardless of concurrent escitalopram treatment, alters behavior and amygdala gene expression of adolescent female rats. Neuropharmacology 2015, 97, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.M.; Pearson, J.N.; Neeley, E.W.; Berger, R.; Leonard, S.; Adams, C.E.; Stevens, K.E. Maternal stress during pregnancy causes sex-specific alterations in offspring memory performance, social interactions, indices of anxiety, and body mass. Physiol. Behav. 2011, 104, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.A.; Terry, A.V., Jr. Variable maternal stress in rats alters locomotor activity, social behavior, and recognition memory in the adult offspring. Pharmacol. Biochem. Behav. 2013, 104, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Said, N.; Lakehayli, S.; El Khachibi, M.; El Ouahli, M.; Nadifi, S.; Hakkou, F.; Tazi, A. Effect of prenatal stress on memory, nicotine withdrawal and 5ht1a expression in raphe nuclei of adult rats. Int. J. Dev. Neurosci. 2015, 43, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiao, J.; Dulawa, S.C. Infant maternal separation impairs adult cognitive performance in BALB/cJ mice. Psychopharmacology 2011, 216, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Niwa, M.; Matsumoto, Y.; Mouri, A.; Ozaki, N.; Nabeshima, T. Vulnerability in early life to changes in the rearing environment plays a crucial role in the aetiopathology of psychiatric disorders. Int. J. Neuropsychopharmacol. 2011, 14, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Aisa, B.; Tordera, R.; Lasheras, B.; Del Rio, J.; Ramirez, M.J. Cognitive impairment associated to hpa axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology 2007, 32, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Salomon, S.; Bejar, C.; Schorer-Apelbaum, D.; Weinstock, M. Corticosterone mediates some but not other behavioural changes induced by prenatal stress in rats. J. Neuroendocrinol. 2011, 23, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Plescia, F.; Marino, R.A.; Navarra, M.; Gambino, G.; Brancato, A.; Sardo, P.; Cannizzaro, C. Early handling effect on female rat spatial and non-spatial learning and memory. Behav. Process. 2014, 103, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Vivinetto, A.L.; Suarez, M.M.; Rivarola, M.A. Neurobiological effects of neonatal maternal separation and post-weaning environmental enrichment. Behav. Brain Res. 2013, 240, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Makena, N.; Bugarith, K.; Russell, V.A. Maternal separation enhances object location memory and prevents exercise-induced mapk/erk signalling in adult sprague-dawley rats. Metab. Brain Dis. 2012, 27, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Klug, M.; Kiss Von Soly, S.; Binder, M.D.; Hannan, A.J.; van den Buuse, M. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus 2014, 24, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.M.; Valero, M.; de la Serna, O.; Aisa, B.; Borcel, E.; Ramirez, M.J.; Viveros, M.P. Maternal deprivation effects on brain plasticity and recognition memory in adolescent male and female rats. Neuropharmacology 2013, 68, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Berzal, A.; Mela, V.; Borcel, E.; Valero, M.; Lopez-Gallardo, M.; Viveros, M.P.; Marco, E.M. Neurobehavioral and metabolic long-term consequences of neonatal maternal deprivation stress and adolescent olanzapine treatment in male and female rats. Neuropharmacology 2012, 62, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Naninck, E.F.; Hoeijmakers, L.; Kakava-Georgiadou, N.; Meesters, A.; Lazic, S.E.; Lucassen, P.J.; Korosi, A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus 2015, 25, 309–328. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.M.; Shrestha Muna, S.; Bagalkot, T.R.; Cui, Y.; Yadav, B.K.; Chung, Y.C. The effects of social defeat on behavior and dopaminergic markers in mice. Neuroscience 2015, 288, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Gumuslu, E.; Mutlu, O.; Sunnetci, D.; Ulak, G.; Celikyurt, I.K.; Cine, N.; Akar, F.; Savli, H.; Erden, F. The antidepressant agomelatine improves memory deterioration and upregulates creb and bdnf gene expression levels in unpredictable chronic mild stress (UCMS)-exposed mice. Drug Target Insights 2014, 8, 11–21. [Google Scholar] [PubMed]
- Haridas, S.; Kumar, M.; Manda, K. Melatonin ameliorates chronic mild stress induced behavioral dysfunctions in mice. Physiol. Behav. 2013, 119, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Elizalde, N.; Gil-Bea, F.J.; Ramirez, M.J.; Aisa, B.; Lasheras, B.; Del Rio, J.; Tordera, R.M. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: Effect of antidepressant treatment. Psychopharmacology 2008, 199, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, C.; Wang, W.; Dong, H.; Hou, P.; Tang, Y. Chronic mild stress impairs cognition in mice: From brain homeostasis to behavior. Life Sci. 2008, 82, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Orsetti, M.; Colella, L.; Dellarole, A.; Canonico, P.L.; Ghi, P. Modification of spatial recognition memory and object discrimination after chronic administration of haloperidol, amitriptyline, sodium valproate or olanzapine in normal and anhedonic rats. Int. J. Neuropsychopharmacol. 2007, 10, 345–357. [Google Scholar] [PubMed]
- Liu, D.; Wang, Z.; Gao, Z.; Xie, K.; Zhang, Q.; Jiang, H.; Pang, Q. Effects of curcumin on learning and memory deficits, bdnf, and erk protein expression in rats exposed to chronic unpredictable stress. Behav. Brain Res. 2014, 271, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Dobarro, M.; Orejana, L.; Aguirre, N.; Ramirez, M.J. Propranolol reduces cognitive deficits, amyloid beta levels, tau phosphorylation and insulin resistance in response to chronic corticosterone administration. Int. J. Neuropsychopharmacol. 2013, 16, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Solas, M.; Gerenu, G.; Gil-Bea, F.J.; Ramirez, M.J. Mineralocorticoid receptor activation induces insulin resistance through c-Jun n-terminal kinases in response to chronic corticosterone: Cognitive implications. J. Neuroendocrinol. 2013, 25, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Kolb, B. Mild prenatal stress-modulated behavior and neuronal spine density without affecting amphetamine sensitization. Dev. Neurosci. 2011, 33, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Baudin, A.; Blot, K.; Verney, C.; Estevez, L.; Santamaria, J.; Gressens, P.; Giros, B.; Otani, S.; Dauge, V.; Naudon, L. Maternal deprivation induces deficits in temporal memory and cognitive flexibility and exaggerates synaptic plasticity in the rat medial prefrontal cortex. Neurobiol. Learn. Mem. 2012, 98, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Grace, L.; Hescham, S.; Kellaway, L.A.; Bugarith, K.; Russell, V.A. Effect of exercise on learning and memory in a rat model of developmental stress. Metab. Brain Dis. 2009, 24, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Frankola, K.A.; Flora, A.L.; Torres, A.K.; Grissom, E.M.; Overstreet, S.; Dohanich, G.P. Effects of early rearing conditions on cognitive performance in prepubescent male and female rats. Neurobiol. Learn. Mem. 2010, 94, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Macri, S.; Chiarotti, F.; Wurbel, H. Maternal separation and maternal care act independently on the development of hpa responses in male rats. Behav. Brain Res. 2008, 191, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.J.; Murrough, J.W.; Han, M.H.; Charney, D.S.; Nestler, E.J. Neurobiology of resilience. Nat. Neurosci. 2012, 15, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, P.A. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Gue, M.; Bravard, A.; Meunier, J.; Veyrier, R.; Gaillet, S.; Recasens, M.; Maurice, T. Sex differences in learning deficits induced by prenatal stress in juvenile rats. Behav. Brain Res. 2004, 150, 149–157. [Google Scholar] [CrossRef]
- Meunier, J.; Gue, M.; Recasens, M.; Maurice, T. Attenuation by a sigma1 (δ1) receptor agonist of the learning and memory deficits induced by a prenatal restraint stress in juvenile rats. Br. J. Pharmacol. 2004, 142, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Liu, J.; Feng, Z. Maternal hydroxytyrosol administration improves neurogenesis and cognitive function in prenatally stressed offspring. J. Nutr. Biochem. 2015, 26, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.H.; de Visser, Y.; Nichols, N.R.; van den Buuse, M. Combined neonatal stress and young-adult glucocorticoid stimulation in rats reduce bdnf expression in hippocampus: Effects on learning and memory. Hippocampus 2008, 18, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Klug, M.; van den Buuse, M. Chronic cannabinoid treatment during young adulthood induces sex-specific behavioural deficits in maternally separated rats. Behav. Brain Res. 2012, 233, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Garner, B.; Wood, S.J.; Pantelis, C.; van den Buuse, M. Early maternal deprivation reduces prepulse inhibition and impairs spatial learning ability in adulthood: No further effect of post-pubertal chronic corticosterone treatment. Behav. Brain Res. 2007, 176, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Schmauss, C. Strain-specific cognitive deficits in adult mice exposed to early life stress. Behav. Neurosci. 2011, 125, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, M.; Garza, J.; Rendon, S.; Sun, X.L.; Zhang, W.; Lu, X.Y. Cognitive and neural correlates of depression-like behaviour in socially defeated mice: An animal model of depression with cognitive dysfunction. Int. J. Neuropsychopharmacol. 2011, 14, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Novick, A.M.; Miiller, L.C.; Forster, G.L.; Watt, M.J. Adolescent social defeat decreases spatial working memory performance in adulthood. Behav. Brain Funct. BBF 2013, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Furuta, M.; Ninomiya-Baba, M.; Chiba, S.; Funabashi, T.; Akema, T.; Kunugi, H. Exposure to social defeat stress in adolescence improves the working memory and anxiety-like behavior of adult female rats with intrauterine growth restriction, independently of hippocampal neurogenesis. Horm. Behav. 2015, 70, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, K.; Andreasen, J.T.; Bouzinova, E.V.; Jayatissa, M.N.; Jensen, M.S.; Redrobe, J.P.; Wiborg, O. Cognitive deficits in the rat chronic mild stress model for depression: Relation to anhedonic-like responses. Behav. Brain Res. 2009, 198, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, K.; Woldbye, D.P.; Wiborg, O. Electroconvulsive stimulation reverses anhedonia and cognitive impairments in rats exposed to chronic mild stress. Eur. Neuropsychopharmacol. 2013, 23, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.T.; Henningsen, K.; Bate, S.; Christiansen, S.; Wiborg, O. Nicotine reverses anhedonic-like response and cognitive impairment in the rat chronic mild stress model of depression: Comparison with sertraline. J. Psychopharmacol. 2011, 25, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, K.; Yuzurihara, M.; Ishige, A.; Sasaki, H.; Chui, D.H.; Tabira, T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J. Neurosci. 2000, 20, 1568–1574. [Google Scholar] [PubMed]
- Palumbo, M.L.; Canzobre, M.C.; Pascuan, C.G.; Rios, H.; Wald, M.; Genaro, A.M. Stress induced cognitive deficit is differentially modulated in BALB/c and C57Bl/6 mice: Correlation with Th1/Th2 balance after stress exposure. J. Neuroimmunol. 2010, 218, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Coburn-Litvak, P.S.; Pothakos, K.; Tata, D.A.; McCloskey, D.P.; Anderson, B.J. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol. Learn. Mem. 2003, 80, 11–23. [Google Scholar] [CrossRef]
- Gururajan, A.; Hill, R.A.; van den Buuse, M. Brain-derived neurotrophic factor heterozygous mutant rats show selective cognitive changes and vulnerability to chronic corticosterone treatment. Neuroscience 2015, 284, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Tata, D.A.; Markostamou, I.; Ioannidis, A.; Gkioka, M.; Simeonidou, C.; Anogianakis, G.; Spandou, E. Effects of maternal separation on behavior and brain damage in adult rats exposed to neonatal hypoxia-ischemia. Behav. Brain Res. 2015, 280, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Patki, G.; Solanki, N.; Atrooz, F.; Allam, F.; Salim, S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013, 1539, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Patki, G.; Solanki, N.; Atrooz, F.; Ansari, A.; Allam, F.; Jannise, B.; Maturi, J.; Salim, S. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiol. Behav. 2014, 130, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Tagliari, B.; Scherer, E.B.; Machado, F.R.; Ferreira, A.G.; Dalmaz, C.; Wyse, A.T. Antioxidants prevent memory deficits provoked by chronic variable stress in rats. Neurochem. Res. 2011, 36, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Trofimiuk, E.; Braszko, J.J. Ciproxifan differentially modifies cognitive impairment evoked by chronic stress and chronic corticosterone administration in rats. Behav. Brain Res. 2015, 283, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, J.J.; Pego, J.M.; Taipa, R.; Bessa, J.M.; Almeida, O.F.; Sousa, N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J. Neurosci. 2005, 25, 7792–7800. [Google Scholar] [CrossRef] [PubMed]
- Workman, J.L.; Chan, M.Y.; Galea, L.A. Prior high corticosterone exposure reduces activation of immature neurons in the ventral hippocampus in response to spatial and nonspatial memory. Hippocampus 2015, 25, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, P.J.; Strupp, B.J. Assessing attention in rodents. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; Buccafusco, J.J., Ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- De Bruin, N.M.; Fransen, F.; Duytschaever, H.; Grantham, C.; Megens, A.A. Attentional performance of (C57Bl/6J × 129Sv)F2 mice in the five-choice serial reaction time task. Physiol. Behav. 2006, 89, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Roige, S.; Pena-Oliver, Y.; Stephens, D.N. Measuring impulsivity in mice: The five-choice serial reaction time task. Psychopharmacology 2012, 219, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Dalley, J.W.; Robbins, T.W. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat. Protoc. 2008, 3, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Van Gaalen, M.M.; Stenzel-Poore, M.; Holsboer, F.; Steckler, T. Reduced attention in mice overproducing corticotropin-releasing hormone. Behav. Brain Res. 2003, 142, 69–79. [Google Scholar] [CrossRef]
- Wilson, C.A.; Schade, R.; Terry, A.V., Jr. Variable prenatal stress results in impairments of sustained attention and inhibitory response control in a 5-choice serial reaction time task in rats. Neuroscience 2012, 218, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Comeau, W.L.; Winstanley, C.A.; Weinberg, J. Prenatal alcohol exposure and adolescent stress—Unmasking persistent attentional deficits in rats. Eur. J. Neurosci. 2014, 40, 3078–3095. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, M.M.; Xie, M.; Taylor, J.R. Chronic corticosterone exposure during adolescence reduces impulsive action but increases impulsive choice and sensitivity to yohimbine in male sprague-dawley rats. Neuropsychopharmacology 2012, 37, 1656–1670. [Google Scholar] [CrossRef] [PubMed]
- Amitai, N.; Markou, A. Comparative effects of different test day challenges on performance in the 5-choice serial reaction time task. Behav. Neurosci. 2011, 125, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Bangasser, D.A.; Kawasumi, Y. Cognitive disruptions in stress-related psychiatric disorders: A role for corticotropin releasing factor (CRF). Horm. Behav. 2015, 76, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, Y.; Yamaguchi, T.; Futami, Y.; Togashi, H.; Izumi, T.; Matsumoto, M.; Yoshida, T.; Yoshioka, M. Corticotropin releasing factor enhances attentional function as assessed by the five-choice serial reaction time task in rats. Behav. Brain Res. 2009, 198, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Van’t Veer, A.; Yano, J.M.; Carroll, F.I.; Cohen, B.M.; Carlezon, W.A., Jr. Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the kappa-opioid receptor antagonist jdtic. Neuropsychopharmacology 2012, 37, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Bondi, C.O.; Rodriguez, G.; Gould, G.G.; Frazer, A.; Morilak, D.A. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 2008, 33, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Naegeli, K.J.; O’Connor, J.A.; Banerjee, P.; Morilak, D.A. Effects of milnacipran on cognitive flexibility following chronic stress in rats. Eur. J. Pharmacol. 2013, 703, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Nikiforuk, A.; Popik, P. Long-lasting cognitive deficit induced by stress is alleviated by acute administration of antidepressants. Psychoneuroendocrinology 2011, 36, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.; Marston, H.M.; McQuade, R.; Gartside, S.E. Evidence that aetiological risk factors for psychiatric disorders cause distinct patterns of cognitive deficits. Eur. Neuropsychopharmacol. 2014, 24, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Shao, S.; Wang, W.; Shao, F. Maternal separation induces alterations in reversal learning and brain-derived neurotrophic factor expression in adult rats. Neuropsychobiology 2013, 68, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, M.; Du, W.; Shao, F.; Wang, W. The different effects of maternal separation on spatial learning and reversal learning in rats. Behav. Brain Res. 2015, 280, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Laredo, S.A.; Steinman, M.Q.; Robles, C.F.; Ferrer, E.; Ragen, B.J.; Trainor, B.C. Effects of defeat stress on behavioral flexibility in males and females: Modulation by the mu-opioid receptor. Eur. J. Neurosci. 2015, 41, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Bisaz, R.; Schachner, M.; Sandi, C. Causal evidence for the involvement of the neural cell adhesion molecule, NCAM, in chronic stress-induced cognitive impairments. Hippocampus 2011, 21, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.M.; Mesquita, A.R.; Oliveira, M.; Pego, J.M.; Cerqueira, J.J.; Palha, J.A.; Almeida, O.F.; Sousa, N. A trans-dimensional approach to the behavioral aspects of depression. Front. Behav. Neurosci. 2009, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.N.; Patel, S.; Carrier, E.J.; Rademacher, D.J.; Ormerod, B.K.; Hillard, C.J.; Gorzalka, B.B. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology 2005, 30, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, J.J.; Mailliet, F.; Almeida, O.F.; Jay, T.M.; Sousa, N. The prefrontal cortex as a key target of the maladaptive response to stress. J. Neurosci. 2007, 27, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Llorente, R.; Miguel-Blanco, C.; Aisa, B.; Lachize, S.; Borcel, E.; Meijer, O.C.; Ramirez, M.J.; De Kloet, E.R.; Viveros, M.P. Long term sex-dependent psychoneuroendocrine effects of maternal deprivation and juvenile unpredictable stress in rats. J. Neuroendocrinol. 2011, 23, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Heisler, J.M.; Morales, J.; Donegan, J.J.; Jett, J.D.; Redus, L.; O’Connor, J.C. The attentional set shifting task: A measure of cognitive flexibility in mice. J. Vis. Exp. JoVE 2015. [Google Scholar] [CrossRef] [PubMed]
- Tait, D.S.; Chase, E.A.; Brown, V.J. Attentional set-shifting in rodents: A review of behavioural methods and pharmacological results. Curr. Pharm. Des. 2014, 20, 5046–5059. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.D. A critical review of chronic stress effects on spatial learning and memory. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Che, W.; Min-Wei, W.; Murakami, Y.; Matsumoto, K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol. Biochem. Behav. 2006, 83, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, B.; Patil, S.; Höger, H.; Luber, G. Barnes maze, a useful task to assess spatial reference memory in the mice. Protocol Exch. 2007, 390, 10–38. [Google Scholar] [CrossRef]
- Olton, D.S. Mazes, maps, and memory. Am. Psychol. 1979, 34, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, X.; Xu, H.; Yang, H.; Chen, T.; Chen, S.; Chen, X. Chronic unpredictable stress before pregnancy reduce the expression of brain-derived neurotrophic factor and n-methyl-d-aspartate receptor in hippocampus of offspring rats associated with impairment of memory. Neurochem. Res. 2010, 35, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Modir, F.; Elahdadi Salmani, M.; Goudarzi, I.; Lashkarboluki, T.; Abrari, K. Prenatal stress decreases spatial learning and memory retrieval of the adult male offspring of rats. Physiol. Behav. 2014, 129, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, M.; Shabani, M.; Ghotbi Ravandi, S.; Aghaei, I.; Nozari, M.; Mazhari, S. Psychological or physical prenatal stress differentially affects cognition behaviors. Physiol. Behav. 2015, 142, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Garcia, I.; Lara-Vasquez, A.; Montiel, J.F.; Diaz-Veliz, G.F.; Sepulveda, H.; Utreras, E.; Montecino, M.; Gonzalez-Billault, C.; Aboitiz, F. Prenatal stress down-regulates reelin expression by methylation of its promoter and induces adult behavioral impairments in rats. PLoS ONE 2015, 10, e0117680. [Google Scholar]
- Benoit, J.D.; Rakic, P.; Frick, K.M. Prenatal stress induces spatial memory deficits and epigenetic changes in the hippocampus indicative of heterochromatin formation and reduced gene expression. Behav. Brain Res. 2015, 281, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Negron-Oyarzo, I.; Neira, D.; Espinosa, N.; Fuentealba, P.; Aboitiz, F. Prenatal stress produces persistence of remote memory and disrupts functional connectivity in the hippocampal-prefrontal cortex axis. Cereb. Cortex 2015, 25, 3132–3143. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Tu, W.Q.; Shi, Y.W.; Xue, L.; Zhao, H. Female-dependent impaired fear memory of adult rats induced by maternal separation, and screening of possible related genes in the hippocampal ca1. Behav. Brain Res. 2014, 267, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Couto, F.S.; Batalha, V.L.; Valadas, J.S.; Data-Franca, J.; Ribeiro, J.A.; Lopes, L.V. Escitalopram improves memory deficits induced by maternal separation in the rat. Eur. J. Pharmacol. 2012, 695, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Mello, P.B.; Benetti, F.; Cammarota, M.; Izquierdo, I. Physical exercise can reverse the deficit in fear memory induced by maternal deprivation. Neurobiol. Learn. Mem. 2009, 92, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, B.; Jin, J.; An, S.; Zeng, Q.; Duan, Y.; Yang, L.; Ma, J.; Cao, X. Early deprivation reduced anxiety and enhanced memory in adult male rats. Brain Res. Bull. 2014, 108, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Rinwa, P.; Kumar, A. Piperine potentiates the protective effects of curcumin against chronic unpredictable stress-induced cognitive impairment and oxidative damage in mice. Brain Res. 2012, 1488, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Q.; Gu, J.; Wang, X.; Xie, K.; Xian, X.; Wang, J.; Jiang, H.; Wang, Z. Resveratrol prevents impaired cognition induced by chronic unpredictable mild stress in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 49, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.N.; Krigbaum, A.; Ortiz, J.B.; Mika, A.; Hutchinson, K.M.; Bimonte-Nelson, H.A.; Conrad, C.D. Recovery after chronic stress within spatial reference and working memory domains: Correspondence with hippocampal morphology. Eur. J. Neurosci. 2011, 34, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.B.; Taylor, S.B.; Hoffman, A.N.; Campbell, A.N.; Lucas, L.R.; Conrad, C.D. Sex-specific impairment and recovery of spatial learning following the end of chronic unpredictable restraint stress: Potential relevance of limbic gad. Behav. Brain Res. 2015, 282, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Shim, I.; Lee, H.; Hahm, D.H. Baicalin improves chronic corticosterone-induced learning and memory deficits via the enhancement of impaired hippocampal brain-derived neurotrophic factor and camp response element-binding protein expression in the rat. J. Nat. Med. 2014, 68, 132–143. [Google Scholar] [CrossRef] [PubMed]
- McLay, R.N.; Freeman, S.M.; Zadina, J.E. Chronic corticosterone impairs memory performance in the barnes maze. Physiol. Behav. 1998, 63, 933–937. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Belzung, C.; Crusio, W.E. Functional implications of decreases in neurogenesis following chronic mild stress in mice. Neuroscience 2007, 150, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Y.; Hu, J.; Cheng, W.; Jiang, H.; Zhang, X.; Li, M.; Ren, J.; Li, X. Prenatal chronic mild stress induces depression-like behavior and sex-specific changes in regional glutamate receptor expression patterns in adult rats. Neuroscience 2015, 301, 363–374. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; Hong, N.S. How does a specific learning and memory system in the mammalian brain gain control of behavior? Hippocampus 2013, 23, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.B.; Grossrubatscher, I.; Frank, L. Dynamic hippocampal circuits support learning- and memory-guided behaviors. Cold Spring Harb. Symp. Quant. Biol. 2014, 79, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.; MacLeod, C. Cognitive vulnerability to emotional disorders. Ann. Rev. Clin. Psychol. 2005, 1, 167–195. [Google Scholar] [CrossRef] [PubMed]
- Gotlib, I.H.; Joormann, J. Cognition and depression: Current status and future directions. Ann. Rev. Clin. Psychol. 2010, 6, 285–312. [Google Scholar] [CrossRef] [PubMed]
- Saxe, M.D.; Battaglia, F.; Wang, J.W.; Malleret, G.; David, D.J.; Monckton, J.E.; Garcia, A.D.; Sofroniew, M.V.; Kandel, E.R.; Santarelli, L.; et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. USA 2006, 103, 17501–17506. [Google Scholar] [CrossRef] [PubMed]
- Imayoshi, I.; Sakamoto, M.; Ohtsuka, T.; Takao, K.; Miyakawa, T.; Yamaguchi, M.; Mori, K.; Ikeda, T.; Itohara, S.; Kageyama, R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008, 11, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Chocyk, A.; Przyborowska, A.; Makuch, W.; Majcher-Maslanka, I.; Dudys, D.; Wedzony, K. The effects of early-life adversity on fear memories in adolescent rats and their persistence into adulthood. Behav. Brain Res. 2014, 264, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.J.; Yang, Y.; Wang, L.P.; Xu, L.; Mao, R.R. Maternal separation exaggerates spontaneous recovery of extinguished contextual fear in adult female rats. Behav. Brain Res. 2014, 269, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Oomen, C.A.; Soeters, H.; Audureau, N.; Vermunt, L.; van Hasselt, F.N.; Manders, E.M.; Joels, M.; Krugers, H.; Lucassen, P.J. Early maternal deprivation affects dentate gyrus structure and emotional learning in adult female rats. Psychopharmacology 2011, 214, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Dulka, B.N.; Lynch, J.F., 3rd; Latsko, M.S.; Mulvany, J.L.; Jasnow, A.M. Phenotypic responses to social defeat are associated with differences in cued and contextual fear discrimination. Behav. Process. 2015, 118, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Shumake, J.; Barrett, D.; Gonzalez-Lima, F. Behavioral characteristics of rats predisposed to learned helplessness: Reduced reward sensitivity, increased novelty seeking, and persistent fear memories. Behav. Brain Res. 2005, 164, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Camp, R.M.; Johnson, J.D. Repeated stressor exposure enhances contextual fear memory in a beta-adrenergic receptor-dependent process and increases impulsivity in a non-beta receptor-dependent fashion. Physiol. Behav. 2015, 150, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Shi, Y.W.; Liu, Q.L.; Zhao, H. Glucocorticoid-induced enhancement of contextual fear memory consolidation in rats: Involvement of D1 receptor activity of hippocampal area CA1. Brain Res. 2013, 1524, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Marks, W.N.; Fenton, E.Y.; Guskjolen, A.J.; Kalynchuk, L.E. The effect of chronic corticosterone on fear learning and memory depends on dose and the testing protocol. Neuroscience 2015, 289, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Monsey, M.S.; Boyle, L.M.; Zhang, M.L.; Nguyen, C.P.; Kronman, H.G.; Ota, K.T.; Duman, R.S.; Taylor, J.R.; Schafe, G.E. Chronic corticosterone exposure persistently elevates the expression of memory-related genes in the lateral amygdala and enhances the consolidation of a pavlovian fear memory. PLoS ONE 2014, 9, e91530. [Google Scholar] [CrossRef] [PubMed]
- Duclot, F.; Hollis, F.; Darcy, M.J.; Kabbaj, M. Individual differences in novelty-seeking behavior in rats as a model for psychosocial stress-related mood disorders. Physiol. Behav. 2011, 104, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Gourley, S.L.; Kedves, A.T.; Olausson, P.; Taylor, J.R. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology 2009, 34, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.; Liu, X.; MacDonald, C.J.; Moffa, A.; Zhou, J.; Redondo, R.L.; Tonegawa, S. Activating positive memory engrams suppresses depression-like behaviour. Nature 2015, 522, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Denny, C.A.; Burghardt, N.S.; Schachter, D.M.; Hen, R.; Drew, M.R. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus 2012, 22, 1188–1201. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.G.; Felker, B.L.; Liu, C.F.; Yano, E.M.; Kirchner, J.E.; Chan, D.; Rubenstein, L.V.; Chaney, E.F. Prevalence of depression-ptsd comorbidity: Implications for clinical practice guidelines and primary care-based interventions. J. General Intern. Med. 2007, 22, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Sotres-Bayon, F.; Cain, C.K.; LeDoux, J.E. Brain mechanisms of fear extinction: Historical perspectives on the contribution of prefrontal cortex. Biol. Psychiatry 2006, 60, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.B.; Bairy, K.L.; Rao, M.S. Chronic prenatal restraint stress induced memory impairment in passive avoidance task in post weaned male and female wistar rats. Indian J. Exp. Biol. 2009, 47, 893–899. [Google Scholar] [PubMed]
- Wu, J.; Song, T.B.; Li, Y.J.; He, K.S.; Ge, L.; Wang, L.R. Prenatal restraint stress impairs learning and memory and hippocampal pkcbeta1 expression and translocation in offspring rats. Brain Res. 2007, 1141, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.H.; Shin, M.S.; Cho, S.; Baik, H.H.; Jin, B.K.; Chang, H.K.; Lee, E.K.; Kim, C.J. Depression-like state in maternal rats induced by repeated separation of pups is accompanied by a decrease of cell proliferation and an increase of apoptosis in the hippocampus. Neuroscience Lett. 2010, 470, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Radahmadi, M.; Alaei, H.; Sharifi, M.R.; Hosseini, N. Effects of different timing of stress on corticosterone, bdnf and memory in male rats. Physiol. Behav. 2015, 139, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Bisagno, V.; Ferrini, M.; Rios, H.; Zieher, L.M.; Wikinski, S.I. Chronic corticosterone impairs inhibitory avoidance in rats: Possible link with atrophy of hippocampal CA3 neurons. Pharmacol. Biochem. Behav. 2000, 66, 235–240. [Google Scholar] [CrossRef]
- Costa-Nunes, J.; Zubareva, O.; Araujo-Correia, M.; Valenca, A.; Schroeter, C.A.; Pawluski, J.L.; Vignisse, J.; Steinbusch, H.; Hermes, D.; Phillipines, M.; et al. Altered emotionality, hippocampus-dependent performance and expression of nmda receptor subunit mrnas in chronically stressed mice. Stress 2014, 17, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; Dong, H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Kheirbek, M.A.; Drew, L.J.; Burghardt, N.S.; Costantini, D.O.; Tannenholz, L.; Ahmari, S.E.; Zeng, H.; Fenton, A.A.; Hen, R. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 2013, 77, 955–968. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, J.T.; Lloyd, A.; McKeith, I.; Gholkar, A.; Ferrier, N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am. J. Psychiatry 2004, 161, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, B.R.; Salvadore, G.; Colon-Rosario, V.; Latov, D.R.; Holroyd, T.; Carver, F.W.; Coppola, R.; Manji, H.K.; Zarate, C.A., Jr.; Grillon, C. Abnormal hippocampal functioning and impaired spatial navigation in depressed individuals: Evidence from whole-head magnetoencephalography. Am. J. Psychiatry 2010, 167, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Gould, N.F.; Holmes, M.K.; Fantie, B.D.; Luckenbaugh, D.A.; Pine, D.S.; Gould, T.D.; Burgess, N.; Manji, H.K.; Zarate, C.A., Jr. Performance on a virtual reality spatial memory navigation task in depressed patients. Am. J. Psychiatry 2007, 164, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Surget, A.; Tanti, A.; Leonardo, E.D.; Laugeray, A.; Rainer, Q.; Touma, C.; Palme, R.; Griebel, G.; Ibarguen-Vargas, Y.; Hen, R.; et al. Antidepressants recruit new neurons to improve stress response regulation. Mol. Psychiatry 2011, 16, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Oomen, C.A.; Bekinschtein, P.; Kent, B.A.; Saksida, L.M.; Bussey, T.J. Adult hippocampal neurogenesis and its role in cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2014, 5, 573–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Christian, K.; Ming, G.L.; Song, H. Time-dependent involvement of adult-born dentate granule cells in behavior. Behav. Brain Res. 2012, 227, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Marin-Burgin, A.; Schinder, A.F. Requirement of adult-born neurons for hippocampus-dependent learning. Behav. Brain Res. 2012, 227, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rabaza, V.; Llorens-Martin, M.; Velazquez-Sanchez, C.; Ferragud, A.; Arcusa, A.; Gumus, H.G.; Gomez-Pinedo, U.; Perez-Villalba, A.; Rosello, J.; Trejo, J.L.; et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience 2009, 159, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Winocur, G.; Wojtowicz, J.M.; Sekeres, M.; Snyder, J.S.; Wang, S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 2006, 16, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Saxe, M.D.; Gallina, I.S.; Gage, F.H. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 2009, 29, 13532–13542. [Google Scholar] [CrossRef] [PubMed]
- Dupret, D.; Revest, J.M.; Koehl, M.; Ichas, F.; De Giorgi, F.; Costet, P.; Abrous, D.N.; Piazza, P.V. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE 2008, 3, e1959. [Google Scholar] [CrossRef] [PubMed]
- Garthe, A.; Kempermann, G. An old test for new neurons: Refining the morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front. Neurosci. 2013, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Clark, R.E.; Broadbent, N.J.; Clemenson, G.D., Jr.; Consiglio, A.; Lie, D.C.; Squire, L.R.; Gage, F.H. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 2009, 16, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Clelland, C.D.; Choi, M.; Romberg, C.; Clemenson, G.D., Jr.; Fragniere, A.; Tyers, P.; Jessberger, S.; Saksida, L.M.; Barker, R.A.; Gage, F.H.; et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009, 325, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Shors, T.J.; Townsend, D.A.; Zhao, M.; Kozorovitskiy, Y.; Gould, E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 2002, 12, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Meshi, D.; Drew, M.R.; Saxe, M.; Ansorge, M.S.; David, D.; Santarelli, L.; Malapani, C.; Moore, H.; Hen, R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat. Neurosci. 2006, 9, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Groves, J.O.; Leslie, I.; Huang, G.J.; McHugh, S.B.; Taylor, A.; Mott, R.; Munafo, M.; Bannerman, D.M.; Flint, J. Ablating adult neurogenesis in the rat has no effect on spatial processing: Evidence from a novel pharmacogenetic model. PLoS Genet. 2013, 9, e1003718. [Google Scholar] [CrossRef] [PubMed]
- Saxe, M.D.; Malleret, G.; Vronskaya, S.; Mendez, I.; Garcia, A.D.; Sofroniew, M.V.; Kandel, E.R.; Hen, R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc. Natl. Acad. Sci. USA 2007, 104, 4642–4646. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Marrone, D.F.; Markus, E.J. Disambiguating the similar: The dentate gyrus and pattern separation. Behav. Brain Res. 2012, 226, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Aimone, J.B.; Deng, W.; Gage, F.H. Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 2011, 70, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Scobie, K.N.; Hill, A.S.; O’Carroll, C.M.; Kheirbek, M.A.; Burghardt, N.S.; Fenton, A.A.; Dranovsky, A.; Hen, R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011, 472, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.S.; Sahay, A.; Hen, R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology 2015, 40, 2368–2378. [Google Scholar] [CrossRef] [PubMed]
- Denny, C.A.; Kheirbek, M.A.; Alba, E.L.; Tanaka, K.F.; Brachman, R.A.; Laughman, K.B.; Tomm, N.K.; Turi, G.F.; Losonczy, A.; Hen, R. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 2014, 83, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Besnard, A.; Sahay, A. Adult hippocampal neurogenesis, fear generalization, and stress. Neuropsychopharmacology 2016, 41, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Saito, D.N.; Yanaka, H.T.; Kosaka, H.; Okazawa, H. Depressive mood modulates the anterior lateral ca1 and dg/ca3 during a pattern separation task in cognitively intact individuals: A functional mri study. Hippocampus 2014, 24, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Akers, K.G.; Martinez-Canabal, A.; Restivo, L.; Yiu, A.P.; De Cristofaro, A.; Hsiang, H.L.; Wheeler, A.L.; Guskjolen, A.; Niibori, Y.; Shoji, H.; et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 2014, 344, 598–602. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darcet, F.; Gardier, A.M.; Gaillard, R.; David, D.J.; Guilloux, J.-P. Cognitive Dysfunction in Major Depressive Disorder. A Translational Review in Animal Models of the Disease. Pharmaceuticals 2016, 9, 9. https://doi.org/10.3390/ph9010009
Darcet F, Gardier AM, Gaillard R, David DJ, Guilloux J-P. Cognitive Dysfunction in Major Depressive Disorder. A Translational Review in Animal Models of the Disease. Pharmaceuticals. 2016; 9(1):9. https://doi.org/10.3390/ph9010009
Chicago/Turabian StyleDarcet, Flavie, Alain M. Gardier, Raphael Gaillard, Denis J. David, and Jean-Philippe Guilloux. 2016. "Cognitive Dysfunction in Major Depressive Disorder. A Translational Review in Animal Models of the Disease" Pharmaceuticals 9, no. 1: 9. https://doi.org/10.3390/ph9010009
APA StyleDarcet, F., Gardier, A. M., Gaillard, R., David, D. J., & Guilloux, J.-P. (2016). Cognitive Dysfunction in Major Depressive Disorder. A Translational Review in Animal Models of the Disease. Pharmaceuticals, 9(1), 9. https://doi.org/10.3390/ph9010009







