Antifungal Activity of 14-Helical β-Peptides against Planktonic Cells and Biofilms of Candida Species
Abstract
:1. Introduction
2. Results
2.1. Design and Synthesis of 14-Helical β-Peptides
2.2. Planktonic Antifungal Activity of β-Peptide Is a Function of Hydrophobicity in Multiple C. albicans Strains

| Peptide # | N-Terminus a (X) | Hydrophobic Residue a (R2) | Cationic Residue a (R3) | Hydrophobicity (HPLC Retention Time, min) b |
|---|---|---|---|---|
| 1 | H | β3-hAla | β3-hLys | 19.3 ± 0.1 |
| 2 | H | β3-Et | β3-hLys | 22.5 ± 0.2 |
| 3 | H | β3-Et | β3-hArg | 23.2 ± 0.1 |
| 4 | H | β3-hVal | β3-hLys | 24.5 ± 0.2 |
| 5 | H | β3-hVal | β3-hArg | 25.4 ± 0.1 |
| 6 | H | ACHC | β3-hLys | 23.1 ± 0.2 |
| 7 | H | ACHC | β3-hArg | 23.8 ± 0.1 |
| 8 | H | β3-hPhe | β3-hLys | 26.2 ± 0.2 |
| 9 | β3-hTyr | β3-hAla | β3-hLys | 20.4 ± 0.2 |
| 10 | β3-hTyr | β3-Et | β3-hLys | 23.5 ± 0.1 |
| 11 | β3-hTyr | β3-Et | β3-hArg | 24.2 ± 0.1 |
| 12 | β3-hTyr | β3-hVal | β3-hLys | 25.7 ± 0.1 |
| 13 | β3-hTyr | β3-hVal | β3-hArg | 26.5 ± 0.2 |
| 14 | β3-hTyr | ACHC | β3-hLys | 24.0 ± 0.2 |
| 15 | β3-hTyr | ACHC | β3-hArg | 24.6 ± 0.2 |
| 16 | β3-hTyr | β3-hPhe | β3-hLys | 27.4 ± 0.2 |
| Peptide # | RT a (min) | MIC b (µg/mL) | ||
|---|---|---|---|---|
| ATCC90028 | K1 | SC5314 | ||
| 1 | 19.3 | >128 | >128 | >128 |
| 2 | 22.5 | 64 | 64 | 64 |
| 6 | 23.1 | 32 | 32 | 16 |
| 3 | 23.2 | 32 | 32 | 32 |
| 7 | 23.8 | 32 | 16 | 16 |
| 4 | 24.5 | 16 | 16 | 8 |
| 5 | 25.4 | 16 | 8 | 8 |
| 8 | 26.2 | 8 | 4 | 8 |
2.3. β-Peptides Kill Planktonic C. glabrata, C. parapsilosis and C. tropicalis Cells in a Hydrophobicity-Dependent Manner
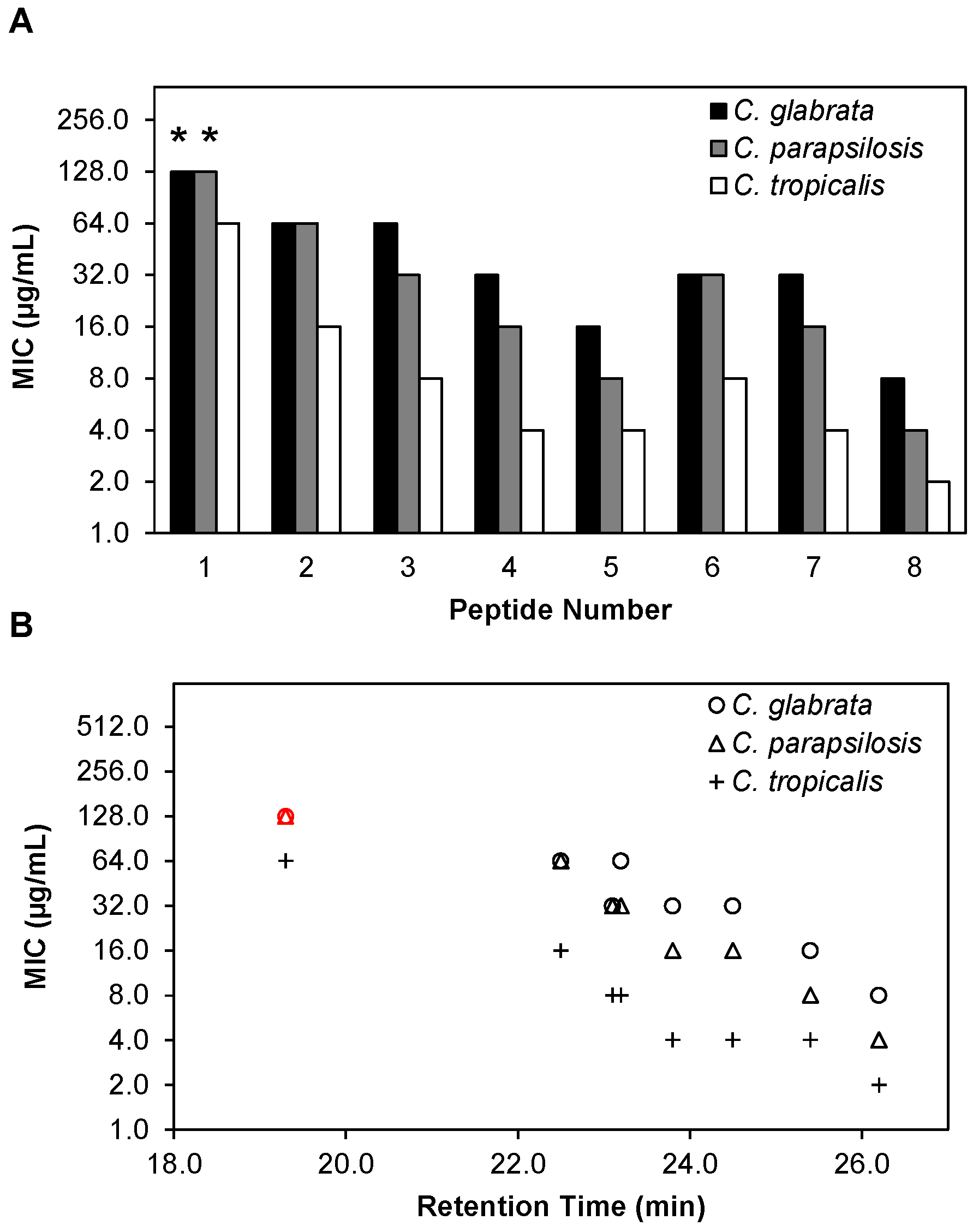
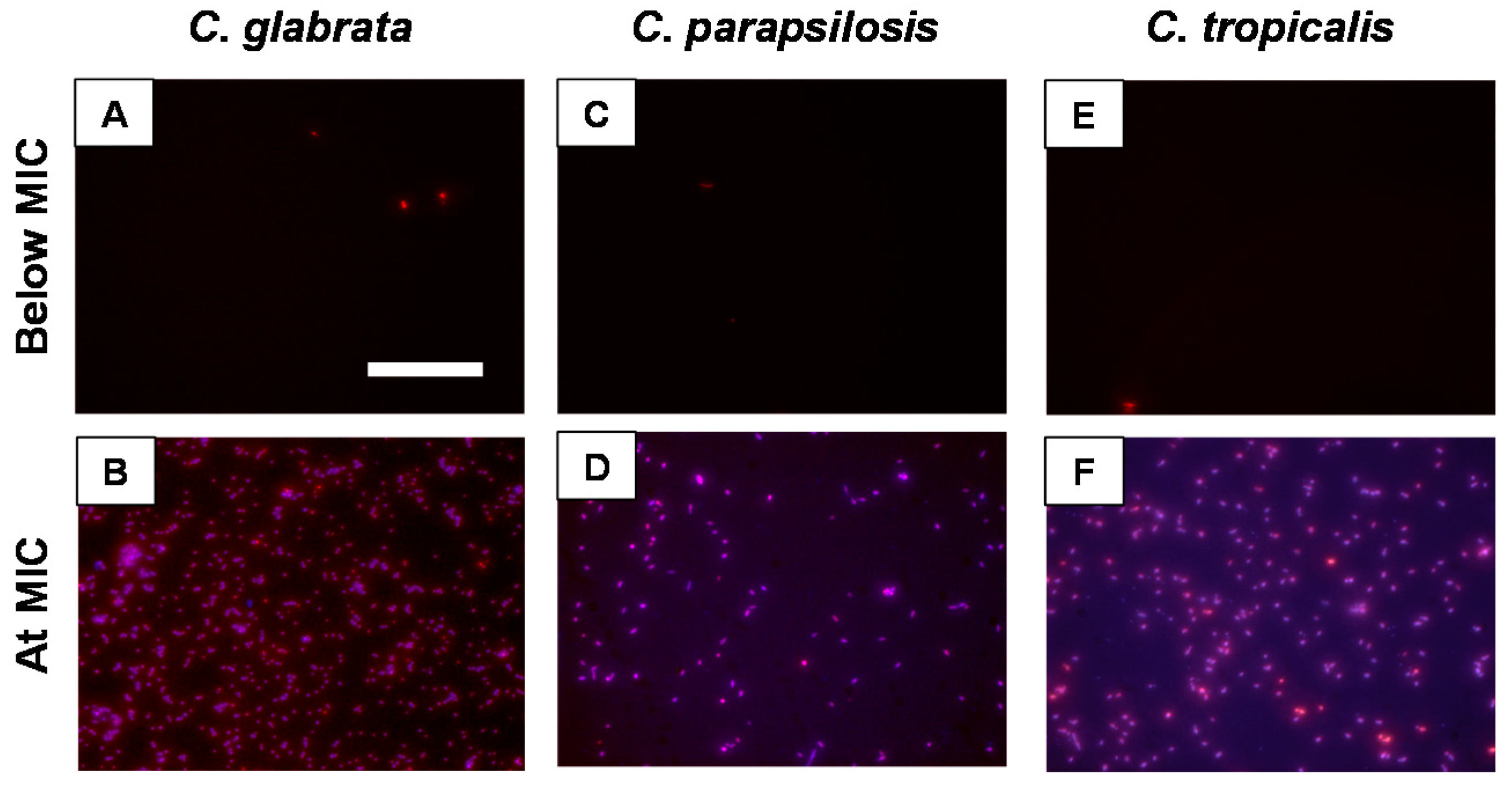
2.4. The Cell Wall Does Not Significantly Affect the Activity of β-Peptides against C. albicans

2.5. β-Peptide Hydrophobicity Does Not Affect Activity against Existing C. albicans Biofilms
2.6. Hydrophobicity of β-Peptide Affects Prevention of C. albicans Biofilm Formation
2.7. β-Peptides Prevent C. glabrata, C. parapsilosis and C. tropicalis Biofilm Formation
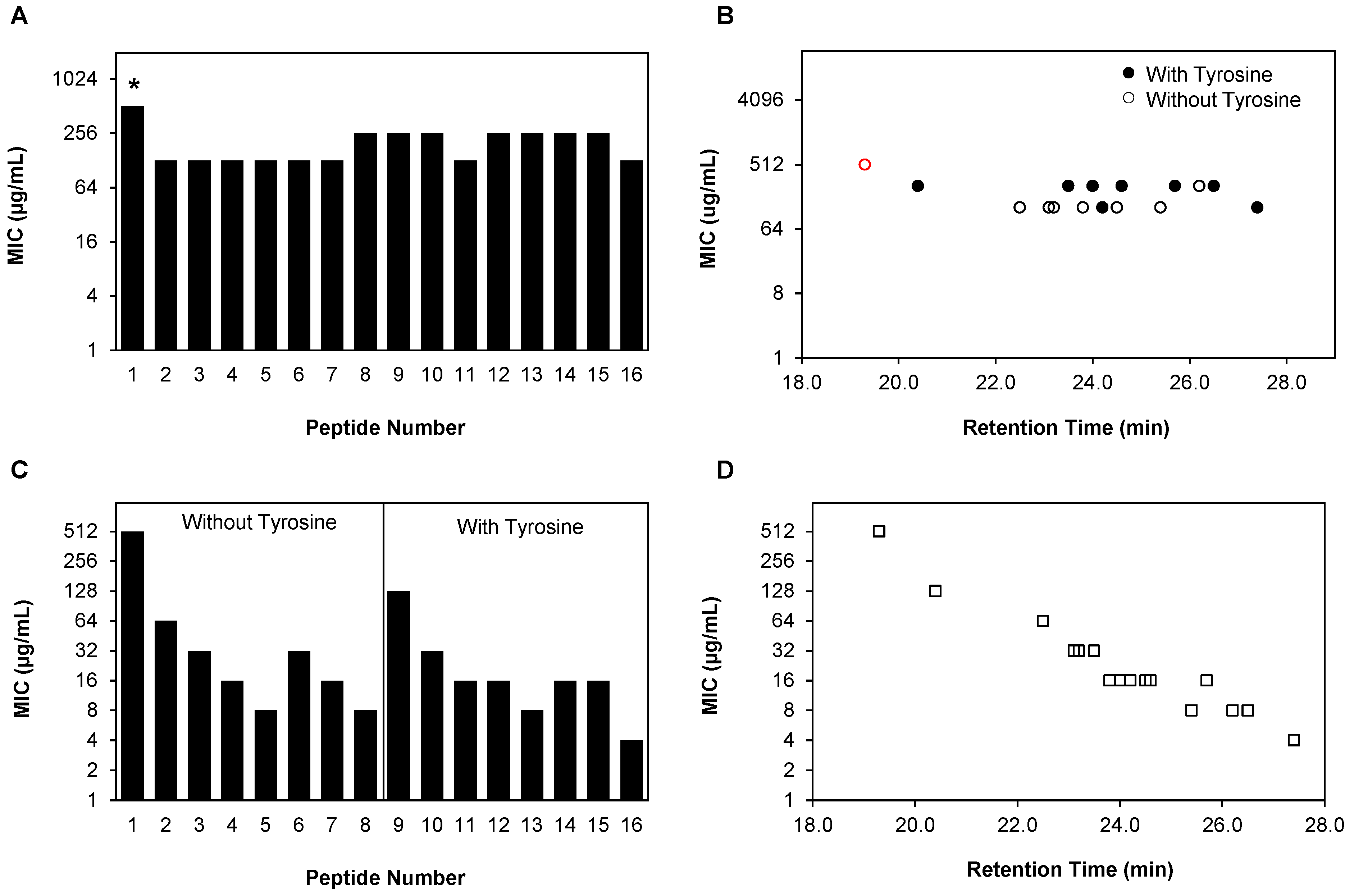
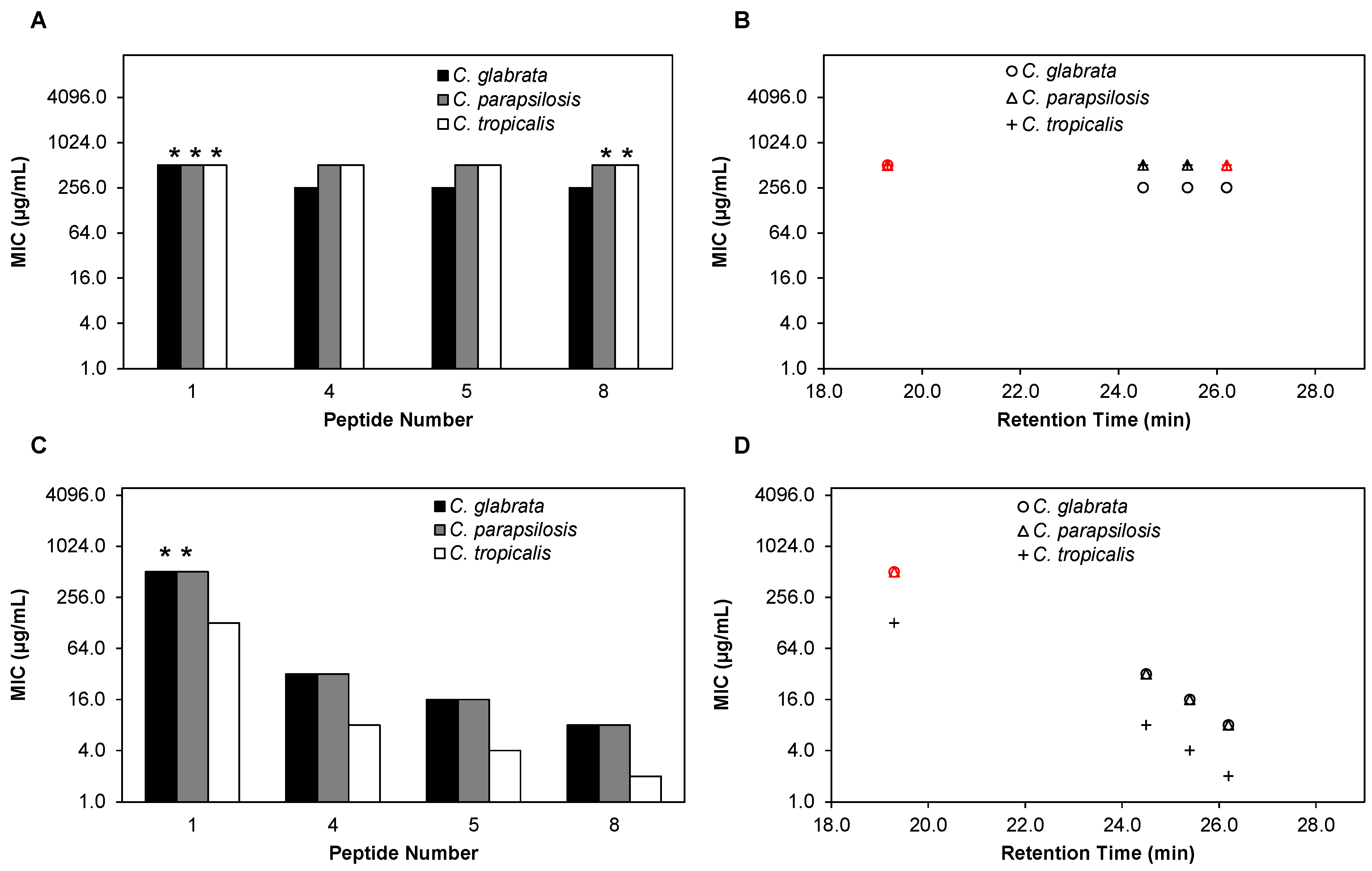
3. Discussion
4. Experimental Section
4.1. Materials
4.2. β-Peptide Synthesis
4.3. Characterization of β-Peptide Hydrophobicity
4.4. Yeast Strain and Culture Conditions
4.5. Planktonic Antifungal Susceptibility Testing
4.6. Fluorescence Imaging of Planktonic C. glabrata, C. parapsilosis and C. tropicalis
4.7. C. albicans Spheroplast Formation and Characterization
4.8. Antifungal Biofilm Susceptibility Testing
4.9. Biofilm Formation in the Presence of β-Peptides
4.10. Quantification of Cell Metabolic Activity Using an XTT Assay
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wenzel, R.P. Nosocomial candidemia: Risk factors and attributable mortality. Clin. Infect. Dis. 1995, 20, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Viscoli, C.; Girmenia, C.; Marinus, A.; Collette, L.; Martino, P.; Vandercam, B.; Doyen, C.; Lebeau, B.; Spence, D.; Krcmery, V.; et al. Candidemia in cancer patients: A prospective, multicenter surveillance study by the invasive fungal infection group (IFIG) of the European organization for research and treatment of cancer (EORTC). Clin. Infect. Dis. 1999, 28, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Concia, E.; Azzini, A.M.; Conti, M. Epidemiology, incidence and risk factors for invasive candidiasis in high-risk patients. Drugs 2009, 69, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Horn, D.; Anaissie, E.; Steinbach, W.; Olyaei, A.; Fishman, J.; Pfaller, M.; Chang, C.; Webster, K.; Marr, K. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: Analysis of multicenter prospective antifungal therapy (path) alliance registry. Clin. Infect. Dis. 2009, 48, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Bodro, M.; Sabe, N.; Gomila, A.; Ayats, J.; Baliellas, C.; Roca, J.; Melilli, E.; Carratala, J. Risk factors, clinical characteristics, and outcomes of invasive fungal infections in solid organ transplant recipients. Transplant. Proc. 2012, 44, 2682–2685. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.; Neofytos, D.; Diekema, D.; Azie, N.; Meier-Kriesche, H.U.; Quan, S.P.; Horn, D. Epidemiology and outcomes of candidemia in 3648 patients: Data from the prospective antifungal therapy (path alliance(r)) registry, 2004–2008. Diagn. Microbiol. Infect. Dis. 2012, 74, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Shoham, S.; Marr, K.A. Invasive fungal infections in solid organ transplant recipients. Future Microbiol. 2012, 7, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, C.A.; Freifeld, A.G.; Andes, D.R.; Baddley, J.W.; Herwaldt, L.; Walker, R.C.; Alexander, B.D.; Anaissie, E.J.; Benedict, K.; Ito, J.I.; et al. Endemic fungal infections in solid organ and hematopoietic cell transplant recipients enrolled in the transplant-associated infection surveillance network (transnet). Transpl. Infect. Dis. 2014, 16, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Cauda, R. Candidaemia in patients with an inserted medical device. Drugs 2009, 69, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Habash, M.; Reid, G. Microbial biofilms: Their development and significance for medical device-related infections. J. Clin. Pharmacol. 1999, 39, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Saville, S.P.; Thomas, D.P.; Lopez-Ribot, J.L. Candida biofilms: An update. Eukaryotic Cell 2005, 4, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Martinez, J.P.; Lopez-Ribot, J.L. Candida biofilms on implanted biomaterials: A clinically significant problem. FEMS Yeast Res. 2006, 6, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [PubMed]
- Sanglard, D.; Odds, F.C. Resistance of Candida species to antifungal agents: Molecular mechanisms and clinical consequences. Lancet. Infect. Dis. 2002, 2, 73–85. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Ciofu, O.; Molin, S.; Givskov, M.; Hoiby, N. Applying insights from biofilm biology to drug development—Can a new approach be developed? Nat. Rev. Drug Discov. 2013, 12, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Mathe, L.; van Dijck, P. Recent insights into Candida albicans biofilm resistance mechanisms. Curr. Genet. 2013, 59, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Chapple, D.S. Peptide antibiotics. Antimicrob. Agents Chemother. 1999, 43, 1317–1323. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Anderson, G.M.; Stolzenberg, E.D.; Kari, U.P.; Zasloff, M.; Wilson, J.M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997, 88, 553–560. [Google Scholar] [CrossRef]
- Chu, H.L.; Yu, H.Y.; Yip, B.S.; Chih, Y.H.; Liang, C.W.; Cheng, H.T.; Cheng, J.W. Boosting salt resistance of short antimicrobial peptides. Antimicrob. Agents Chemother. 2013, 57, 4050–4052. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.A.; Wang, X.; Lee, H.S.; Weisblum, B.; Gellman, S.H. Non-haemolytic beta-amino-acid oligomers. Nature 2000, 404, 565. [Google Scholar] [CrossRef] [PubMed]
- Chongsiriwatana, N.P.; Patch, J.A.; Czyzewski, A.M.; Dohm, M.T.; Ivankin, A.; Gidalevitz, D.; Zuckermann, R.N.; Barron, A.E. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2008, 105, 2794–2799. [Google Scholar] [CrossRef] [PubMed]
- Chongsiriwatana, N.P.; Miller, T.M.; Wetzler, M.; Vakulenko, S.; Karlsson, A.J.; Palecek, S.P.; Mobashery, S.; Barron, A.E. Short alkylated peptoid mimics of antimicrobial lipopeptides. Antimicrob. Agents Chemother. 2011, 55, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Ong, Z.Y.; Wiradharma, N.; Yang, Y.Y. Strategies employed in the design and optimization of synthetic antimicrobial peptide amphiphiles with enhanced therapeutic potentials. Adv. Drug Deliv. Rev. 2014, 78, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.A.; Weisblum, B.; Gellman, S.H. Mimicry of host-defense peptides by unnatural oligomers: Antimicrobial β-peptides. J. Am. Chem. Soc. 2002, 124, 7324–7330. [Google Scholar] [CrossRef] [PubMed]
- Raguse, T.L.; Porter, E.A.; Weisblum, B.; Gellman, S.H. Structure-activity studies of 14-helical antimicrobial β-peptides: Probing the relationship between conformational stability and antimicrobial potency. J. Am. Chem. Soc. 2002, 124, 12774–12785. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.J.; Pomerantz, W.C.; Weisblum, B.; Gellman, S.H.; Palecek, S.P. Antifungal activity from 14-helical β-peptides. J. Am. Chem. Soc. 2006, 128, 12630–12631. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.J.; Pomerantz, W.C.; Neilsen, K.J.; Gellman, S.H.; Palecek, S.P. Effect of sequence and structural properties on 14-helical β-peptide activity against Candida albicans planktonic cells and biofilms. ACS Chem. Biol. 2009, 4, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Raman, N.; Gellman, S.H.; Lynn, D.M.; Palecek, S.P. Hydrophobicity and helicity regulate the antifungal activity of 14-helical β-peptides. ACS Chem. Biol. 2014, 9, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol. 2011, 19, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: Data from the prospective antifungal therapy (path) registry 2004–2008. PloS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Fidel, P.L.; Vazquez, J.A.; Sobel, J.D. Candida glabrata: Review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 1999, 12, 80–96. [Google Scholar] [PubMed]
- Bizerra, F.C.; Nakamura, C.V.; de Poersch, C.; Estivalet Svidzinski, T.I.; Borsato Quesada, R.M.; Goldenberg, S.; Krieger, M.A.; Yamada-Ogatta, S.F. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008, 8, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Van Asbeck, E.C.; Clemons, K.V.; Stevens, D.A. Candida parapsilosis: A review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Crit. Rev. Microbiol. 2009, 35, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Cruz, M.; Lopez-Romero, E.; Villagomez-Castro, J.C.; Ruiz-Baca, E. Candida species: New insights into biofilm formation. Future Microbiol. 2012, 7, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Hamuro, Y.; Schneider, J.P.; DeGrado, W.F. De novo design of antibacterial beta-peptides. J. Am. Chem. Soc. 1999, 121, 12200–12201. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.S.; Lee, B.J. Correlation between the activities of alpha-helical antimicrobial peptides and hydrophobicities represented as RP HPLC retention times. Peptides 2005, 26, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Kullberg, B.J.; van der Lee, H.; Vasil, A.I.; Hale, J.D.; Mant, C.T.; Hancock, R.E.; Vasil, M.L.; Netea, M.G.; Hodges, R.S. Effects of hydrophobicity on the antifungal activity of α-helical antimicrobial peptides. Chem. Biol. Drug Des. 2008, 72, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Chen, X.; Falk, S.P.; Mowery, B.P.; Karlsson, A.J.; Weisblum, B.; Palecek, S.P.; Masters, K.S.; Gellman, S.H. Structure-activity relationships among antifungal nylon-3 polymers: Identification of materials active against drug-resistant strains of Candida albicans. J. Am. Chem. Soc. 2014, 136, 4333–4342. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, X.; Falk, S.P.; Masters, K.S.; Weisblum, B.; Gellman, S.H. Nylon-3 polymers active against drug-resistant Candida albicans biofilms. J. Am. Chem. Soc. 2015, 137, 2183–2186. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Nett, J.; Oschel, P.; Albrecht, R.; Marchillo, K.; Pitula, A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 2004, 72, 6023–6031. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.A.; Tsyshevskaya-Hoover, I.; Hinsley, H.; Logan, S.; Nguyen, M.; Nguyen, T.T.; Pohl, J.; Wozniak, K.; Fidel, P.L. Candidacidal activity of synthetic peptides based on the antimicrobial domain of the neutrophil-derived protein, CAP37. Med. Mycol. 2010, 48, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Tavanti, A.; Maisetta, G.; del Gaudio, G.; Petruzzelli, R.; Sanguinetti, M.; Batoni, G.; Senesi, S. Fungicidal activity of the human peptide hepcidin 20 alone or in combination with other antifungals against Candida glabrata isolates. Peptides 2011, 32, 2484–2487. [Google Scholar] [CrossRef] [PubMed]
- Grieco, P.; Carotenuto, A.; Auriemma, L.; Limatola, A.; Di Maro, S.; Merlino, F.; Mangoni, M.L.; Luca, V.; Di Grazia, A.; Gatti, S.; et al. Novel α-msh peptide analogues with broad spectrum antimicrobial activity. PloS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef] [PubMed]
- Raguse, T.L.; Lai, J.R.; Gellman, S.H. Environment-independent 14-helix formation in short β-peptides: Striking a balance between shape control and functional diversity. J. Am. Chem. Soc. 2003, 125, 5592–5593. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Raguse, T.L.; Schinnerl, M.; Pomerantz, W.C.; Wang, X.; Wipf, P.; Gellman, S.H. Origins of the high 14-helix propensity of cyclohexyl-rigidified residues in beta-peptides. Org. Lett. 2007, 9, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Vaz, E.; Pomerantz, W.C.; Geyer, M.; Gellman, S.H.; Brunsveld, L. Comparison of design strategies for promotion of beta-peptide 14-helix stability in water. Chembiochem. 2008, 9, 2254–2259. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Bajwa, J.S.; Sun, J.N.; Edgerton, M. Salivary histatin 5 internalization by translocation, but not endocytosis, is required for fungicidal activity in Candida albicans. Mol. Microbiol. 2010, 77, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Edgerton, M. How does it kill?: Understanding the candidacidal mechanism of salivary histatin 5. Eukaryotic Cell 2014, 13, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Mohanram, H.; Bhattacharjya, S. Resurrecting inactive antimicrobial peptides from the lipopolysaccharide trap. Antimicrob. Agents Chemother. 2014, 58, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Nunez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, J.; d’Enfert, C. Candida albicans biofilms: Building a heterogeneous, drug-tolerant environment. Curr. Opin. Microbiol. 2013, 16, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Taff, H.T.; Nett, J.E.; Zarnowski, R.; Ross, K.M.; Sanchez, H.; Cain, M.T.; Hamaker, J.; Mitchell, A.P.; Andes, D.R. A Candida biofilm-induced pathway for matrix glucan delivery: Implications for drug resistance. PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.J.; Flessner, R.M.; Gellman, S.H.; Lynn, D.M.; Palecek, S.P. Polyelectrolyte multilayers fabricated from antifungal β-peptides: Design of surfaces that exhibit antifungal activity against Candida albicans. Biomacromolecules 2010, 11, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.J.; Venuleo, C.; Beri, A.; Oppenheim, F.G. Candida glabrata is unusual with respect to its resistance to cationic antifungal proteins. Yeast 2005, 22, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Prasad, T.; Kapoor, K.; Mandal, A.; Roth, M.; Welti, R.; Prasad, R. Phospholipidome of Candida: Each species of Candida has distinctive phospholipid molecular species. Omics 2010, 14, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.K.; Gellman, S.H. Application of microwave irradiation to the synthesis of 14-helical beta-peptides. Org. Lett. 2005, 7, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.F.; Taff, H.T.; Cuevas, M.A.; Reinicke, E.L.; Sanchez, H.; Andes, D.R. Role of matrix beta-1,3 glucan in antifungal resistance of non-albicans Candida biofilms. Antimicrob. Agents Chemother. 2013, 57, 1918–1920. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raman, N.; Lee, M.-R.; Lynn, D.M.; Palecek, S.P. Antifungal Activity of 14-Helical β-Peptides against Planktonic Cells and Biofilms of Candida Species. Pharmaceuticals 2015, 8, 483-503. https://doi.org/10.3390/ph8030483
Raman N, Lee M-R, Lynn DM, Palecek SP. Antifungal Activity of 14-Helical β-Peptides against Planktonic Cells and Biofilms of Candida Species. Pharmaceuticals. 2015; 8(3):483-503. https://doi.org/10.3390/ph8030483
Chicago/Turabian StyleRaman, Namrata, Myung-Ryul Lee, David M. Lynn, and Sean P. Palecek. 2015. "Antifungal Activity of 14-Helical β-Peptides against Planktonic Cells and Biofilms of Candida Species" Pharmaceuticals 8, no. 3: 483-503. https://doi.org/10.3390/ph8030483
APA StyleRaman, N., Lee, M.-R., Lynn, D. M., & Palecek, S. P. (2015). Antifungal Activity of 14-Helical β-Peptides against Planktonic Cells and Biofilms of Candida Species. Pharmaceuticals, 8(3), 483-503. https://doi.org/10.3390/ph8030483





