A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters?
Abstract
:1. Alpha Emitting Radionuclides in the Clinic
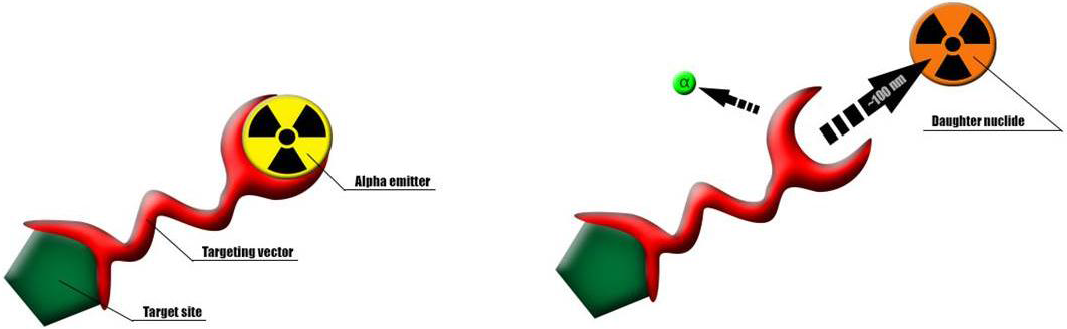
2. In Vivo Alpha Generator Radionuclides and Their Decay Chains
| Radionuclides and Their Decay Chain | Half-Life | Decay | Eα (MeV) * | Calculated ER (keV) * |
|---|---|---|---|---|
| 225Ac | 9.9 days ** | α(100%) | 5.8 | - |
| 221Fr | 4.9 min | α(100%) | 6.3 | 105.5 |
| 217At | 32 ms | α(99.98%)/β−(0.01%) | 7.1 | 116.9 |
| 213Bi | 45.6 min | α(2%)/β−(98%) | - | 132.8 |
| 213Po | 4.2 µs | α(100%) | 8.4 | - |
| 209Pb | 3.3 h | β− (100%) | - | 160.4 |
| 209Bi | stable | - | - | - |
| 227Th | 18.7 days | α(100%) | 6 | - |
| 223Ra | 11.4 days | α(100%) | 5.7 | 108.4 |
| 219Rn | 4.0 s | α(100%) | 6.8 | 104.5 |
| 215Po | 1.8 ms | α(100%) | 7.4 | 126.9 |
| 211Bi | 2.2 min | α(99.7%)/β−(0.3%) | 6.6 | 140.1 |
| 207Tl | 4.8 min | β− (100%) | - | 128.1 |
| 207Pb | stable | - | - | |
| 228Th | 1.9 years | α(100%) | 5.4 | - |
| 224Ra | 3.7 days | α(100%) | 5.7 | 96.9 |
| 220Rn | 55.6 s | α(100%) | 6.3 | 103.4 |
| 216Po | 0.15 s | α(100%) | 6.8 | 116.5 |
| 212Pb | 10.6 h | β−(100%) | - | 128 |
| 212Bi | 60.6 min | α(36%)/β−(64%) | 6.1/- | - |
| 208Tl/212Po | 3.0 min/0.3 µs | β−(100%)/α(100%) | -/8.8 | 116.5/- |
| 208Pb | stable | - | - | - |
| 230U | 20.8 days | α(100%) | 5.9 | - |
| 226Th | 31 min | α(100%) | 6.3 | 104.3 |
| 222Ra | 38 s | α(100%) | 6.5 | 114.2 |
| 218Rn | 35 ms | α(100%) | 7.1 | 120.4 |
| 214Po | 164 µs | α(100%) | 7.7 | 133.4 |
| 210Pb | 22.3 years | β−(100%) | 146.5 | |
| 210Bi | 5.0 days | β−(100%) | - | |
| 210Po | 138.4 days | α(100%) | 5.3 | - |
| 206Pb | stable | - |
3. Distribution of Recoil Daughters in the Body
| Element | Major Targeted Organs * |
|---|---|
| Francium | primarily kidneys [38] |
| Bismuth | 30% urine, 40% kidney, 30% other organs [39] |
| Radium | 25% bone surface, 45% soft tissue, 30% excreted via large intestine [12,24] |
| Radon | soft tissue to blood: 100 day−1, exhaled 1 min−1, bone to blood: 0.36 d−1 [40] |
| Lead | 55% blood, 15% liver, 10%–15% skeleton, kidneys 4% after 1 day [40] |
| Polonium | 28% liver, 28% kidneys, 10% red bone marrow, 5% spleen [41] |
4. Approaches to Deal with the Recoil Problem
4.1. Encapsulation in A Nano-Carrier
4.2. Fast Uptake in Tumour Cells
4.3. Local Administration
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Carlise, R.; Bunch, P.; Yoder, D.; Alvarez, R. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.J.; Singla, A.A.; Rizvi, S.M.A.; Graham, P.; Bruchertseifer, F.; Apostolidis, C.; Morgenstern, A. Analysis of patient survival in a Phase I trial of systemic targeted α-therapy for metastatic melanoma. Immunotherapy 2011, 3, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Raja, C.; Graham, P.; Rizvi, S.; Song, E.; Goldsmith, H.; Thompson, J.; Bosserhoff, A.; Morgenstern, A.; Apostolidis, C.; Kearsley, J.; et al. Interim analysis of toxicity and response in phase 1 trial of systemic targeted alpha therapy for metastatic melanoma. Cancer Biol. Ther. 2007, 6, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Mcdevitt, M.R.; Finn, R.D.; Ma, D.; Larson, S.M.; Scheinberg, D.A. Preparation of alpha-Emitting 213Bi-Labeled Antibody Constructs for Clinical Use. J. Nucl. Med. 1999, 40, 1722–1727. [Google Scholar] [PubMed]
- Jurcic, J.G.; Larson, S.M.; Sgouros, G.; Mcdevitt, M.R.; Finn, R.D.; Divgi, C.R.; Ballangrud, Å.M.; Hamacher, K.A.; Ma, D.; Humm, J.L.; et al. Targeted alpha particle immunotherapy for myeloid leukemia. Blood 2002, 100, 1233–1240. [Google Scholar] [PubMed]
- Rosenblat, T.L.; McDevitt, M.R.; Mulford, D.A.; Pandit-Taskar, N.; Divgi, C.R.; Panageas, K.S.; Heaney, M.L.; Chanel, S.; Morgenstern, A.; Sgouros, G.; et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin. Cancer Res. 2010, 16, 5303–5311. [Google Scholar] [CrossRef] [PubMed]
- Cordier, D.; Forrer, F.; Bruchertseifer, F.; Morgenstern, A.; Apostolidis, C.; Good, S.; Müller-Brand, J.; Mäcke, H.; Reubi, J.C.; Merlo, A. Targeted alpha-radionuclide therapy of functionally critically located gliomas with 213Bi-DOTA-[Thi8,Met(O2)11]-substance P: A pilot trial. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical Experience with α-Particle–Emitting 211At: Treatment of Recurrent Brain Tumor Patients with 211At-Labeled Chimeric Antitenascin Monoclonal Antibody 81C6. J. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Cederkrantz, E.; Back, T.; Divgi, C.; Elgqvist, J.; Himmelman, J.; Horvath, G.; Jacobsson, L.; Jensen, H.; Lindegren, S.; et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of (211)At-MX35 F(ab’)2-a phase I study. J. Nucl. Med. 2009, 50, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Targeted Atomic Nano-Generators (Actinium-225-Labeled Humanized Anti-CD33 Monoclonal Antibody HuM195) in Patients With Advanced Myeloid Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT00672165 (accessed on 11 May 2015).
- Jadvar, H.; Quinn, D. Targeted α-Particle Therapy of Bone Metastases in Prostate Cancer. Clin. Nucl. Med. 2013, 38, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, M.; Nosske, D.; Reiners, C. Therapy of ankylosing spondylitis with 224Ra-radium chloride: Dosimetry and risk considerations. Radiat. Environ. Biophys. 2002, 41, 173–178. [Google Scholar] [PubMed]
- Morgenstern, A.; Bruchertseifer, F.; Apostolidis, C. Bismuth-213 and Actinium-225—Generator Performance and Evolving Therapeutic Applications of Two Generator-Derived Alpha-Emitting Radioisotopes. Curr. Radiopharm. 2012, 49, 221–227. [Google Scholar] [CrossRef]
- Müller, C.; Reber, J.; Haller, S.; Dorrer, H.; Köster, U.; Johnston, K.; Zhernosekov, K.; Türler, A.; Schibli, R. Folate receptor targeted alpha-therapy using terbium-149. Pharmaceuticals 2014, 7, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Hobbs, R.F.; Vajravelu, R.; Huso, D.L.; Esaias, C.; Morgenstern, A.; Sgouros, G. Radioimmunotherapy of breast cancer metastases with alpha-particle emitter 225Ac: Comparing efficacy with 213Bi and 90Y. Cancer Res. 2009, 69, 8941–8948. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Atkinson, M.J.; Nekolla, E.A. Incidence of leukaemia and other malignant diseases following injections of the short-lived alpha-emitter 224Ra into man. Radiat. Environ. Biophys. 2009, 48, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Nekolla, E.A.; Gaubitz, M.; Schulte, T.L. Increased risk of myeloid leukaemia in patients with ankylosing spondylitis following treatment with radium-224. Rheumatology (Oxford) 2008, 47, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Kommission Pharmakotherapie. Stellungnahme der Deutschen Gesellschaft für Rheumatologie zur Therapie der ankylosierenden Spondylitis (AS) mit Radiumchlorid (224SpondylAT®). Z. Rheumatol. 2001, 60, 84–87. [Google Scholar]
- Zhang, Z.; Siegert, J.; Maywald, U.; Kirch, W. Cost-benefit analysis of [224Ra] radium chloride therapy for ankylosing spondylitis (Bekhterev’s disease). Med. Klin. (Munich) 2007, 102, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Schulte, T.L.; Nekolla, E.A.; Wick, R.R. Long-term investigation of the risk of malignant diseases following intravenous radium-224 treatment for ankylosing spondylitis. Strahlentherapie und Onkol. 2009, 185, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Eckert & Ziegler Eckert & Ziegler stoppt klinische Entwicklung von SpondylAT®. Available online: http://www.ezag.com/de/print/startseite/presse/pressemeldungen/detail/article/eckert-ziegler-stoppt-klinische-entwicklung-von-spondylatR.html (accessed on 11 May 2015).
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.; Song, H.; Watchman, C.; Bolch, W.; Aksnes, A.-K.; Ramdahl, T.; Flux, G.D.; Sgouros, G. A bone marrow toxicity model for 223Ra alpha-emitter radiopharmaceutical therapy. Phys. Med. Biol. 2013, 57, 3207–3222. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, G.; Fisher, D.R.; Roeske, J.C.; Bruland, Ø.S.; Larsen, R.H. Targeting of Osseous Sites with Alpha-Emitting 223Ra: Comparison with the Beta-Emitter 89Sr in Mice. J. Nucl. Med. 2003, 44, 252–260. [Google Scholar] [PubMed]
- Phase II Trial of Ra-223 Dichloride and Hormonal Treatment. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02366130?term=radium+223+breast&rank=4 (accessed on 11 May 2015).
- Study of Radium-223 Dichloride Versus Placebo and Treatment With Exemestane / Everolimus in Subjects With Bone Predominant HER2 (Human Epidermal Growth Factor Receptor 2) Negative Hormone Receptor Positive Metastatic Breast Cancer. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02258451?term=radium+223+breast&rank=2 (accessed on 11 May 2015).
- Jurcic, J.G.; Rosenblat, T.L.; McDevitt, M.R.; Pandit-Taskar, N.; Carrasquillo, J.A.; Chanel, S.M.; Ryan, C.; Frattini, M.G.; Cicic, D.; Larson, S.M.; et al. Phase I trial of the targeted alpha-particle nano-generator actinium-225 (225Ac-lintuzumab) (anti-CD33; HuM195) in acute myeloid leukemia (AML). In Proceedings of 2011 ASCO Annual Meeting, Chicago, IL, USA, June 2011.
- Jurcic, J.G. What happened to anti-CD33 therapy for acute myeloid leukemia? Curr. Hematol. Malig. Rep. 2012, 7, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G.; Ravandi, F.; Pagel, J.M.; Park, J.H.; Douer, D.; Estey, E.H.; Kantarjian, H.M.; Cicic, D.; Scheinberg, D.A. Phase I Trial Of The Targeted Alpha-Particle Nano-Generator Actinium-225 (225Ac)-Lintuzumab (Anti-CD33) In Combination With Low-Dose Cytarabine (LDAC) For Older Patients With Untreated Acute Myeloid Leukemia (AML). In Proceedings of 55th ASH Annual Meeting and Exposition, San Francisco, CA, USA, 7–10 December 2013; p. 1460.
- Magill, J.; Pfennig, G.; Galy, J. Karlsruher Nuklidkarte, 7th ed.; The Joint Research Centre: Brussels, Belgium, 2006. [Google Scholar]
- Pommé, S.; Marouli, M.; Suliman, G.; Dikmen, H.; Van Ammel, R.; Jobbágy, V.; Dirican, A.; Stroh, H.; Paepen, J.; Bruchertseifer, F.; et al. Measurement of the 225Ac half-life. Appl. Radiat. Isot. 2012, 70, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, S.; Kumar, K.; Gansow, O.A. The Chemical Fate of 212Bi-DOTA Formed by β-Decay of 212Pb(DOTA)2. Radiochim. Acta 1993, 60, 1–10. [Google Scholar]
- Dahle, J.; Borrebæk, J.; Melhus, K.B.; Bruland, Ø.S.; Salberg, G.; Olsen, D.R.; Larsen, R.H. Initial evaluation of 227Th-p-benzyl-DOTA-rituximab for low-dose rate α-particle radioimmunotherapy. Nucl. Med. Biol. 2006, 33, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, G.; Alstad, J.; Hoff, P.; Larsen, R.H. 223Ra for endotherapeutic applications prepared from an immobilized 227Ac/227Th source. Radiochim. Acta 2001, 89, 661–666. [Google Scholar] [CrossRef]
- Morgenstern, A.; Abbas, K.; Bruchertseifer, F.; Apostolidis, C. Production of Alpha Emitters for Targeted Alpha Therapy. Curr. Radiopharm. 2008, 1, 135–143. [Google Scholar] [CrossRef]
- Weidner, J.W.; Mashnik, S.G.; John, K.D.; Hemez, F.; Ballard, B.; Bach, H.; Birnbaum, E.R.; Bitteker, L.J.; Couture, A.; Dry, D.; et al. Proton-induced cross sections relevant to production of 225Ac and 223Ra in natural thorium targets below 200 MeV. Appl. Radiat. Isot. 2012, 70, 2602–2607. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, L.; Schaart, D.R.; de Vries, D.; Morgenstern, A.; Bruchertseifer, F.; Denkova, A.G. Polymersomes as nano-carriers to retain harmful recoil nuclides in alpha radionuclide therapy: A feasibility study. Radiochim. Acta 2012, 100, 473. [Google Scholar] [CrossRef]
- Jaggi, J.S.; Kappel, B.J.; Mcdevitt, M.R.; Sgouros, G.; Flombaum, C.D.; Cabassa, C.; Scheinberg, D.A. Efforts to Control the Errant Products of a Targeted In vivo Generator. Cancer Res. 2005, 65, 4888–4896. [Google Scholar] [CrossRef] [PubMed]
- Beninson, D.J.; Dunster, H.J.; Jacobi, W.; Jammet, H.P.; Liniecki, J.; Meinhold, C.B.; Moiseev, A.A.; Rowley, K.A.; Sinclair, W.K.; Takahashi, S.; et al. Limits for Intakes of Radionuclides by Workers. In Annals of the ICRP; Sowby, F.D., Ed.; Pergamon Press: Oxford, UK, 1980; p. 67. [Google Scholar]
- Beninson, D.; Dunster, H.J.; Ilyin, I.A.; Jacobi, W.; Jammet, H.P.; Kaul, A.; Li, D.; Liniecki, J.; Matsudaira, H.; Mettler, F.; et al. Age-dependent Doses to Members of the Public from Intake of Radionuclides: Part 2 Ingestion Dose Coefficients. In Annals of the ICRP; Smith, H., Ed.; Pergamon Press: Oxford, UK, 1993; pp. 75–87. [Google Scholar]
- Keverling Buisman, A.S. Handboek Radionucliden; BetaText: Bergen, Netherlands, 1996; pp. 226–227. [Google Scholar]
- Davis, I.A.; Glowienka, K.A.; Boll, R.A.; Deal, K.A.; Brechbiel, M.W.; Stabin, M.; Bochsler, P.N.; Mirzadeh, S.; Kennel, S.J. Comparison of Actinium Chelates: Tissue Distribution and Radiotoxicity. Nucl. Med. Biol. 1999, 26, 581–589. [Google Scholar] [CrossRef]
- Beyer, G.J.; Bergmann, R.; Schomäcker, K.; Rösch, F.; Schäfer, G.; Kulikov, E.V.; Novgorodov, A.F. Comparison of the Biodistribution of 225Ac and Radio-Lanthanides as Citrate Complexes. Isotopenpraxis 1990, 26, 111–114. [Google Scholar]
- Lloyd, R.D.; Mays, C.W.; Taylor, G.N.; Atherton, D.R.; Bruenger, F.W.; Jones, C.W. Radium-224 Retention, Distribution, and Dosimetry in Beagles. Radiat. Res. 1982, 295, 280–295. [Google Scholar] [CrossRef]
- Piotrowska, A.; Leszczuk, E.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized NaA nanozeolites labeled with (224,225)Ra for targeted alpha therapy. J. Nanoparticle Res. 2013, 15, 2082. [Google Scholar] [CrossRef] [PubMed]
- Sofou, S.; Thomas, J.L.; Lin, H.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Engineered liposomes for potential alpha-particle therapy of metastatic cancer. J. Nucl. Med. 2004, 45, 253–260. [Google Scholar] [PubMed]
- Wang, G.; de Kruijff, R.M.; Rol, A.; Thijssen, L.; Mendes, E.; Morgenstern, A.; Bruchertseifer, F.; Stuart, M.C.A.; Wolterbeek, H.T.; Denkova, A.G. Retention studies of recoiling daughter nuclides of 225Ac in polymer vesicles. Appl. Radiat. Isot. 2014, 85, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jonasdottir, T.J.; Fisher, D.R.; Borrebaek, J.; Bruland, O.S.; Larsen, R.H. First in vivo evaluation of liposome-encapsulated 223Ra as a potential alpha-particle-emitting cancer therapeutic agent. Anticancer Res. 2006, 26, 2841–2848. [Google Scholar] [PubMed]
- Woodward, J.; Kennel, S.J.; Stuckey, A.; Osborne, D.; Wall, J.; Rondinone, A.J.; Standaert, R.F.; Mirzadeh, S. LaPO4 nanoparticles doped with actinium-225 that partially sequester daughter radionuclides. Bioconjug. Chem. 2011, 22, 766–776. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.F.; Woodward, J.; Boll, R.A.; Wall, J.S.; Rondinone, A.J.; Kennel, S.J.; Mirzadeh, S.; Robertson, J.D. Gold coated lanthanide phosphate nanoparticles for targeted alpha generator radiotherapy. PLoS ONE 2013, 8, e54531. [Google Scholar] [CrossRef] [PubMed]
- Essler, M.; Gärtner, F.C.; Neff, F.; Blechert, B.; Senekowitsch-Schmidtke, R.; Bruchertseifer, F.; Morgenstern, A.; Seidl, C. Therapeutic efficacy and toxicity of 225Ac-labelled vs. 213Bi-labelled tumour-homing peptides in a preclinical mouse model of peritoneal carcinomatosis. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Miederer, M.; Henriksen, G.; Alke, A.; Mossbrugger, I.; Quintanilla-Martinez, L.; Senekowitsch-Schmidtke, R.; Essler, M. Preclinical evaluation of the alpha-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin. Cancer Res. 2008, 14, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Mcdevitt, M.R.; Ma, D.; Lai, L.T.; Simon, J.; Borchardt, P.; Frank, R.K.; Wu, K.; Pellegrini, V.; Curcio, M.J.; Miederer, M.; et al. Tumor Therapy with Targeted Atomic Nanogenerators. Science 2001, 294, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Ballangrud, A.M.; Jurcic, J.G.; Mcdevitt, M.R.; Humm, J.L.; Erdi, Y.E.; Mehta, B.M.; Finn, R.D.; Larson, S.M.; Scheinberg, D.A. Pharmacokinetics and Dosimetry of an a-Particle Emitter Labeled Antibody: 213Bi-HuM195 ( Anti-CD33 ) in Patients with Leukemia. J. Nucl. Med. 1999, 40, 1935–1946. [Google Scholar] [PubMed]
- Miederer, M.; Mcdevitt, M.R.; Sgouros, G.; Kramer, K.; Cheung, N.V.; Scheinberg, D.A. Pharmacokinetics, Dosimetry, and Toxicity of the Tagetable Atomic Generator, 225Ac-HuM195, in Nonhuman Primates. J. Nucl. Med. 2004, 45, 129–137. [Google Scholar] [PubMed]
- Borchardt, P.E.; Yuan, R.R.; Miederer, M.; Mcdevitt, M.R.; Scheinberg, D.A. Targeted Actinium-225 in Vivo Generators for Therapy of Ovarian Cancer. Cancer Res. 2003, 63, 5084–5090. [Google Scholar] [PubMed]
- Staudacher, A.H.; Bezak, E.; Borysenko, A.; Brown, M.P. Targeted α-therapy using 227Th-APOMAB and cross-fire antitumour effects: Preliminary in vivo evaluation. Nucl. Med. Commun. 2014, 35, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Arazi, L.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. Treatment of solid tumors by interstitial release of recoiling short-lived alpha emitters. Phys. Med. Biol. 2007, 52, 5025–5042. [Google Scholar] [CrossRef] [PubMed]
- Cooks, T.; Schmidt, M.; Bittan, H.; Lazarov, E.; Arazi, L.; Kelson, I.; Keisari, Y. Local control of lung derived tumors by diffusing alpha-emitting atoms released from intratumoral wires loaded with radium-224. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Cooks, T.; Tal, M.; Raab, S.; Efrati, M.; Reitkopf, S.; Lazarov, E.; Etzyoni, R.; Schmidt, M.; Arazi, L.; Kelson, I.; et al. Intratumoral 224Ra-Loaded Wires Spread Alpha-Emitters Inside Solid Human Tumors in Athymic Mice Achieving Tumor Control. Anticancer Res. 2012, 32, 5315–5321. [Google Scholar] [PubMed]
- Horev-Drori, G.; Cooks, T.; Bittan, H.; Lazarov, E.; Schmidt, M.; Arazi, L.; Efrati, M.; Kelson, I.; Keisari, Y. Local control of experimental malignant pancreatic tumors by treatment with a combination of chemotherapy and intratumoral 224radium-loaded wires releasing alpha-emitting atoms. Transl. Res. 2012, 159, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Confino, H.; Hochman, I.; Efrati, M.; Schmidt, M.; Umansky, V.; Kelson, I.; Keisari, Y. Tumor ablation by intratumoral Ra-224-loaded wires induces anti-tumor immunity against experimental metastatic tumors. Cancer Immunol. Immunother. 2014, 64, 191–199. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321-336. https://doi.org/10.3390/ph8020321
De Kruijff RM, Wolterbeek HT, Denkova AG. A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters? Pharmaceuticals. 2015; 8(2):321-336. https://doi.org/10.3390/ph8020321
Chicago/Turabian StyleDe Kruijff, Robin M., Hubert T. Wolterbeek, and Antonia G. Denkova. 2015. "A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters?" Pharmaceuticals 8, no. 2: 321-336. https://doi.org/10.3390/ph8020321
APA StyleDe Kruijff, R. M., Wolterbeek, H. T., & Denkova, A. G. (2015). A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters? Pharmaceuticals, 8(2), 321-336. https://doi.org/10.3390/ph8020321





