Edaravone Enhances Brain-Derived Neurotrophic Factor Production in the Ischemic Mouse Brain
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals
2.2. Global Cerebral Ischemia Procedure
2.3. Drug Treatment
2.4. Immunofluorescence for Confocal Microscopy
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results and Discussion
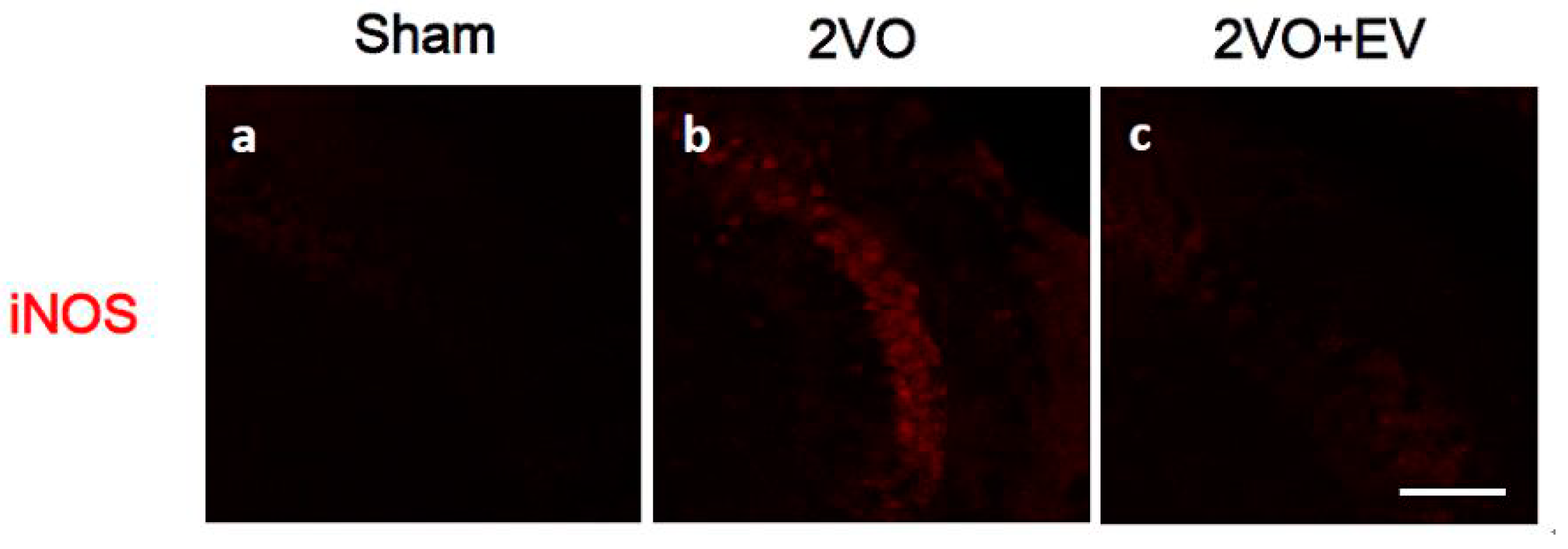
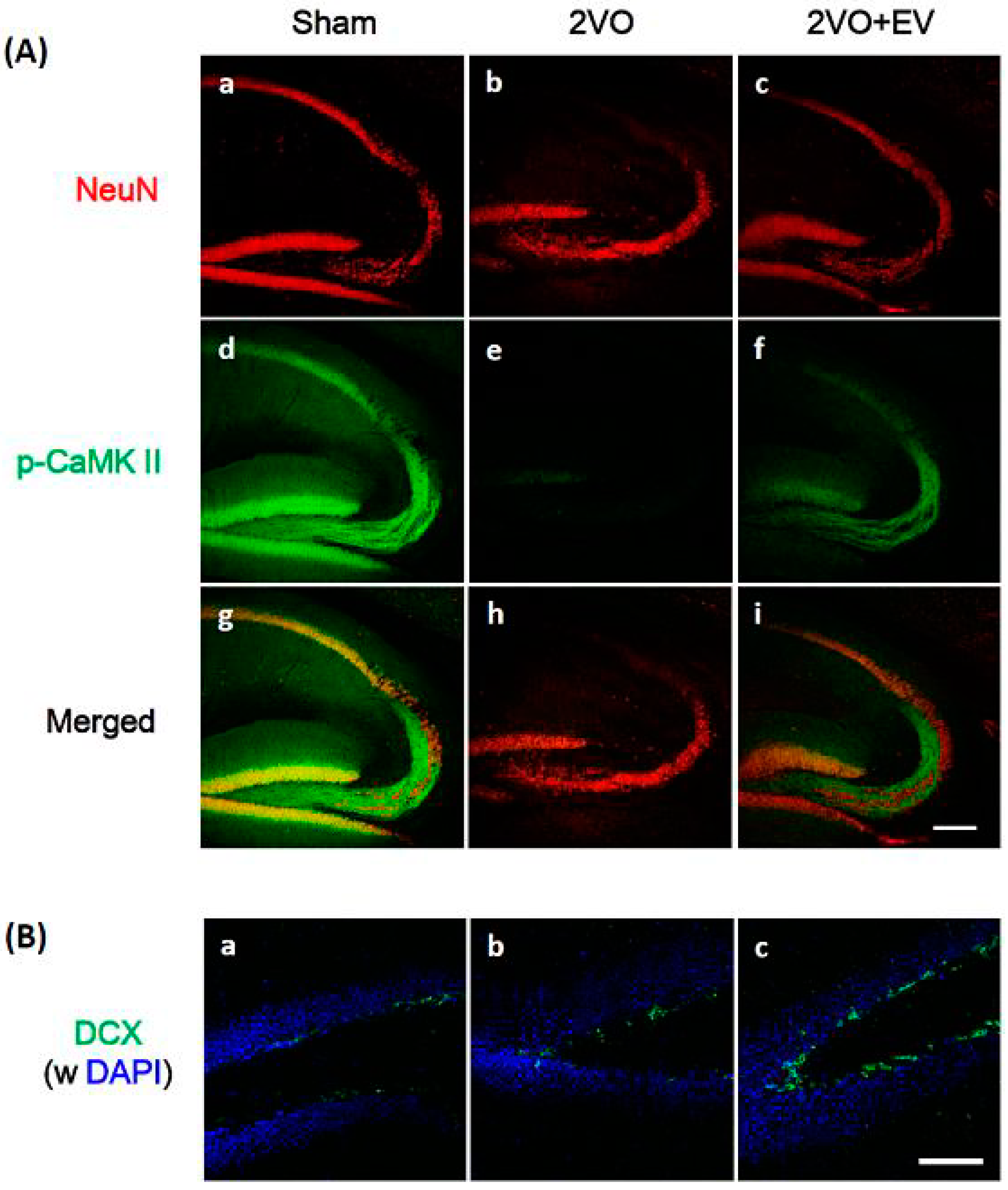
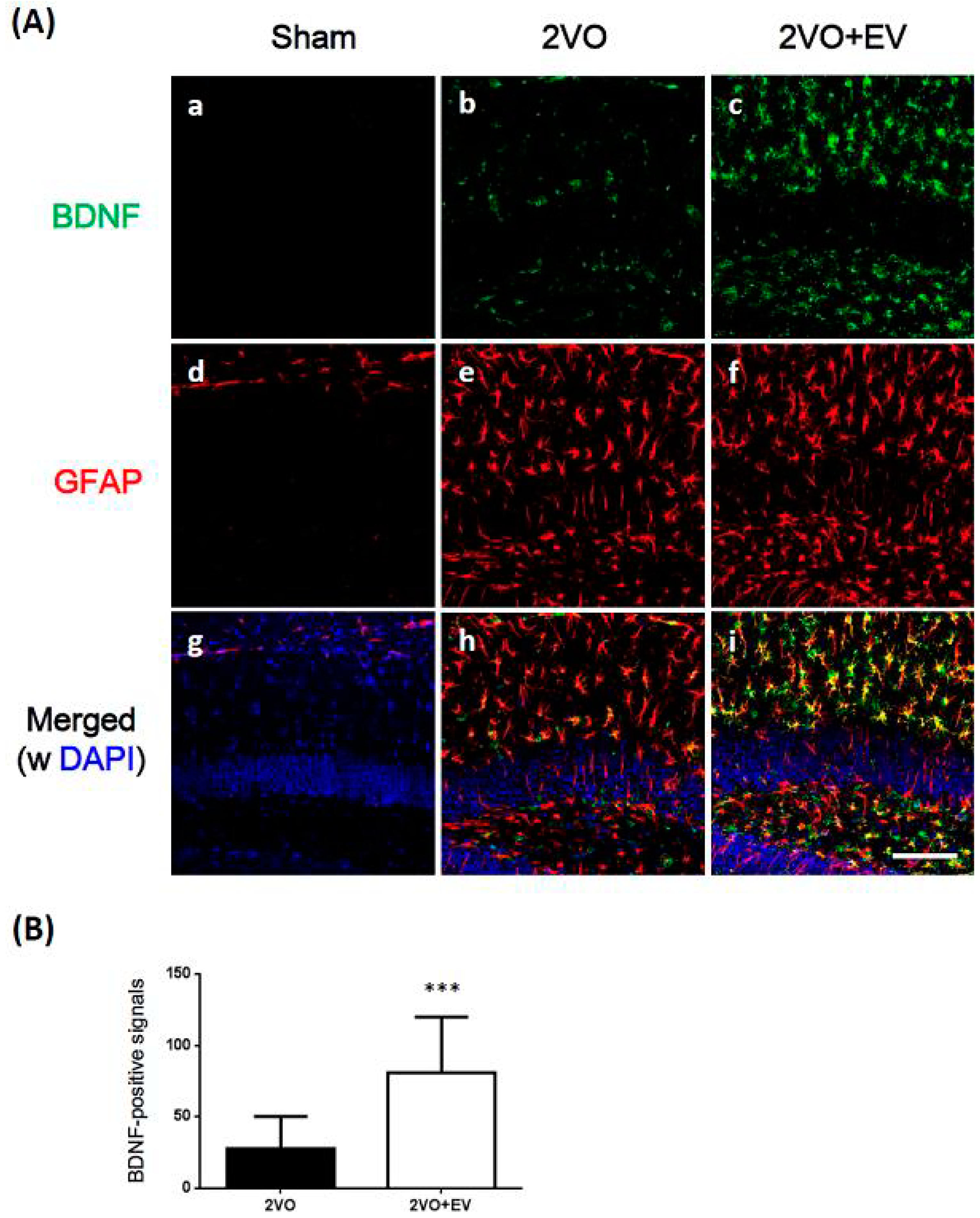
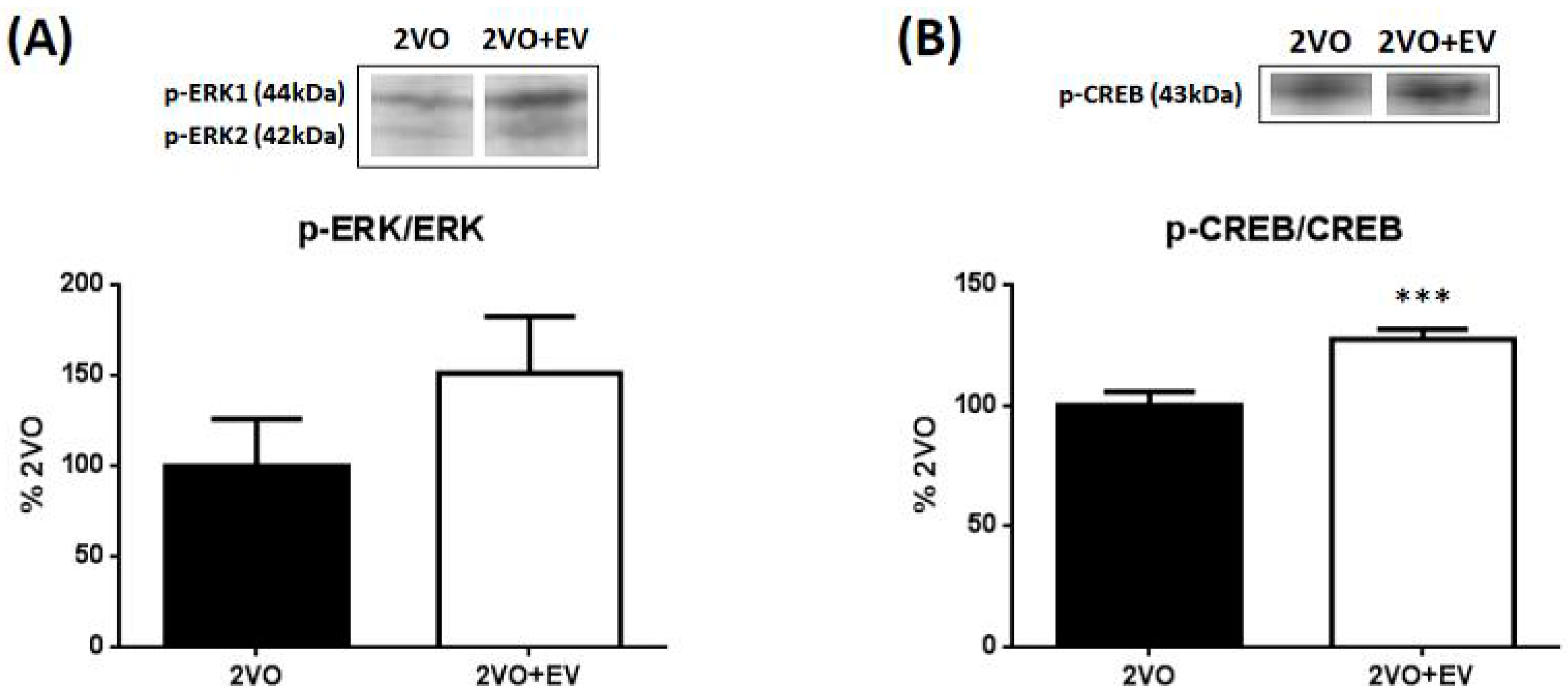
4. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Horn, M.; Schlote, W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathol. 1992, 85, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zola-Morgan, S.; Squire, L.R.; Amaral, D.G. Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J. Neurosci. 1986, 6, 2950–2967. [Google Scholar] [PubMed]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Juurlink, B.H.; Sweeney, M.I. Mechanisms that result in damage during and following cerebral ischemia. Neurosci. Biobehav. Rev. 1997, 21, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Nakamura, T.; Toyoshima, T.; Liu, Y.; Hirooka, K.; Kawai, N.; Okabe, N.; Shiraga, F.; Tamiya, T.; Miyamoto, O.; et al. Edaravone, a free radical scavenger, attenuates behavioral deficits following transient forebrain ischemia by inhibiting oxidative damage in gerbils. Neurosci. Lett. 2012, 506, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yuki, S.; Egawa, M.; Nishi, H. Protective effects of MCI-186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J. Pharmacol. Exp. Ther. 1994, 268, 1597–1604. [Google Scholar] [PubMed]
- Cheng, Y.D.; Al-Khoury, L.; Zivin, J.A. Neuroprotection for ischemic stroke: Two decades of success and failure. NeuroRx 2004, 1, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.; Nanobashvili, A.; Kokaia, Z.; Lindvall, O. Evidence for neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J. Cereb. Blood Flow Metab. 1999, 19, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Schabitz, W.R.; Schwab, S.; Spranger, M.; Hacke, W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 1997, 17, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Su, J.; Li, L.; Feng, J.; Shi, L.; He, W.; Liu, Y. Edaravone alleviates hypoxia-acidosis/reoxygenation-induced neuronal injury by activating ERK1/2. Neurosci. Lett. 2013, 543, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Grãos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell. Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Shimada, N.; Kaji, M.; Morita, M.; Miyoshi, K.; Minami, S.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Watanabe, S.; et al. Heptamethoxyflavone, a citrus flavonoid, enhances brain-derived neurotrophic factor production and neurogenesis in the hippocampus following cerebral global ischemia in mice. Neurosci. Lett. 2012, 528, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Morita, M.; Miyoshi, K.; Nishigawa, Y.; Kaji, M.; Sawamoto, A.; Terugo, T.; Toyoda, N.; Makihata, N.; Amakura, Y.; et al. 3,5,6,7,8,3',4'-Heptamethoxyflavone, a citrus flavonoid, on protection against memory impairment and neuronal cell death in a global cerebral ischemia mouse model. Neurochem. Int. 2014, 70, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Minami, S.; Shimada, N.; Makihata, N.; Nakajima, M.; Furukawa, Y. Anti-inflammatory and neuroprotective effects of auraptene, a citrus coumarin, following cerebral global ischemia in mice. Eur. J. Pharmacol. 2013, 699, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Harukuni, I.; Bhardwaj, A. Mechanisms of Brain Injury after Global Cerebral Ischemia. Neurol. Clin. 2006, 24, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W.; Becker, L.B. State of the Art in Therapeutic Hypothermia. Annu. Rev. Med. 2011, 62, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, S.; Zhang, F.; Ross, M.E.; Iadecola, C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J. Neurosci. 1997, 17, 2746–2755. [Google Scholar] [PubMed]
- Zhang, N.; Komine-Kobayashi, M.; Tanaka, R.; Liu, M.; Mizuno, Y.; Urabe, T. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke 2005, 36, 2220–2225. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.O.; Kim, S.K.; Cho, Y.J.; Sung, K.W.; Kim, S.Y. Regional differences in the neuroprotective effect of minocycline in a mouse model of global forebrain ischemia. Life Sci. 2007, 80, 2030–2035. [Google Scholar] [CrossRef] [PubMed]
- Olsson, T.; Wieloch, T.; Smith, M.L. Brain damage in a mouse model of global cerebral ischemia. Effect of NMDA receptor blockade. Brain Res. 2003, 982, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.S.; Lee, S.H.; Jeon, S.H.; Kwon, Y.S.; Lee, H.J.; Kim, S.S.; Kim, Y.M.; Kim, M.J.; Chun, W. Kainic Acid-induced Neuronal Death is Attenuated by Aminoguanidine but Aggravated by L-NAME in Mouse Hippocampus. Korean J. Physiol. Pharmacol. 2009, 13, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Jin, Y.; Kim, I.D.; Lee, J.K. Glycyrrhizin attenuates kainic Acid-induced neuronal cell death in the mouse hippocampus. Exp. Neurobiol. 2013, 22, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Shioda, N.; Han, F.; Moriguchi, S.; Nakajima, A.; Yokosuka, A.; Mimaki, Y.; Sashida, Y.; Yamakuni, T.; Ohizumi, Y.; et al. Nobiletin improves brain ischemia-induced learning and memory deficits through stimulation of CaMKII and CREB phosphorylation. Brain Res. 2009, 1295, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.K.; Grotta, J.C.; Waxham, M.N.; Aronowski, J.; Ostrow, P. Calcium/calmodulin-dependent protein kinase II activity in focal ischemia with reperfusion in rats. Stroke 1994, 25, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, R.D.; Wong, T.; Nouranifar, R.; Iyengar, R.; Landau, E.M. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron 1995, 15, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Blanquet, P.R.; Lamour, Y. Brain-Derived neurotrophic factor increases Ca2+/calmodulin-dependent protein kinase 2 activity in hippocampus. J. Biol. Chem. 1997, 272, 24133–24136. [Google Scholar] [CrossRef] [PubMed]
- Kee, N.J.; Preston, E.; Wojtowicz, J.M. Enhanced neurogenesis after transient global ischemia in the dentate gyrus of the rat. Exp. Brain Res. 2001, 136, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Solway, K.; Messing, R.O.; Sharp, F.R. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J. Neurosci. 1998, 18, 7768–7778. [Google Scholar] [PubMed]
- Sharp, F.R.; Lu, A.; Tang, Y.; Millhorn, D.E. Multiple molecular penumbras after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2000, 20, 1011–1032. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Brambilla, R.; Thomas, K.L. A simple role for BDNF in learning and memory? Front. Mol. Neurosci. 2010, 3, 1. [Google Scholar] [PubMed]
- Samuels, I.S.; Karlo, J.C.; Faruzzi, A.N.; Pickering, K.; Herrup, K.; Sweatt, J.D.; Saitta, S.C.; Landreth, G.E. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J. Neurosci. 2008, 28, 6983–6995. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Endo, S.; Ikeda, T.; Yamada, K.; Ito, M.; Kuroki, M.; Hiramoto, T.; Imamura, O.; Kobayashi, Y.; Watanabe, Y.; et al. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J. Neurosci. 2007, 27, 10765–10776. [Google Scholar]
- Ying, S.W.; Futter, M.; Rosenblum, K.; Webber, M.J.; Hunt, S.P.; Bliss, T.V.; Bramham, C.R. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J. Neurosci. 2002, 22, 1532–1540. [Google Scholar] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuyama, S.; Morita, M.; Sawamoto, A.; Terugo, T.; Nakajima, M.; Furukawa, Y. Edaravone Enhances Brain-Derived Neurotrophic Factor Production in the Ischemic Mouse Brain. Pharmaceuticals 2015, 8, 176-185. https://doi.org/10.3390/ph8020176
Okuyama S, Morita M, Sawamoto A, Terugo T, Nakajima M, Furukawa Y. Edaravone Enhances Brain-Derived Neurotrophic Factor Production in the Ischemic Mouse Brain. Pharmaceuticals. 2015; 8(2):176-185. https://doi.org/10.3390/ph8020176
Chicago/Turabian StyleOkuyama, Satoshi, Mayu Morita, Atsushi Sawamoto, Tsukasa Terugo, Mitsunari Nakajima, and Yoshiko Furukawa. 2015. "Edaravone Enhances Brain-Derived Neurotrophic Factor Production in the Ischemic Mouse Brain" Pharmaceuticals 8, no. 2: 176-185. https://doi.org/10.3390/ph8020176
APA StyleOkuyama, S., Morita, M., Sawamoto, A., Terugo, T., Nakajima, M., & Furukawa, Y. (2015). Edaravone Enhances Brain-Derived Neurotrophic Factor Production in the Ischemic Mouse Brain. Pharmaceuticals, 8(2), 176-185. https://doi.org/10.3390/ph8020176





