γδ T Cell Immunotherapy—A Review
Abstract
:1. Introduction
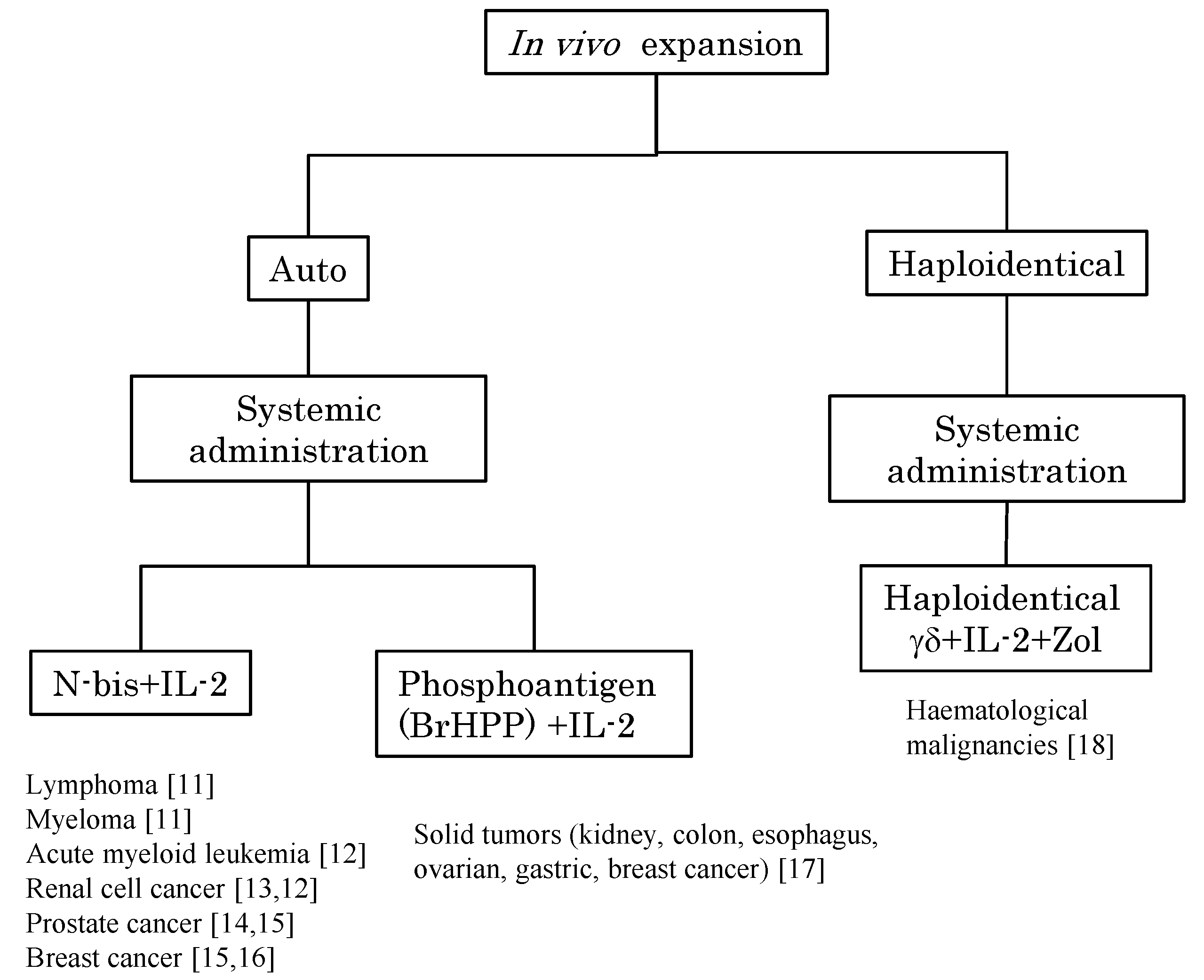
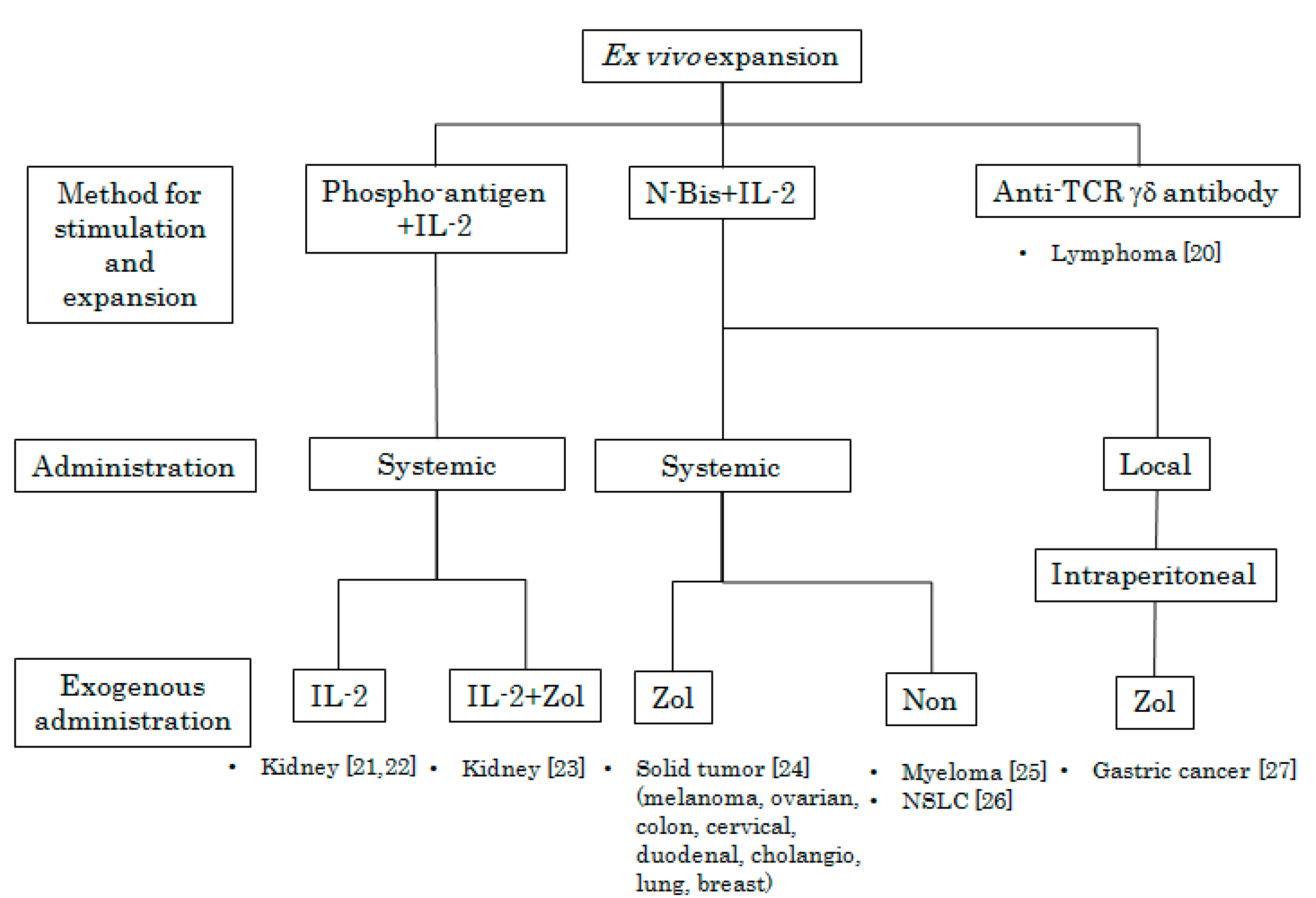
2. In Vivo Stimulation of Vγ9Vδ2 T Cells Using Synthetic Antigens and IL-2
| Reference | Number of Patients | Disease | Stimulant | Exogenous IL-2 | Cycle | Numbers of Cycles | Clinical Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| Dose | Schedule | Dose (IU), Route, Patients | Schedule | ||||||
| Wilhelm et al., 2003 Blood [11] | 10 | MM: 4 | Pam (90 mg) | d1 | 0.25–3 × 106, iv | d3–d8 | at least 3 weeks | 1–6 | PD: 8, SD: 1, NE: 1 |
| CLL: 4 | |||||||||
| IC: 1 | |||||||||
| MZL: 1 | |||||||||
| 9 | MM: 4 | 0.25 × 106, iv, n = 3, 0.5–2 × 106, iv, n = 3, 1–2 × 106, iv, n = 3 | d1–6 | 1–2 | PD: 3 | ||||
| FCL: 4 | 1–9 | PD: 1, SD: 2, PR: 1 | |||||||
| MZL: 1 | 4–8 | SD: 1, PR: 2 | |||||||
| Dieli et al., 2007 Cancer Res. [14] | 9 | HRPC | Zol (4 mg) | d1 | No | 21 days | 2–17 (at 12 months) | SD: 1, PR: 1, PD: 1, death: 6 | |
| 9 | 0.6 × 106, sc | d1 | 7–17 (at 12 months) | SD: 4, PR: 2, PD: 1, death: 2 | |||||
| Lang et al., 2011 Cancer Immunol. Immunother. [13] | 6 | RCC | Zol (4 mg) | d1, d8, d15 | 7 × 106 U/m2/day, sc | d1–5, d8–12, d15–19 | 28 days | <1: n = 2, | SD:3, PD:1, NA: 3 |
| 2–10: n = 4, | |||||||||
| NA: n = 1 | |||||||||
| 3 | 1–2 × 106 U/m2/day, sc | <1 | NA:1, SD:2 | ||||||
| 3 | |||||||||
| 33 | |||||||||
| 3 | Zol (0.4–3.0 mg) | 1–2 × 106 U/m2/day, sc | 4 | SD: 3 | |||||
| 7 | |||||||||
| 16 | |||||||||
| Meraviglia et al., 2010 Clin. Exp. Immunol. [16] | 10 | Breast cancer | Zol (4 mg) | d1 | 1 × 106 U/m2/day, sc | d1 | 21 days | <4: n = 2 | PD: 2, SD: 2, PR: 1 at 9 months |
| 5–13: n = 3 | |||||||||
| 14–18: n = 1 | PD: 1, SD: 2, PR: 1 at 12 months | ||||||||
| 18<, n = 4 | |||||||||
| Bennouna et al., 2010 Cancer Immunol. Immunother. [17] | 28 | RCC: 18 | BrHPP (200 mg/m2) | d1 | 1 × 106 U/m2/day, sc, from 2 cycles | d1–7 | 21 days | 18 | SD: 12, PD: 16 at 3 cycles |
| Colon Ca: 3 | (600 mg/m2) | ||||||||
| Esophagus Ca: 3 | (1200 mg/m2) | 10 | |||||||
| Gastric Ca: 1 | (1500 mg/m2) | 26 | |||||||
| Ovarian Ca: 1 | (1500 mg/m2) | 1 × 106 U/m2/day, sc, from 1 cycle | 39 | ||||||
| Breast Ca: 2 | (1800 mg/m2) | 1 × 106 U/m2/day, sc, from 2 cycles | 16 cycles | ||||||
2.1. In Vivo Activation of γδ T Cells After Adoptive Cell Therapy
2.2. Theoretical Concern and Appropriate Dosages of N-bis/IL-2
3. Adoptive Immunotherapy Using Ex Vivo Activated Vγ9Vδ2 T Cells
3.1. Role of Vδ1 T Cells
| Reference | Number of Patients | Disease | Cell Source | Culture condition | ||||
|---|---|---|---|---|---|---|---|---|
| IL-2 | Stimulant (Concentration) | Serum | Culture days | |||||
| Kobayashi et al., 2007 Cancer Immunol. Immunother. [22] | 7 | RCC | PB | 100 U/mL | 2M3B1-PP | 100 μM | 2% Auto serum | 14 days |
| Bennouna et al., 2008 Cancer Immunol. Immunother. [21] | 10 | RCC | Leukapheresis | 328 U/mL: d1, 984 U/mL: d4–14 | BrHPP | 3 μM | 9% FCS | 14 days |
| Kobayashi et al., 2011 Cancer Immunol. Immunother. [23] | 11 | RCC | Leukapheresis (1 leukapheresis for 2 treatments) | 100 U/mL | 2M3B1-PP | 100 μM | 2% Auto serum | 11 days |
| Nicol et al., 2011 Br. J. Cancer [24] | 18 | Melanoma: 4, Ovarian cancer: 1, Colon cancer: 1 | Luekapheresis 1 leukapheresis for 8 treatments | 700 IU/mL, d0; 350 IU/mL, every 2–3 days | Zol | 1 μM | 10% AB serum | 7–14 days |
| Melanoma: 3 | ||||||||
| Adenocarcinoma: 1 | ||||||||
| Cholangiocarcinoma: 1 | ||||||||
| Ovarian carcinoma: 1 | ||||||||
| Colon cancer: 2 | ||||||||
| Duodenal cancer: 1 | ||||||||
| Breast cancer: 2, Cervical cancer: 1 | ||||||||
| Nakajima et al., 2010 Eur. J. Cardiothorac. Surg. [26] | 10 | Non-small-cell lung cancer | Peripheral blood 70 mL | 1000 U/mL | Zol | 5 μM | 10% Auto serum | 14 days |
| Abe et al., 2009 Exp. Hematol. [25] | 6 | Multiple myeloma | Peripheral blood | 1000 U/mL | Zol | 5 μM | Auto serum | 14 days |
| Wada et al., 2014 Cancer Med. [27] | 7 | Gastric cancer | Leukapheresis | 1000 U/mL | Zol | 5 μM | Auto serum | 14 days |
| References | No. of Transferred γδ T Cells | Cycle | Route | Exogenous Administration | Clinical Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|
| Each Cycle | Total | Interval | Cycles | Zol | IL-2: Dose/Schedule | ||||
| Kobayashi et al., 2007 Cancer Immunol. Immunother. [22] | 5 × 106 to 3.57 × 109 | 0.1–40.8 × 109 | Weekly | 6–12 | iv | No | 7 × 105 IU, iv | d1 | |
| Bennouna et al., 2008 Cancer Immunol. Immunother. [21] | 1 × 109, n = 1 | 1.45–18.3 × 109 | 3 weeks | 3 | iv | No | Cycle 1: no, cycle 2: 2 × 106 IU/m2, sc | d1–d7 | SD: 6, PD: 4 |
| 4 × 109, n = 6 | 16 | ||||||||
| 8 × 109, n = 3 | 8 | ||||||||
| Kobayashi et al., 2011 Cancer Immunol. Immunother. [23] | 9.4 × 106–24.0 × 109 | 1.5×109–46.7 × 109 | 3 weeks | Average 4.2 | iv | 4 mg, iv. d1 | 1.4 × 106 IU, iv | d1–5 | SD: 5, PD: 5, CR: 1 |
| mean: 1.4 × 109 | mean: 22.0 × 109 | ||||||||
| Nicol et al., 2011 Br. J. Cancer [24] | 0.04–2.8 × 109 | 0.1–5.5 × 109 | Up to 8 | iv | 1 mg, iv, 24 h before cell transfer; 1 mg, iv, just before cell transfer | No | No | SD: 2, PD: 4 | |
| 0.3–2.2 × 109 | 1.0–7.2 × 109 | SD: 1, PD: 7, NE: 1 | |||||||
| 0.3–1.9 × 109 | 0.9–4.0 × 109 | PR: 2, CR: 1 | |||||||
| Nakajima et al., 2010 Eur. J. Cardiothorac. Surg. [26] | 2.6–31.4 × 109 | 2 weeks | 3–12 (mean 6) | iv | No | No | SD: 3, PD: 5 NE: 2 | ||
| Abe et al., 2009 Exp. Hematol. [25] | 0.07–5.2 × 109 | 3.0–20.0 × 109 | 2 weeks | 4–8 (mean 7) | iv | No | No | Decreased M-protein levels: 4 | |
| Wada et al., 2014 Cancer Med [27] | 0.06–6.49 × 109 | 0.06–25.0 × 109 | weekly | 1–4 (mean 3) | ip | 1 mg, iv at 1st therapy, 1 mg intraperitoneal injection, after 2nd therapy | No | Ascites, disappeared: 1, Reduced: 1, No change: 5 | |
3.2. Clinical Trials Using Ex Vivo Activated Vγ9Vδ2 T Cells
4. Discussion
Conflicts of Interest
References
- Saito, H.; Kranz, D.M.; Takagaki, Y.; Hayday, A.C.; Eisen, H.N.; Tonegawa, S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. Nature 1984, 309, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.B.; McLean, J.; Dialynas, D.P.; Strominger, J.L.; Smith, J.A.; Owen, F.L.; Seidman, J.G.; Ip, S.; Rosen, F.; Krangel, M.S. Identification of a putative second T-cell receptor. Nature 1986, 322, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.H.; Iwashima, M.; Kaplan, K.B.; Elliott, J.F.; Davis, M.M. A new T-cell receptor gene located within the alpha locus and expressed early in T-cell differentiation. Nature 1987, 327, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, S.; Brenner, M.B.; Band, H. Biology of human γδ T cell receptor. Immunol. Rev. 1991, 120, 137–183. [Google Scholar] [CrossRef] [PubMed]
- Constant, P.; Davodeau, F.; Peyrat, M.A.; Poquet, Y.; Puzo, G.; Bonneville, M.; Fournié, J.J. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science 1994, 264, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Morita, C.T.; Tanaka, Y.; Nieves, E.; Brenner, M.B.; Bloom, B.R. Natural and synthetic nonpeptide antigens recognized by human γδ T cells. Nature 1995, 375, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, J.F.; Morita, C.T.; Brenner, M.B. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: Implications for innate immunity. Immunity 1999, 11, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Harly, C.; Guillaume, Y.; Nedellec, S.; Peigne, C.M.; Monkkonen, H.; Monkkonen, J.; Li, J.; Kuball, J.; Adams, E.J.; Netzer, S.; et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 2012, 120, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Sandstrom, A.; Peigne, C.M.; Leger, A.; Crooks, J.E.; Konczak, F.; Gesnel, M.C.; Breathnach, R.; Bonneville, M.; Scotet, E.; Adams, E.J. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 2014, 40, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Henry, O.; Distefano, M.D.; Wang, Y.C.; Raikkonen, J.; Monkkonen, J.; Tanaka, Y.; Morita, C.T. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T cells. J. Immunol. 2013, 191, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Kunzmann, V.; Eckstein, S.; Reimer, P.; Weissinger, F.; Ruediger, T.; Tony, H.P. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood 2003, 102, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, V.; Smetak, M.; Kimmel, B.; Weigang-Koehler, K.; Goebeler, M.; Birkmann, J.; Becker, J.; Schmidt-Wolf, I.G.; Einsele, H.; Wilhelm, M. Tumor-promoting versus tumor-antagonizing roles of γδ T cells in cancer immunotherapy: Results from a prospective phase I/II trial. J. Immunother. 2012, 35, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.M.; Kaikobad, M.R.; Wallace, M.; Staab, M.J.; Horvath, D.L.; Wilding, G.; Liu, G.; Eickhoff, J.C.; McNeel, D.G.; Malkovsky, M. Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Dieli, F.; Vermijlen, D.; Fulfaro, F.; Caccamo, N.; Meraviglia, S.; Cicero, G.; Roberts, A.; Buccheri, S.; D’Asaro, M.; Gebbia, N.; et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007, 67, 7450–7457. [Google Scholar] [CrossRef] [PubMed]
- Dieli, F.; Gebbia, N.; Poccia, F.; Caccamo, N.; Montesano, C.; Fulfaro, F.; Arcara, C.; Valerio, M.R.; Meraviglia, S.; di Sano, C.; et al. Induction of γδ T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood 2003, 102, 2310–2311. [Google Scholar] [CrossRef] [PubMed]
- Meraviglia, S.; Eberl, M.; Vermijlen, D.; Todaro, M.; Buccheri, S.; Cicero, G.; la Mendola, C.; Guggino, G.; D’Asaro, M.; Orlando, V.; et al. In vivo manipulation of Vγ9Vδ2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin. Exp. Immunol. 2010, 161, 290–297. [Google Scholar] [PubMed]
- Bennouna, J.; Levy, V.; Sicard, H.; Senellart, H.; Audrain, M.; Hiret, S.; Rolland, F.; Bruzzoni-Giovanelli, H.; Rimbert, M.; Galéa, C.; et al. Phase I study of bromohydrin pyrophosphate (BrHPP, IPH 1101), a Vγ9Vδ2 T lymphocyte agonist in patients with solid tumors. Cancer Immunol. Immunother. 2010, 59, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Smetak, M.; Schaefer-Eckart, K.; Kimmel, B.; Birkmann, J.; Einsele, H.; Kunzmann, V. Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells. J. Transl. Med. 2014, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, V.; Bauer, E.; Wilhelm, M. Gamma/delta T-cell stimulation by pamidronate. N. Engl. J. Med. 1999, 340, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kang, N.; Cui, L.; Ba, D.; He, W. Anti- γδ TCR antibody-expanded γδ T cells: A better choice for the adoptive immunotherapy of lymphoid malignancies. Cell Mol. Immunol. 2012, 9, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, J.; Bompas, E.; Neidhardt, E.M.; Rolland, F.; Philip, I.; Galéa, C.; Salot, S.; Saiagh, S.; Audrain, M.; Rimbert, M.; et al. Phase-I study of Innacell γδ, an autologous cell-therapy product highly enriched in Vγ9Vδ2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2008, 57, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tanaka, Y.; Yagi, J.; Osaka, Y.; Nakazawa, H.; Takehiko Uchiyama, T.; Minato, N.; Toma, H. Safety profile and anti-tumor effects of adoptive immunotherapy using γδ T cells against advanced renal cell carcinoma: A pilot study. Cancer Immunol. Immunother. 2007, 56, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tanaka, Y.; Yagi, J.; Minato, N.; Tanabe, K. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Nicol, A.J.; Tokuyama, H.; Mattarollo, S.R.; Hagi, T.; Suzuki, K.; Yokokawa, K.; Nieda, M. Clinical evaluation of autologous γδ T cell-based immunotherapy for metastatic solid tumours. Br. J. Cancer 2011, 105, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Muto, M.; Nieda, M.; Nakagawa, Y.; Nicol, A.; Kaneko, T.; Goto, S.; Yokokawa, K.; Suzuki, K. Clinical and immunological evaluation of zoledronate-activated Vγ9 γδ T-cell-based immunotherapy for patients with multiple myeloma. Exp. Hematol. 2009, 37, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, J.; Murakawa, T.; Fukami, T.; Goto, S.; Kaneko, T.; Yoshida, Y.; Takamoto, S.; Kakimi, K. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous γδ T cells. Eur. J. Cardiothorac. Surg. 2010, 37, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Wada, I.; Matsushita, H.; Noji, S.; Mori, K.; Yamashita, H.; Nomura, S.; Shimizu, N.; Seto, Y.; Kakimi, K. Intraperitoneal injection of in vitro expanded Vγ9Vδ2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med. 2014, 3, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Adami, S.; Viapiana, O.; Ortolani, R.; Vella, A.; Fracassi, E.; Gatti, D. Circulating γδ T cells and the risk of acute-phase response after zoledronic acid administration. J. Bone Miner. Res. 2012, 27, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Casetti, R.; Perretta, G.; Taglioni, A.; Mattei, M.; Colizzi, V.; Dieli, F.; D’Offizi, G.; Malkovsky, M.; Poccia, F. Drug-induced expansion and differentiation of Vγ9Vδ2 T cells in vivo: The role of exogenous IL-2. J. Immunol. 2005, 175, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, V.; Bauer, E.; Feurle, J.; Weissinger, F.; Tony, H.P.; Wilhelm, M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000, 96, 384–392. [Google Scholar] [PubMed]
- Espinosa, E.; Belmant, C.; Pont, F.; Luciani, B.; Poupot, R.; Romagné, F.; Brailly, H.; Bonneville, M.; Fournié, J.J. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human γδ T cells. J. Biol. Chem. 2001, 276, 18337–18344. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Izumi, T.; Fujieda, N.; Kondo, A.; Morishita, T.; Matsushita, H.; Kakimi, K. Expansion of human peripheral blood γδ T cells using zoledronate. J. Vis. Exp. 2011, 55, 3182. [Google Scholar] [PubMed]
- Miyagawa, F.; Tanaka, Y.; Yamashita, S.; Minato, N. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human γδ T cells by aminobisphosphonate antigen. J. Immunol. 2001, 166, 5508–5514. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Adami, S.; Viapiana, O.; Fracassi, E.; Ortolani, R.; Vella, A.; Zanotti, R.; Tripi, G.; Idolazzi, L.; Gatti, D. Long-term effects of amino-bisphosphonates on circulating γδ T cells. Calcif. Tissue Int. 2012, 91, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, J.; Li, W.; Yamanishi, H.; Yamamoto, H.; Okuda, A.; Kubo, S.; Ma, Z.; Terada, N.; Tanaka, Y.; Okamura, H. Involvement of CD56brightCD11c+ cells in IL-18-mediated expansion of human γδ T cells. J. Immunol. 2011, 186, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, O.; Gruenbacher, G.; Gander, H.; Komuczki, J.; Rahm, A.; Thurnher, M. Essential requirements of zoledronate-induced cytokine and γδ T cell proliferative responses. J. Immunol. 2013, 191, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Sugie, T.; Murata-Hirai, K.; Iwasaki, M.; Morita, C.T.; Li, W.; Okamura, H.; Minato, N.; Toi, M.; Tanaka, Y. Zoledronic acid-induced expansion of γδ T cells from early-stage breast cancer patients: Effect of IL-18 on helper NK cells. Cancer Immunol. Immunother. 2013, 62, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Thedrez, A.; Harly, C.; Morice, A.; Salot, S.; Bonneville, M.; Scotet, E. IL-21-mediated potentiation of antitumor cytolytic and proinflammatory responses of human Vγ9Vδ2 T cells for adoptive immunotherapy. J. Immunol. 2009, 182, 3423–3431. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.J.; Kline, J.; Struemper, H.; Koch, K.M.; Bauman, J.W.; Gardner, O.S.; Murray, S.C.; Germaschewski, F.; Weisenbach, J.; Jonak, Z.; et al. A dose-escalation study of recombinant human interleukin-18 in combination with rituximab in patients with non-Hodgkin lymphoma. J. Immunother. 2013, 36, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Petrella, T.M.; Tozer, R.; Belanger, K.; Savage, K.J.; Wong, R.; Smylie, M.; Kamel-Reid, S.; Tron, V.; Chen, B.E.; Hunder, N.N.; et al. Interleukin-21 has activity in patients with metastatic melanoma: A phase II study. J. Clin. Oncol. 2012, 30, 3396–3401. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Wang, H.Y.; Peng, W.; Kiniwa, Y.; Seo, K.H.; Wang, R.F. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 2007, 27, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Arnouk, H.; Britt, W.; Gillespie, G.Y.; Cloud, G.A.; Harkins, L.; Su, Y.; Lowdell, M.W.; Lamb, L.S. CMV-independent lysis of glioblastoma by ex vivo expanded/activated Vδ1+ γδ T cells. PLoS One 2013, 8, e68729. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kobayashi, H.; Terasaki, T.; Toma, H.; Aruga, A.; Uchiyama, T.; Mizutani, K.; Mikami, B.; Morita, C.T.; Minato, N. Synthesis of pyrophosphate-containing compounds that stimulate Vγ2Vδ2 T cells: Application to cancer immunotherapy. Med. Chem. 2007, 3, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jenö, P.; Mori, L.; de Libero, G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Tanaka, Y.; Miyagawa, F.; Yamashita, S.; Minato, N. Targeting of tumor cells for human γδ T cells by nonpeptide antigens. J. Immunol. 2001, 167, 5092–5098. [Google Scholar] [CrossRef] [PubMed]
- Benzaïd, I.; Mönkkönen, H.; Stresing, V.; Bonnelye, E.; Green, J.; Mönkkönen, J.; Touraine, J.L.; Clézardin, P. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vγ9Vδ2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 2011, 71, 4562–4572. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, V.; Kimmel, B.; Herrmann, T.; Einsele, H.; Wilhelm, M. Inhibition of phosphoantigen-mediated γδ T-cell proliferation by CD4+CD25+FoxP3+ regulatory T cells. Immunology 2009, 126, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Koreth, J.; Matsuoka, K.; Kim, H.T.; McDonough, S.M.; Bindra, B.; Alyea, E.P., 3rd; Armand, P.; Cutler, C.; Ho, V.T.; Treister, N.S.; et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 2011, 365, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Shao, L.; Wang, Y.; Chen, C.Y.; Huang, D.; Yao, S.; Zhan, X.; Sicard, H.; Wang, R.; Chen, Z.W. Phosphoantigen-activated Vγ2Vδ2 T cells antagonize IL-2-induced CD4+CD25+Foxp3+ T regulatory cells in mycobacterial infection. Blood 2009, 113, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tanaka, Y.; Shimmura, H.; Minato, N.; Tanabe, K. Complete remission of lung metastasis following adoptive immunotherapy using activated autologous γδ T-cells in a patient with renal cell carcinoma. Anticancer Res. 2010, 30, 575–579. [Google Scholar] [PubMed]
- Sakamoto, M.; Nakajima, J.; Murakawa, T.; Fukami, T.; Yoshida, Y.; Murayama, T.; Takamoto, S.; Matsushita, H.; Kakimi, K. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded γδ Tcells: A phase I clinical study. J. Immunother. 2011, 34, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tanaka, Y.; Nakazawa, H.; Yagi, J.; Minato, N.; Tanabe, K. A new indicator of favorable prognosis in locally advanced renal cell carcinomas: γδ T-cells in peripheral blood. Anticancer Res. 2011, 31, 1027–1031. [Google Scholar] [PubMed]
- Dopfer, E.P.; Hartl, F.A.; Oberg, H.-H.; Siegers, G.M.; Yousefi, O.S.; Kock, S.; Fiala, G.J.; Garcillan, B.; Sandstrom, A.; Alarcon, B.; et al. The CD3 conformational change in the γδ T cell receptor is not triggered by antigens but can be reforced to enhance tumor killing. Cell Rep. 2014, 7, 1704–1705. [Google Scholar] [CrossRef] [PubMed]
- Santolaria, T.; Robard, M.; Leger, A.; Catros, V.; Bonneville, M.; Scotet, E. Repeated systemic administrations of both aminobisphosphonates and human Vγ9Vδ2 T cells efficiently control tumor development in vivo. J. Immunol. 2013, 191, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, T.; Sato, K.; Ashihara, E.; Takeuchi, M.; Maita, S.; Tsuchiya, N.; Habuchi, T.; Maekawa, T.; Kimura, S. Intravesical administration of γδ T cells successfully prevents the growth of bladder cancer in the murine model. Cancer Immunol. Immunother. 2009, 58, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Lamb, L.S., Jr.; Bowersock, J.; Dasgupta, A.; Gillespie, G.Y.; Su, Y.; Johnson, A.; Spencer, H.T. Engineered drug resistant γδ T cells kill glioblastoma cell lines during a chemotherapy challenge: A strategy for combining chemo- and immunotherapy. PLoS One 2013, 8, e51805. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.L.; Gillespie, G.Y.; Lopez, R.D.; Markert, J.M.; Cloud, G.A.; Langford, C.P.; Arnouk, H.; Su, Y.; Haines, H.L.; Suarez-Cuervo, C.; et al. Preclinical evaluation of ex vivo expanded/activated γδ T cells for immunotherapy of glioblastoma multiforme. J. Neurooncol. 2011, 101, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Tanaka, Y.; Kobayashi, H.; Murata-Hirai, K.; Miyabe, H.; Sugie, T.; Toi, M.; Minato, N. Expression and function of PD-1 in human γδ T cells that recognize phosphoantigens. Eur. J. Immunol. 2011, 41, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Kudchadkar, R.R.; Yu, B.; Gallenstein, D.; Horak, C.E.; Inzunza, H.D.; Zhao, X.; Martinez, A.J.; Wang, W.; Gibney, G.; et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J. Clin. Oncol. 2013, 31, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, H.; Tanaka, Y. γδ T Cell Immunotherapy—A Review. Pharmaceuticals 2015, 8, 40-61. https://doi.org/10.3390/ph8010040
Kobayashi H, Tanaka Y. γδ T Cell Immunotherapy—A Review. Pharmaceuticals. 2015; 8(1):40-61. https://doi.org/10.3390/ph8010040
Chicago/Turabian StyleKobayashi, Hirohito, and Yoshimasa Tanaka. 2015. "γδ T Cell Immunotherapy—A Review" Pharmaceuticals 8, no. 1: 40-61. https://doi.org/10.3390/ph8010040
APA StyleKobayashi, H., & Tanaka, Y. (2015). γδ T Cell Immunotherapy—A Review. Pharmaceuticals, 8(1), 40-61. https://doi.org/10.3390/ph8010040







