Enhanced Antimicrobial Activity of AamAP1-Lysine, a Novel Synthetic Peptide Analog Derived from the Scorpion Venom Peptide AamAP1
Abstract
:1. Introduction
2. Experimental Section
2.1. Design of AamAP1-Lysine Based on Structural Determinants
2.2. Molecular Modeling and In Silico Analysis of AamAP1-Lysine
2.3. Peptide Synthesis and Purification
2.4. Bacterial Strains
2.5. Bacterial Susceptibility Assay
2.6. β-Galactosidase Assay
2.7. Extraction of Genomic DNA
2.8. DNA Gel Retardation
2.9. Erythrocyte Hemolysis Assay
2.10. Cell Culture
2.11. Cell Proliferation Assay
3. Results
3.1. AamAP1-Lysine Design and Structural Modeling
| Peptide | Sequence | Hydrophobicity (H) | Hydrophobic moment (MH) | Helicity |
|---|---|---|---|---|
| AamAP1 | FLFSLIPHAIGGLISAFK | 0.9 | 0.44 | 66.60% |
| AamAP1-Lysine | 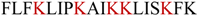 | 0.61 | 0.61 | 88.3% |
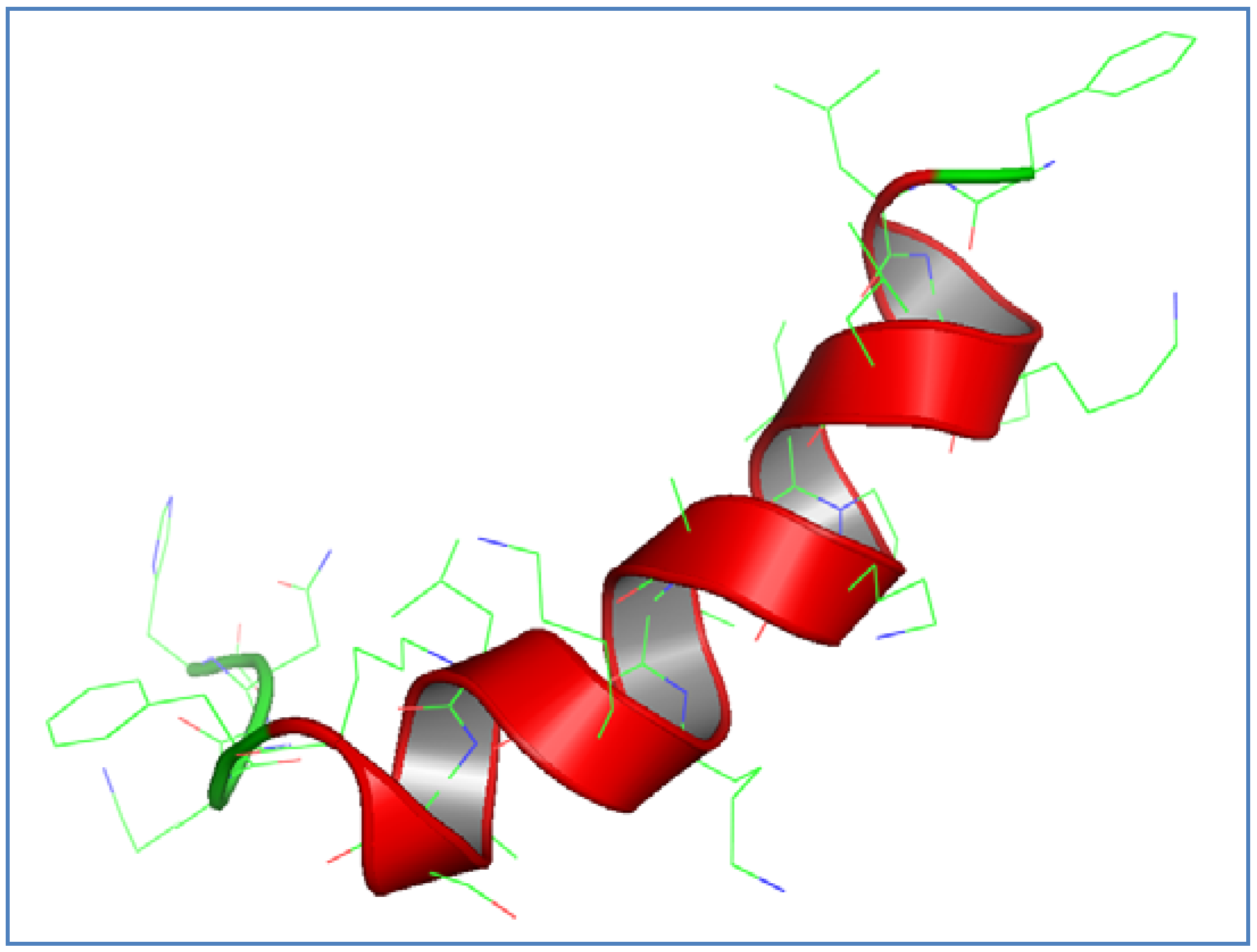
3.2. Antimicrobial Activity of AamAP1-Lysine
| Strain (Gram positive) | ATCC | MIC (µM) |
|---|---|---|
| Staphylococcus epidermidis | 12228 | 5 |
| Staphylococcus aureus | 29213 | 5 |
| Staphylococcus aureus | 43300 | 5 |
| Staphylococcus aureus | 33591 | 5 |
| Enterococcus faecalis | 19433 | 5 |
| Strain(Gram negative) | ATCC | |
| Eshereschia coli | 25922 | 7.5 |
| Salmonella enterica | 10708 | 7.5 |
| Pseudomonas aeruginosa | 9027 | 5 |
| Klebsiella pneumoniae | 13883 | 5 |
3.3. Hemolytic Activity of AamAP1-Lysine against Human Erythrocytes
| Peptide concentration (µM) | Hemolysis (%) |
|---|---|
| 1 | 0 |
| 5 | 0 |
| 10 | 1.38 |
| 20 | 7.25 |
| 40 | 16.58 |
| 60 | 21.29 |
| 80 | 29.32 |
| 100 | 38.25 |
3.4. Viability of Eukaryotic Cells in Culture
3.5. Cytoplasmic Membrane Permeability
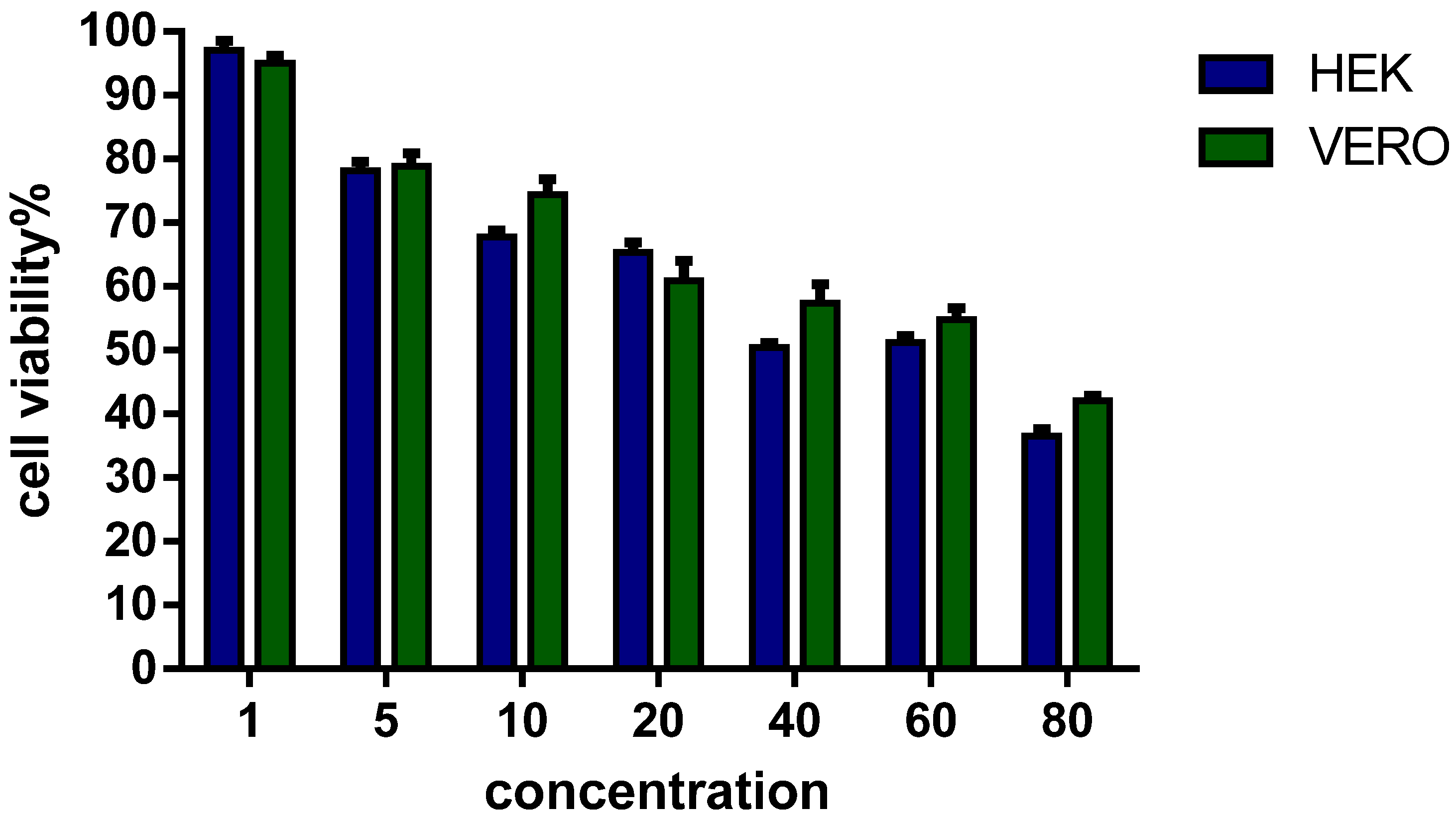
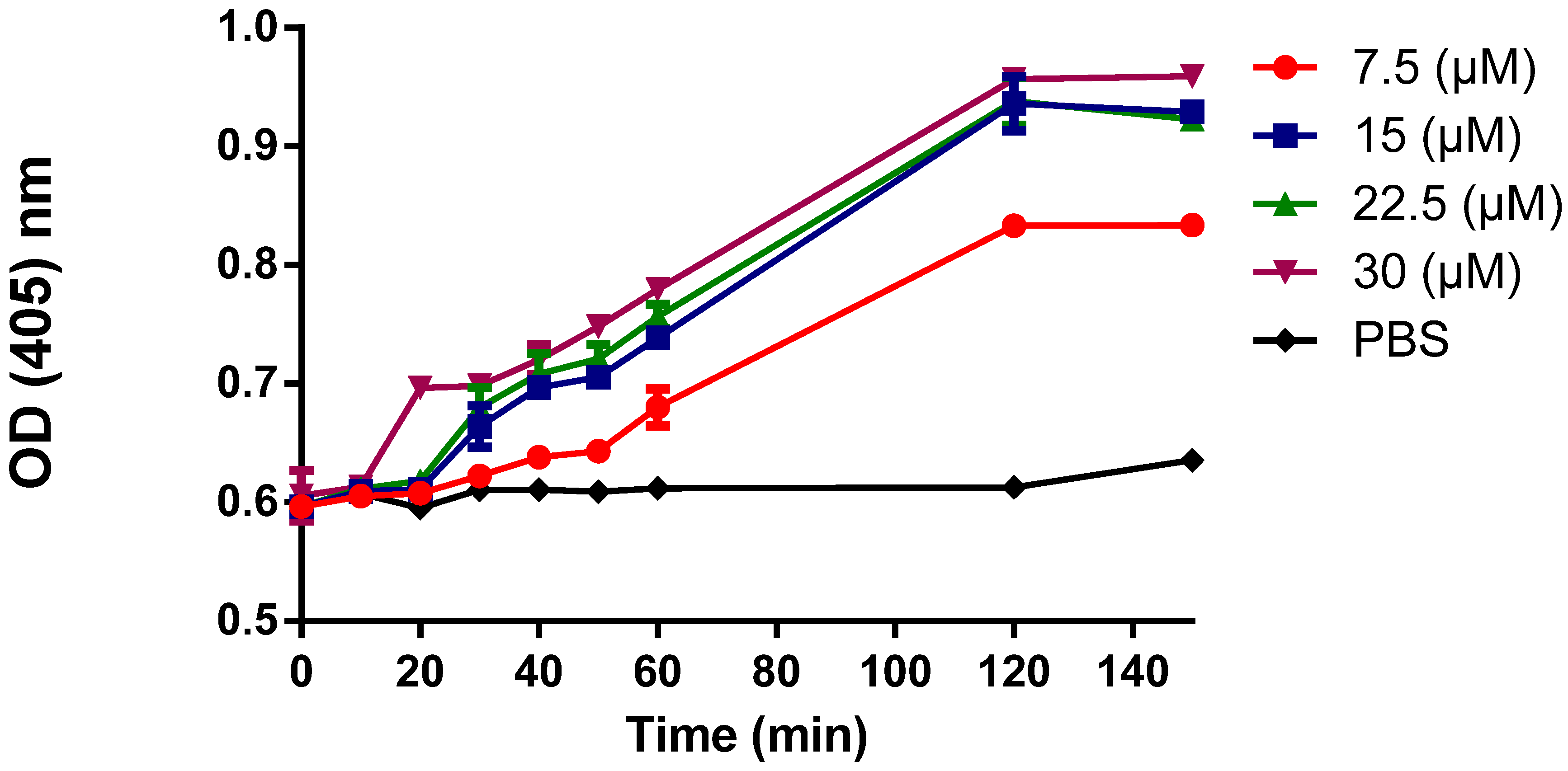
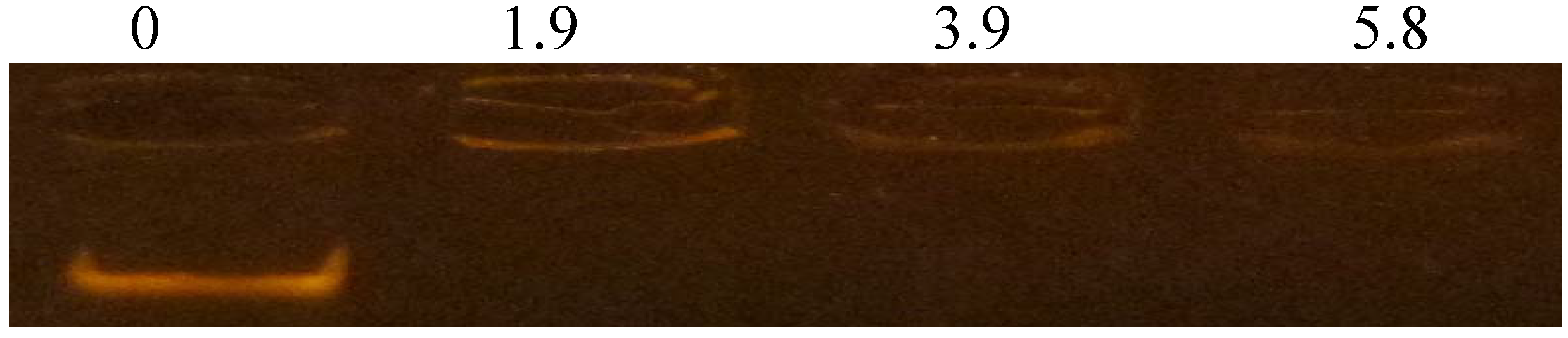
3.6. DNA-Binding Activity
4. Discussion
5. Conclusions
Conflicts of Interest
References
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef]
- Heymann, D.L. Resistance to anti-infective drugs and the threat to public health. Cell 2006, 124, 671–675. [Google Scholar] [CrossRef]
- Paphitou, N.I. Antimicrobial resistance: action to combat the rising microbial challenges. Int. J. Antimicrob. Agents 2013, 42, S25–S28. [Google Scholar] [CrossRef]
- Hawkey, P.M.; Jones, A.M. The changing epidemiology of resistance. J. Antimicrob. Chemother. 2009, 64 (Suppl. 1), i3–i10. [Google Scholar] [CrossRef]
- Rice, L.B. The clinical consequences of antimicrobial resistance. Curr. Opin. Microbiol. 2009, 12, 476–481. [Google Scholar] [CrossRef]
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J., Jr.; the Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef]
- Spellberg, B.; Powers, J.H.; Brass, E.P.; Miller, L.G.; Edwards, J.E., Jr. Trends in antimicrobial drug development: Implications for the future. Clin. Infect. Dis. 2004, 38, 1279–1286. [Google Scholar] [CrossRef]
- Chen, Y.; Vasil, A.I.; Rehaume, L.; Mant, C.T.; Burns, J.L.; Vasil, M.L.; Hancock, R.E.W.; Hodges, R.S. Comparison of biophysical and biologic properties of α-helical enantiomeric antimicrobial peptides. Chem. Boil. Drug Des. 2006, 67, 162–173. [Google Scholar] [CrossRef]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of peptide hydrophobicity in the mechanism of action of α-helical antimicrobial peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef]
- Kraus, D.; Peschel, A. Molecular mechanisms of bacterial resistance to antimicrobial peptides. In Antimicrobial Peptides and Human Disease; Springer: Berlin/Heidelberg, Germany, 2006; pp. 231–250. [Google Scholar]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide antimicrobial agents. Clin. Microbial. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Nicolas, P.; Mor, A. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 1995, 49, 277–304. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Wiradharma, N.; Khoe, U.; Hauser, C.A.E.; Seow, S.V.; Zhang, S.; Yanga, Y.-Y. Synthetic cationic amphiphilic α-helical peptides as antimicrobial agents. Biomaterials 2011, 32, 2204–2212. [Google Scholar] [CrossRef]
- Stark, M.; Liu, L.P.; Deber, C.M. Cationic hydrophobic peptides with antimicrobial activity. Antimicrob. Agents Chemother. 2002, 46, 3585–3590. [Google Scholar] [CrossRef]
- Lee, E.K.; Kim, Y.C.; Nan, Y.H.; Shin, S.Y. Cell selectivity, mechanism of action and LPS-neutralizing activity of bovine myeloid antimicrobial peptide-18 (BMAP-18) and its analogs. Peptides 2011, 32, 1123–1130. [Google Scholar]
- Li, L.; Shi, Y.H.; Cheserek, M.J.; Su, G.F.; Le, G.W. Antibacterial activity and dual mechanisms of peptide analog derived from cell-penetrating peptide against Salmonella typhimurium and Streptococcus pyogenes. Appl. Microbiol. Biotechnol. 2013, 97, 1711–1723. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, Y.; Dai, C.; Zhao, R.; Li, S.R.; Wu, Y.; Cao, Z.; Li, W. Imcroporin, a new cationic antimicrobial peptide from the venom of the scorpion Isometrus maculates. Antimicrob. Agents Chemother. 2009, 53, 3472–3477. [Google Scholar]
- Yuan, W.; Cao, L.; Ma, Y.; Mao, P.; Wang, W.; Zhao, R.; Wu, Y.; Cao, Z.; Li, W. Cloning and functional characterization of a new antimicrobial peptide gene StCT1 from the venom of the scorpion Scorpiops tibetanus. Peptides 2010, 31, 22–26. [Google Scholar] [CrossRef]
- Silva, É.C.N.; Camargos, T.S.; Maranhão, A.Q.; Silva-Pereira, L.; Silva, L.P.; Possani, L.D.; Schwartz, E.F. Cloning and characterization of cDNA sequences encoding for new venom peptides of the Brazilian scorpion Opisthacanthus cayaporum. Toxicon 2009, 54, 252–261. [Google Scholar]
- Luna-Ramírez, K.; Quintero-Hernández, V.; Vargas-Jaimes, L.; Batista, C.V.F.; Winkel, K.D.; Possani, L.D. Characterization of the venom from the Australian scorpion Urodacus yaschenkoi: Molecular mass analysis of components, cDNA sequences and peptides with antimicrobial activity. Toxicon 2013, 63, 44–54. [Google Scholar] [CrossRef]
- Torres-Larios, A.; Gurrola, G.B.; Zamudio, F.Z.; Possani, L.D. Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus. Eur. J. Biochem. 2000, 267, 5023–5031. [Google Scholar] [CrossRef]
- Almaaytah, A.; Zhou, M.; Wang, L.; Chen, T.; Walker, B.; Shaw, C. Antimicrobial/cytolytic peptides from the venom of the North African scorpion, Androctonus amoreuxi: Biochemical and functional characterization of natural peptides and a single site-substituted analog. Peptides 2012, 35, 291–299. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Söding, J.; Biegert, A.; Lupas, A.N. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005, 33 (Suppl. 2), W244–W248. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35 (Suppl. 2), W407–W410. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9. [Google Scholar] [CrossRef]
- Šali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Komatsuzawa, H.; Ohtaa, K.; Sugaia, M.; Fujiwaraa, T.; Glanzmannb, P.; Berger-Bächib, B.; Suginaka, H. Tn551-mediated insertional inactivation of the fmtB gene encoding a cell wall-associated protein abolishes methicillin resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 2000, 45, 421–431. [Google Scholar] [CrossRef]
- Ouhara, K.; Komatsuzawa, K.; Kawai, T.; Nishi, H.; Fujiwara, T.; Fujiue, Y.; Kuwabara, M.; Sayama, K.; Hashimoto, K.; Sugai, M. Increased resistance to cationic antimicrobial peptide LL-37 in methicillin-resistant strains of Staphylococcus aureus. J. Antimicrob. Chemother. 2008, 61, 1266–1269. [Google Scholar] [CrossRef]
- Je, J.Y.; Kim, S.K. Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. J. Agric. Food Chem. 2006, 54, 6629–6633. [Google Scholar] [CrossRef]
- Imura, Y.; Nishida, M; Ogawa, Y.; Takakura, Y.; Matsuzaki, K. Action mechanism of tachyplesin I and effects of PEGylation. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 1160–1169. [Google Scholar] [CrossRef]
- Almaaytah, A.; Tarazi, S.; Mhaidat, N.; Al-Balas, Q.; Mukattash, T.L. Mauriporin, a novel cationic α-helical peptide with selective cytotoxic activity against prostate cancer cell lines from the venom of the scorpion Androctonus mauritanicus. Int. J. Pept. Res. Ther. 2013, 19, 281–293. [Google Scholar] [CrossRef]
- Hmed, B.N.; Serria, H.T.; Mounir, Z.K. Scorpion peptides: Potential use for new drug development. J. Toxicol. 2013, 2013, 958797. [Google Scholar]
- Almaaytah, A.; Albalas, O. Scorpion venom peptides with no disulfide bridges: A review. Peptides 2014, 51, 35–45. [Google Scholar] [CrossRef]
- Wimley, W.C.; Hristova, K. Antimicrobial peptides: Successes, challenges and unanswered questions. J. Membr. Biol. 2011, 239, 27–34. [Google Scholar] [CrossRef]
- Brogden, N.K.; Brogden, K.A. Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? Int. J. Antimicrob. Agents 2011, 38, 217–225. [Google Scholar]
- Tagai, C.; Morita, S.; Shiraishi, T.; Miyaji, K.; Iwamuro, S. Antimicrobial properties of arginine-and lysine-rich histones and involvement of bacterial outer membrane protease T in their differential mode of actions. Peptides 2011, 32, 2003–2009. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: Relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef]
- Findlay, B.; Zhanel, G.G.; Schweizer, F. Cationic amphiphiles, a new generation of antimicrobials inspired by the natural antimicrobial peptide scaffold. Antimicrobi. Agents Chemother 2010, 54, 4049–4058. [Google Scholar]
- Yeaman, M.R.; Nannette, Y.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Takahashi, D.; Sanjeev, K.S.; Om, P.; Guolong, Z. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie. 2010, 92(9), 1236–1241. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Almaaytah, A.; Tarazi, S.; Abu-Alhaijaa, A.; Altall, Y.; Alshar'i, N.; Bodoor, K.; Al-Balas, Q. Enhanced Antimicrobial Activity of AamAP1-Lysine, a Novel Synthetic Peptide Analog Derived from the Scorpion Venom Peptide AamAP1. Pharmaceuticals 2014, 7, 502-516. https://doi.org/10.3390/ph7050502
Almaaytah A, Tarazi S, Abu-Alhaijaa A, Altall Y, Alshar'i N, Bodoor K, Al-Balas Q. Enhanced Antimicrobial Activity of AamAP1-Lysine, a Novel Synthetic Peptide Analog Derived from the Scorpion Venom Peptide AamAP1. Pharmaceuticals. 2014; 7(5):502-516. https://doi.org/10.3390/ph7050502
Chicago/Turabian StyleAlmaaytah, Ammar, Shadi Tarazi, Ahmad Abu-Alhaijaa, Yara Altall, Nizar Alshar'i, Khaldon Bodoor, and Qosay Al-Balas. 2014. "Enhanced Antimicrobial Activity of AamAP1-Lysine, a Novel Synthetic Peptide Analog Derived from the Scorpion Venom Peptide AamAP1" Pharmaceuticals 7, no. 5: 502-516. https://doi.org/10.3390/ph7050502
APA StyleAlmaaytah, A., Tarazi, S., Abu-Alhaijaa, A., Altall, Y., Alshar'i, N., Bodoor, K., & Al-Balas, Q. (2014). Enhanced Antimicrobial Activity of AamAP1-Lysine, a Novel Synthetic Peptide Analog Derived from the Scorpion Venom Peptide AamAP1. Pharmaceuticals, 7(5), 502-516. https://doi.org/10.3390/ph7050502





