Ferric Citrate Hydrate as a Phosphate Binder and Risk of Aluminum Toxicity
Abstract
:1. Introduction
2. Dietary Intake and Metabolism of Aluminum
3. Citrate Promotes Absorption of Dietary Aluminum
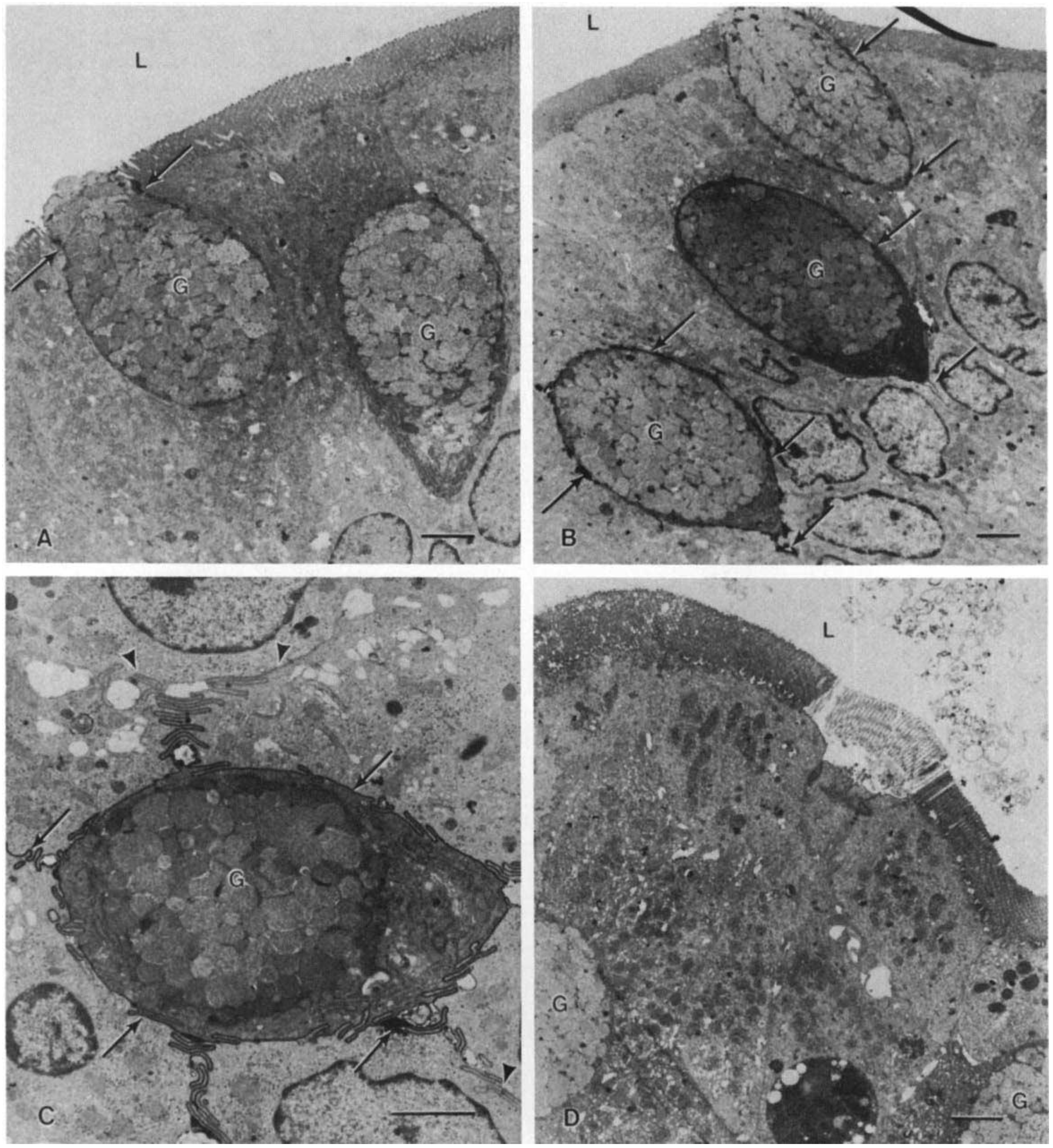
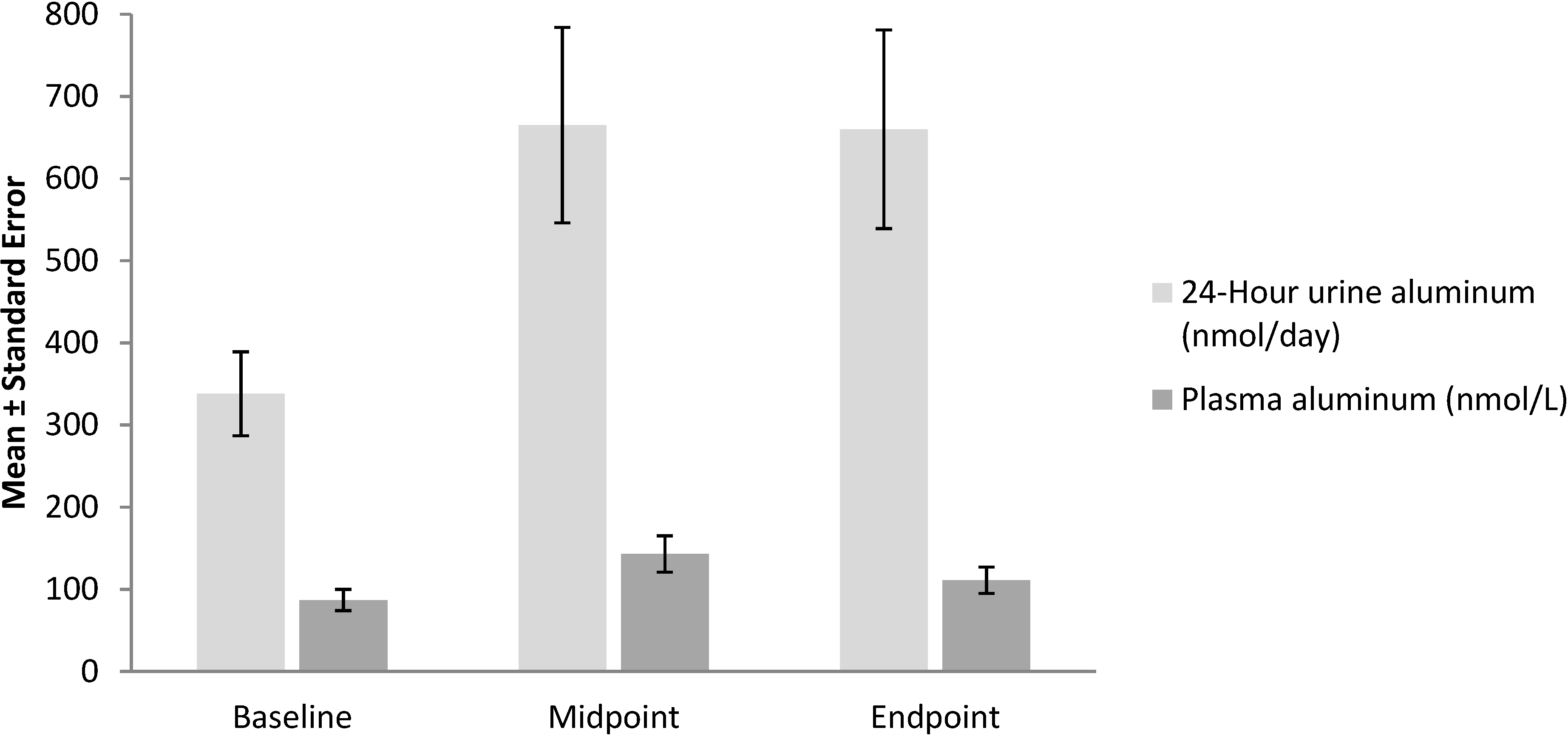
4. Citrate Leads to Accumulation of Aluminum in Brain and Bones
5. Use of Ferric Citrate Hydrate as a Phosphate Binder in Patients with CKD: Source of Citrate and a Risk Factor for Aluminum Toxicity
6. Interaction between Aluminum and Iron
7. Conclusions
Conflicts of Interest
References
- Yokoyama, K.; Hirakata, H.; Akiba, T.; Fukagawa, M.; Nakayama, M.; Sawada, K.; Kumagai, Y.; Block, G.A. Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent ckd. Clin. J. Am. Soc. Nephrol. 2014, 9, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Akiba, T.; Fukagawa, M.; Nakayama, M.; Sawada, K.; Kumagai, Y.; Chertow, G.M.; Hirakata, H. A randomized trial of jtt-751 versus sevelamer hydrochloride in patients on hemodialysis. Nephrol. Dial. Transplant. 2014, 29, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.R.; DeGoes, J.J.; Alfrey, A.C. Aluminum and lead absorption from dietary sources in women ingesting calcium citrate. South. Med. J. 1994, 87, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004.
- Pennington, J.A. Aluminium content of foods and diets. Food Addit. Contam. 1988, 5, 161–232. [Google Scholar] [CrossRef]
- Greger, J.L. Aluminum content of the american diet. Food Technol. 1985, 39, 73–80. [Google Scholar]
- Rajwanshi, P.; Singh, V.; Gupta, M.K.; Kumari, V.; Shrivastav, R.; Ramanamurthy, M.; Dass, S. Studies on aluminium leaching from cookware in tea and coffee and estimation of aluminium content in toothpaste, baking powder and paan masala. Sci. Total Environ. 1997, 193, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.R.; Pougnet, M.A.; de Villiers, S.; Monteagudo, F. Increased urinary excretion of al after drinking tea. Nature 1988, 333, 122. [Google Scholar] [CrossRef] [PubMed]
- Aluminium in Drinking-Water: Background Document for Development of Who Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003; pp. 1–14.
- Lione, A. The prophylactic reduction of aluminium intake. Food Chem. Toxicol. 1983, 21, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Partridge, N.A.; Regnier, F.E.; White, J.L.; Hem, S.L. Influence of dietary constituents on intestinal absorption of aluminum. Kidney Int. 1989, 35, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol Environ. Health B Crit Rev. 2007, 10 (Suppl. 1), 1–269. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, I.S.; Ward, M.K.; Kerr, D.N. Dialysis encephalopathy, bone disease and anaemia: The aluminum intoxication syndrome during regular haemodialysis. J. Clin. Pathol. 1981, 34, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.M.; Polinsky, M.S.; Gruskin, A.B.; Baluarte, H.J.; Grover, W.D. Encephalopathy in infants and children with chronic renal disease. Arch. Neurol. 1981, 38, 656–658. [Google Scholar] [CrossRef]
- Froment, D.H.; Buddington, B.; Miller, N.L.; Alfrey, A.C. Effect of solubility on the gastrointestinal absorption of aluminum from various aluminum compounds in the rat. J. Lab. Clin. Med. 1989, 114, 237–242. [Google Scholar] [PubMed]
- Froment, D.P.; Molitoris, B.A.; Buddington, B.; Miller, N.; Alfrey, A.C. Site and mechanism of enhanced gastrointestinal absorption of aluminum by citrate. Kidney Int. 1989, 36, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Slanina, P.; Falkeborn, Y.; Frech, W.; Cedergren, A. Aluminium concentrations in the brain and bone of rats fed citric acid, aluminium citrate or aluminium hydroxide. Food Chem. Toxicol. 1984, 22, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Alfrey, A.C. Aluminum. Adv. Clin. Chem. 1983, 23, 69–91. [Google Scholar] [PubMed]
- Kausz, A.T.; Antonsen, J.E.; Hercz, G.; Pei, Y.; Weiss, N.S.; Emerson, S.; Sherrard, D.J. Screening plasma aluminum levels in relation to aluminum bone disease among asymptomatic dialysis patients. Am. J. Kidney Dis. 1999, 34, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Hercz, G.; Greenwood, C.; Sherrard, D.; Segre, G.; Manuel, A.; Saiphoo, C.; Fenton, S. Non-invasive prediction of aluminum bone disease in hemo- and peritoneal dialysis patients. Kidney Int. 1992, 41, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Van Landeghem, G.F.; D'Haese, P.C.; Lamberts, L.V.; Djukanovic, L.; Pejanovic, S.; Goodman, W.G.; De Broe, M.E. Low serum aluminum values in dialysis patients with increased bone aluminum levels. Clin. Nephrol. 1998, 50, 69–76. [Google Scholar] [PubMed]
- D'Haese, P.C.; Couttenye, M.M.; De Broe, M.E. Diagnosis and treatment of aluminium bone disease. Nephrol Dial. Transplant. 1996, 1 (Suppl. 3), 74–79. [Google Scholar] [CrossRef]
- Malluche, H.H. Aluminium and bone disease in chronic renal failure. Nephrol. Dial. Transplant. 2002, 17 (Suppl. 2), 21–24. [Google Scholar] [CrossRef] [PubMed]
- D'Haese, P.C.; Couttenye, M.M.; Goodman, W.G.; Lemoniatou, E.; Digenis, P.; Sotornik, I.; Fagalde, A.; Barsoum, R.S.; Lamberts, L.V.; De Broe, M.E. Use of the low-dose desferrioxamine test to diagnose and differentiate between patients with aluminium-related bone disease, increased risk for aluminium toxicity, or aluminium overload. Nephrol. Dial. Transplant. 1995, 10, 1874–1884. [Google Scholar] [PubMed]
- Heinrich, H.C. Intestinal absorption of 59fe from neutron-activated commercial oral iron(iii)-citrate and iron(iii)-hydroxide-polymaltose complexes in man. Arzneimittelforschung 1987, 37, 105–107. [Google Scholar] [PubMed]
- Yang, W.C.; Yang, C.S.; Hou, C.C.; Wu, T.H.; Young, E.W.; Hsu, C.H. An open-label, crossover study of a new phosphate-binding agent in haemodialysis patients: Ferric citrate. Nephrol. Dial. Transplant. 2002, 17, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.; Pregi, N.; Vittori, D.; Di Risio, C.; Garbossa, G.; Nesse, A. Aluminum exposure affects transferrin-dependent and -independent iron uptake by k562 cells. Biochim. Biophys. Acta 2005, 1745, 124–130. [Google Scholar] [PubMed]
- Ward, R.J.; Zhang, Y.; Crichton, R.R. Aluminium toxicity and iron homeostasis. J. Inorg. Biochem. 2001, 87, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Du, Y.; Xue, H.; Wu, Y.; Zhou, B. Aluminum induces neurodegeneration and its toxicity arises from increased iron accumulation and reactive oxygen species (ros) production. Neurobiol. Aging 2012, 33, 199 e191–112. [Google Scholar]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimers Dis. 2011, 2011, 276393. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, A. Ferric Citrate Hydrate as a Phosphate Binder and Risk of Aluminum Toxicity. Pharmaceuticals 2014, 7, 990-998. https://doi.org/10.3390/ph7100990
Gupta A. Ferric Citrate Hydrate as a Phosphate Binder and Risk of Aluminum Toxicity. Pharmaceuticals. 2014; 7(10):990-998. https://doi.org/10.3390/ph7100990
Chicago/Turabian StyleGupta, Ajay. 2014. "Ferric Citrate Hydrate as a Phosphate Binder and Risk of Aluminum Toxicity" Pharmaceuticals 7, no. 10: 990-998. https://doi.org/10.3390/ph7100990
APA StyleGupta, A. (2014). Ferric Citrate Hydrate as a Phosphate Binder and Risk of Aluminum Toxicity. Pharmaceuticals, 7(10), 990-998. https://doi.org/10.3390/ph7100990



