Effect of Essential Oils on Pathogenic Bacteria
Abstract
:1. Introduction
2. Activity of Essential Oils against Bacteria
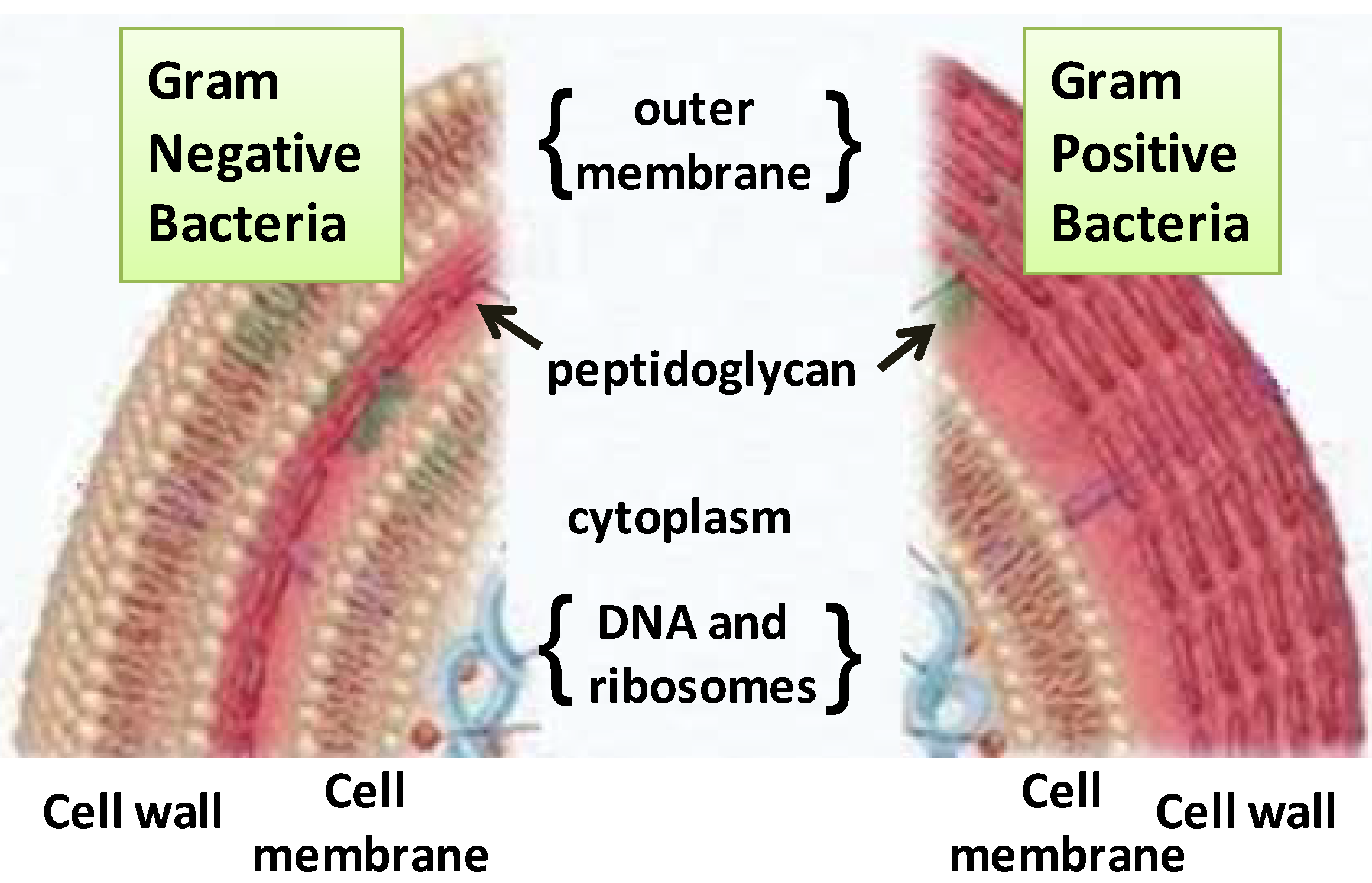
2.1. Terpenes
2.2. Terpenoids
2.3. Phenylpropenes
3. Mechanisms of Action of the Essential Oils and/or Their Components
4. What are the Possible Mechanisms of Action of the EOs and/or Their Components against Microbes?
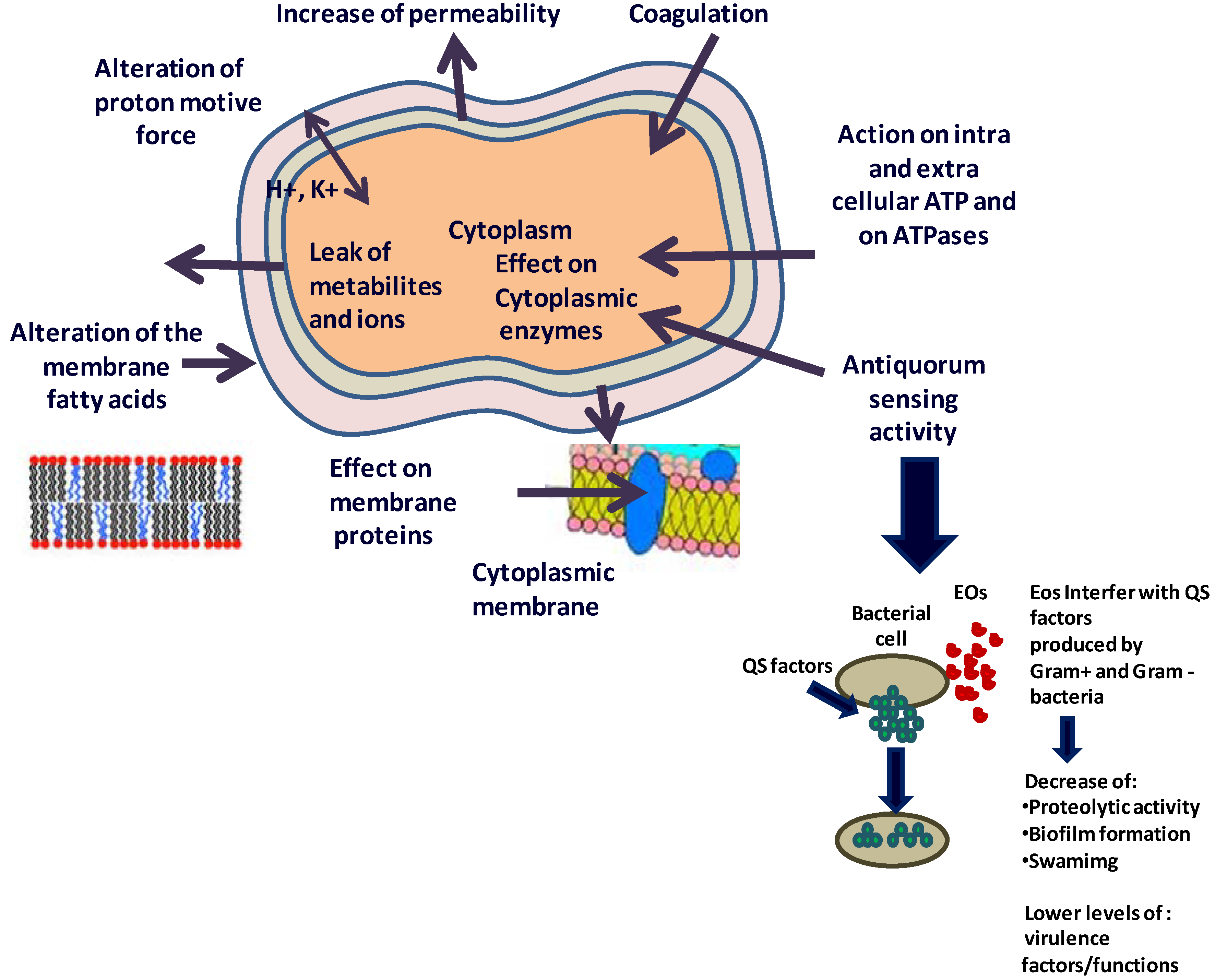
4.1. Effect on the Fatty Acids Profile of the Cell Membrane
4.2. Action on Proteins
4.3. Effect on ATP and ATPases
4.4. Effect on the Metabolome
4.5. Effects on Cell Morphology
4.6. Anti-Quorum Sensing Activity
5. Conclusions
Conflicts of Interest
References
- Abad, M.J.; Ansuategui, M.; Bermejo, P. Active antifungal substances from natural sources. ARCHIVOC 2007, 2007, 116–145. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Chorianopoulos, N.G.; Giaouris, E.D.; Skandamis, P.N.; Haroutounian, S.A.; Nychas, G.J.E. Disinfectant test against monoculture and mixed-culture biofilms composed of technological, spoilage and pathogenic bacteria: Bactericidal effect of essential oil and hydrosol of Satureja thymbra and comparison with standard acid-base sanitizers. J. Appl. Microbiol. 2008, 104, 1586–1599. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef]
- De Martino, L.; de Feo, V.; Nazzaro, F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven lamiaceae essential oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnel, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef]
- Nikaido, H. Prevention of drug access to bacterial targets: Permeability barriers and active efflux. Science 1994, 264, 382–388. [Google Scholar]
- Vaara, M. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar]
- Plesiat, P.; Nikaido, H. Outer membranes of Gram-negative bacteria are permeable to steroid probes. Mol. Microbiol. 1992, 6, 1323–1333. [Google Scholar] [CrossRef]
- Nikaido, H. Outer Membrane. In Escherichia coli and Salmonella: Cellular and Molecular biology; Neidhardt, F.C., Ed.; ASM Press: Washington, DC, USA, 1996; pp. 29–47. [Google Scholar]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.L.; Latva, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.M.; von Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Koski, P. Polyethyleneimine is an effective permeabilizer of Gram negative bacteria. Microbiology 1997, 143, 3193–3199. [Google Scholar] [CrossRef]
- De Martino, L.; de Feo, V.; Fratianni, F.; Nazzaro, F. Chemistry, antioxidant, antibacterial and antifungal activities of volatile oils and their components. Nat. Prod. Comm. 2009, 4, 1741–1746. [Google Scholar]
- Caballero, B.; Trugo, L.C.; Finglas, P.M. Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar]
- Mann, C.M.; Cox, S.D.; Markham, J.L. The outer membrane of Pseudomonas aeruginosa NCTC 6749 contributes to its tolerance to the essential oil of Melaleuca alternifolia (tea tree oil). Lett. Appl. Microbiol. 2000, 30, 294–297. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J. Biosci. Bioeng. 2010, 110, 614–619. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes:implications for their antibacterialactivity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Burt, S.A.; van der Zee, R.; Koets, A.P.; de Graaff, A.M.; Van Knapen, F.; Gaastra, W.; Haagsman, H.P.; Veldhuizen, E.J.A. Carvacrol induces heat shock protein and inhibits synthesis of flagellin in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 4484–4490. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.M.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar]
- Walsh, S.E.; Maillard, J.Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Activity and mechanisms of action of selected biocidal agents on Gram-positive and-negative bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Turina, A.D.V.; Nolan, M.V.; Zygadlo, J.A.; Perillo, M.A. Natural terpenes: Self-assembly and brane partitioning. Biophys. Chem. 2006, 122, 101–113. [Google Scholar] [CrossRef]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that Interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- La Storia, A.; Ercolini, D.; Marinello, F.; di Pasqua, R.; Villani, F.; Mauriello, G. Atomic force microscopy analysis shows surface structure changes in carvacrol-treated bacterial cells. Res. Microbiol. 2011, 162, 164–172. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.A.; Tjeerdsma-Van Bokhoven, J.L.M.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural requirements for the antimicrobial activity of carvacrol. J. Agric. Food Chem. 2006, 54, 1874–1879. [Google Scholar]
- Ultee, A.; Kets, E.P.W.; Alberda, M.; Hoekstra, F.A.; Smid, E.J. Adaptation of the food-borne pathogen Bacillus cereus to carvacrol. Arch. Microbiol. 2000, 174, 233–238. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar]
- Horváth, G.; Kovács, K.; Kocsis, B.; Kustos, I. Effect of thyme (Thymus vulgaris L.) essential oil and its main constituents on the outer membrane protein composition of Erwinia strains studied with microfluid chip technology. Chromatographia 2009, 70, 1645–1650. [Google Scholar] [CrossRef]
- Gabel, C.V.; Berg, H.C. The speed of the flagellar rotary motor of Escherichia coli varies linearly with proton motive force. Proc. Natl. Acad. Sci. USA 2003, 100, 8748–8751. [Google Scholar] [CrossRef]
- Laekeman, G.M.; VanHoof, L.; Haemers, A.; Berghe, D.A.V.; Herman, A.G.; Vlietinck, A.J. Eugenol a valuable compound for in vitro experimental research and worthwhile for further in vivo investigation. Phytother. Res. 1990, 4, 90–96. [Google Scholar] [CrossRef]
- Jung, H.G.; Fahey, G.C. Nutritional implications of phenolic monomers and lignin: A review. J. Anim. Sci. 1983, 57, 206–219. [Google Scholar]
- Pauli, A.; Kubeczka, K.H. Antimicrobial properties of volatile phenylpropanes. Nat. Prod. Commun. 2010, 5, 1387–1394. [Google Scholar]
- Zemek, J.; Kosikova, B.; Augustin, J.; Joniak, D. Antibiotic properties of lignin components. Folia Microbiol. 1979, 24, 483–486. [Google Scholar] [CrossRef]
- Zemek, J.; Valent, M.; Pódová, M.; Košíková, B.; Joniak, D. Antimicrobial properties of aromatic compounds of plant origin. Folia Microbiol. 1987, 32, 421–425. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Rikke, L.M. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 1–24. [Google Scholar]
- Gill, A.O.; Holley, R.A. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef]
- Thoroski, J. Eugenol induced inhibition of extracellular enzyme production by Bacillus cereus. J. Food Prot. 1989, 52, 399–403. [Google Scholar]
- Wendakoon, C.N.; Sakaguchi, M. Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components in spices. J. Food Prot. 1995, 58, 280–283. [Google Scholar]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Ueckert, J.; Bos, A.; Narbad, A. Mode of antimicrobial of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J. Appl. Microbiol. 2004, 97, 104–113. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Narbad, A. Structure-function analysis of the vanillin molecule and its antifungal properties. J. Agric. Food Chem. 2005, 53, 1769–1775. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Poolman, B.; Smid, E.J. S-carvone as a natural potato sprout inhibiting, fungistatic and bacteristatic compound. Ind. Crops Prod. 1995, 4, 23–31. [Google Scholar] [CrossRef]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Khkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Deans, S.G.; Eaglesham, E. Relationship between bioactivity and chemical composition of commercial essential oils. Flavour Fragr. J. 1998, 13, 98–104. [Google Scholar] [CrossRef]
- Davidson, P.M. Chemical Preservatives and Naturally Antimicrobial Compounds. In Food Microbiology. Fundamentals and Frontiers, 2nd ed.; Beuchat, M.P., Montville, L.R., Eds.; ASM Press: Washington, DC, USA, 2001; pp. 593–628. [Google Scholar]
- Pina-Vaz, C.; Gonçalves Rodrigues, A.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Martinez-de-Oliveira, J. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The anti-microbial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Antimicrobial activity of the essential oils of Thymus vulgaris L. measured using a bioimpedimetric method. Int. J. Food Microbiol. 2001, 67, 187–195. [Google Scholar] [CrossRef]
- Poolman, B.; Driessen, A.J.M.; Konings, W.N. Regulation of solute transport in Streptococci by external and internal pH values. Microbiol. Rev. 1987, 51, 498–508. [Google Scholar]
- Trumpower, B.L.; Gennis, R.B. Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. Ann. Rev. Biochem. 1994, 63, 675–716. [Google Scholar] [CrossRef]
- Andrews, R.E.; Parks, L.W.; Spence, K.D. Some effects of Douglas fir terpenes on certain microorganisms. Appl. Environ. Microbiol. 1980, 40, 301–304. [Google Scholar]
- Uribe, S.; Ramirez, T.; Pena, A. Effects of β-pinene on yeast membrane functions. J. Bacteriol. 1985, 161, 1195–1200. [Google Scholar]
- Knobloch, K.; Pauli, A.; Iberl, B. Antibacterial activity and antifungal properties of essential oil components. J. Essent. Oils Res. 1988, 1, 119–128. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Disruption of E. coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef]
- Gustafson, J.E.; Liew, Y.C.; Chew, S.; Markham, J.L.; Bell, H.C.; Wyllie, S.G.; Warmington, J.R. Effects of tea tree oil on Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 194–198. [Google Scholar]
- Ultee, A.; Smid, E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef]
- Kim, J.; Marshal, M.R.; Wie, C.I. Antibacterial activity of some essential oils components against five foodborne pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Tassou, C.; Koutsoumanis, K.; Nychas, J.E. Inhibition of Salmonella enteritidis and Staphylococcus aureus in nutrient broth by mint essential oil. Food Res. Int. 2000, 33, 273–280. [Google Scholar] [CrossRef]
- Mrozik, A.; Pietrovska-Seget, Z.; Labuzek, S. Changes in whole cell-derived fatty acids induced by naphthalene in bacteria from genus Pseudomonas. Microbiol. Res. 2004, 159, 87–95. [Google Scholar] [CrossRef]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef]
- Heath, R.J.; Rock, C.O. Fatty acid biosynthesis as a target for novel antibacterials. Curr. Opin. Invest. Drugs 2004, 5, 146–153. [Google Scholar]
- Heath, R.J.; White, S.W.; Rock, C.O. Lipid biosynthesis as a target for antibacterial agents. Prog. Lipid Res. 2001, 40, 467–497. [Google Scholar] [CrossRef]
- Campbell, J.W.; Cronan, J.E. Bacterial fatty acids biosynthesis: Targets for antibacterial drug discovery. Ann. Rev. Microbiol. 2001, 55, 305–332. [Google Scholar] [CrossRef]
- Bayer, A.S.; Presad, R.; Chandra, J.; Smirti, A.M.; Varma, A.; Skurray, R.A.; Firth, N.; Brown, M.H.; Koo, S.P.; Yeaman, M.R. In vitro resistance of Staphylococcus aureus to thrombin induced platelet microbicidal protein is associated with alterations in cytoplasmic membrane fluidity. Infect. Immun. 2000, 68, 3548–3553. [Google Scholar] [CrossRef]
- Heath, R.J.; Jackowski, S.; Rock, C.O. Fatty Acid and Phospholipid Metabolism in Prokaryotes. In Biochemistry of Lipids, Lipoproteins and Membranes, 4th ed.; Vance, J.E., Vance, D.E., Eds.; Elsevier: New York, NY, USA, 2002. [Google Scholar]
- Russell, N.J. Mechanism of thermal adaptation in bacteria: Blueprints for survival. Tr. Biochem. Sci. 1984, 9, 108–112. [Google Scholar] [CrossRef]
- Russell, N.J. Psychrophilic bacteria: Molecular adaptations of membrane lipids. Comp. Biochem. Physiol. 1997, 118, 489–493. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Meinhardt, F.; Segura, A. The cis-trans isomerase of unsaturated fatty acids in Pseudomonas and Vibrio: Biochemistry, molecular biology and physiological function of a unique stress adaptive mechanism. FEMS Microbiol. Lett. 2003, 229, 1–7. [Google Scholar] [CrossRef]
- Domadia, P.; Swarup, S.; Bhunia, A.; Sivaraman, J.; Dasgupta, D. Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem. Pharmacol. 2007, 74, 831–840. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Mamone, G.; Ferranti, P.; Ercolini, D.; Mauriello, G. Changes in the proteome of Salmonella enterica serovar Thompson as stress adaptation to sublethal concentrations of thymol. Proteomics 2010, 10, 1040–1049. [Google Scholar]
- Kumar, M.; Berwal, J.S. Sensitivity of food pathogens to garlic (Allium sativum). J. Appl. Microbiol. 1998, 84, 213–215. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Mauriello, G.; Mamone, G.; Ercolini, D. Expression of DnaK, HtpG, GroEL and Tf chaperones and the corresponding encoding genes during growth of Salmonella Thompson in presence of thymol alone or in combination with salt and cold stress. Food Res. Int. 2013, 52, 153–159. [Google Scholar] [CrossRef]
- Baucheron, S.; Mouline, C.; Praud, K.; Chlaus-Dancla, E.; Cloeckaert, A. TolC but not AcrB is essential for multidrugresistant Salmonella enterica serotype Typhimurium colonization of chicks. J. Antimicrob. Chem. 2005, 55, 707–712. [Google Scholar] [CrossRef]
- Klose, K.E.; Mekalanos, J.J. Simultaneous prevention of glutamine synthesis and high-affinity transport attenuates Salmonella typhimurium virulence. Infect. Immun. 1997, 65, 587–596. [Google Scholar]
- Miesel, L.; Greene, J.; Black, T.A. Genetic strategies for antibacterial drug discovery. Nat. Rev. Gen. 2003, 4, 442–456. [Google Scholar] [CrossRef]
- Xu, H.H.; Trawick, J.D.; Haselbeck, R.J.; Forsyth, R.; Yamamoto, R.T.; Archer, R.; Patterson, J.; Allen, M.; Froelich1, J.M. ; Taylor, I.; et al. Staphylococcus aureus Target Array: Comprehensive differential essential gene expression as a mechanistic tool to profile antibacterials. Antimicrob. Agents Chemother. 2010, 54, 3659–3670. [Google Scholar] [CrossRef]
- Scotti, P.A.; Urbanus, M.L.; Brunner, J.; de Gier, J.W.L.; von Heijne, G.; van der Does, C.; Driessen, A.J.M.; Oudega, B.; Luirink, J. YidC, the Escherichia coli homologue of mitochondrial Oxa1p, is a component of the Sec translocase. EMBO J. 2000, 19, 542–549. [Google Scholar] [CrossRef]
- Serek, J.; Bauer-Manz, G.; Struhalla, G.; van den Berg, L.; Kiefer, D.; Dalbey, R.; Kuhn, A. Escherichia coli YidC is a membrane insertase for Sec-independent proteins. EMBO J. 2004, 23, 294–301. [Google Scholar] [CrossRef]
- Samuelson, J.C.; Chen, M.; Jiang, F.; Möller, I.; Wiedmann, M.; Kuhn, A.; Phillips, G.J.; Dalbey, R.E. YidC mediates membrane protein insertion in bacteria. Nature 2000, 406, 637–641. [Google Scholar] [CrossRef]
- Van der Laan, M.; Urbanus, M.; Ten Hagen-Jongman, C.; Nouwen, N.; Oudega, B.; Harms, N.; Driessen, A.J.M.; Luirink, J. A conserved function of YidC in the biogenesis of respiratory chain complexes. Proc. Nat. Acad. Sci. USA 2003, 100, 5801–5806. [Google Scholar] [CrossRef]
- Patil, S.D.; Sharma, R.; Srivastava, S.; Navani, N.K.; Pathania, R. Down regulation of yidC in Escherichia coli by antisense RNA expression results in sensitization to antibacterial essential oils eugenol and carvacrol. PLoS One 2013, 8, 57370. [Google Scholar]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 2006, 73, 236–244. [Google Scholar] [CrossRef]
- Turgis, M.; Han, J.; Caillet, S.; Lacroix, M. Antimicrobial activity of mustard essential oil against Escherichia coli O157:H7 and Salmonella typhi. Food Control. 2009, 20, 1073–1079. [Google Scholar] [CrossRef]
- Caillet, S.; Ursachi, L.; Shareck, F.; Lacroix, M. Effect of gamma radiation and oregano essential oil on murein and ATP concentration of Staphylococcus aureus. J. Food Sci. 2009, 74, M499–M508. [Google Scholar] [CrossRef]
- Caillet, S.; Lacroix, M. Effect of gamma radiation and oregano essential oil on murein and ATP concentration of Listeria monocytogenes. J. Food Prot. 2006, 69, 2961–2969. [Google Scholar]
- Abee, T.; Klaenhammer, T.R.; Letellier, L. Kinetic studies of the action of lactacin F, a bacteriocin produced by Lactobacillus johnsonii that forms poration complexes in the cytoplasmic membrane. Appl. Env. Microbiol. 1994, 60, 1006–1013. [Google Scholar]
- Shabala, L.; Budde, L.; Ross, B.; Siegumfeldt, T.; Jakobsen, H.; McMeekin, M. Responses of Listeria monocytogenes to acid stress and glucose availability revealed by a novel combination of fluorescence microscopy and microelectrode ion-selective techniques. Appl. Env. Microbiol. 2002, 68, 1794–1802. [Google Scholar] [CrossRef]
- Kwon, J.A.; Yu, C.B.; Park, H.D. Bactericidal effects and inhibition of cell separation of cinnamic aldehyde on Bacillus cereus. Lett. Appl. Microbiol. 2003, 37, 61–65. [Google Scholar] [CrossRef]
- Carneiro, S.; Villas-Bôas, S.G.; Ferreira, E.C.; Rocha, I. Metabolic footprint analysis of recombinant Escherichia coli strains during fed-batch fermentations. Mol. Bio. Syst. 2011, 7, 899–910. [Google Scholar]
- Van der Werf, M.J.; Overkamp, K.M.; Muilwijk, B.; Coulier, L.; Hankemeier, T. Microbial metabolomics: Toward a platform with full metabolome coverage. Anal. Biochem. 2007, 370, 17–25. [Google Scholar] [CrossRef]
- Rabinowitz, J.D. Cellular metabolomics of Escherichia coli. Exp. Rev. Prot. 2007, 4, 187–198. [Google Scholar] [CrossRef]
- Mapelli, V.; Olsson, L.; Nielsen, J. Metabolic footprinting in microbiology: Methods and applications in functional genomics and biotechnology. Trends Biotech. 2008, 26, 490–497. [Google Scholar] [CrossRef]
- Jozefczuk, S.; Klie, S.; Catchpole, G.; Szymanski, J.; Cuadros-Inostroza, A.; Steinhauser, D.; Selbig, J.; Willmitzer, L. Metabolomic and transcriptomic stress response of Escherichia coli. Mol. Syst. Biol. 2010, 6, 364–381. [Google Scholar]
- Gunasekera, T.S.; Csonka, L.N.; Paliy, O. Genome-wide transcriptional responses of Escherichia coli K-12 to continuous osmotic and heat stresses. J. Bacteriol. 2008, 190, 3712–3720. [Google Scholar] [CrossRef]
- Durfee, T.; Hansen, A.M.; Zhi, H.; Blattner, F.R.; Jin, D.J. Transcription profiling of the stringent response in Escherichia coli. J. Bacteriol. 2008, 190, 1084–1096. [Google Scholar] [CrossRef]
- Malin, G.; Lapidot, A. Induction of synthesis of tetrahydropyrimidine derivatives in Streptomyces strains and their effect on Escherichia coli in response to osmotic and heat stress. J. Bacteriol. 1996, 178, 385–395. [Google Scholar]
- Picone, G.; Laghi, L.; Gardini, F.; Lanciotti, R.; Siroli, L.; Capozzi, F. Evaluation of the effect of carvacrol on the Escherichia coli 555 metabolome by using 1H-NMR spectroscopy. Food Chem. 2013, 141, 4367–4374. [Google Scholar] [CrossRef]
- Cox, S.D.; Gustafson, J.E.; Mann, C.M.; Markham, J.L.; Liew, Y.C.; Hartland, R.P.; et al. Tea tree oil causes K+ leakage and inhibits respiration in Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 355–358. [Google Scholar]
- Hossain, Z.S.M.; Bojko, B.; Pawliszyn, J. Automated SPME–GC–MS monitoring of headspace metabolomic responses of E. coli to biologically active components extracted by the coating. An. Chim. Acta 2013, 776, 41–49. [Google Scholar] [CrossRef]
- Hafedh, H.; Najla, T.; Emira, N.; Mejdi, S.; Hanen, F.; Riadh, K.; Amina, B. Biological activities of the essential oils and methanol extract of two cultivated mint species (Mentha longifolia and Mentha pulegium) used in the Tunisian folkloric medicine. World J. Biotec. Microbiol. 2009, 25, 2227–2238. [Google Scholar] [CrossRef]
- Kalchayanand, N.; Dunneb, P.; Sikes, A.; Ray, B. Viability loss and morphology change of foodborne pathogens following exposure to hydrostatic pressures in the presence and absence of bacteriocins. Int. J. Food Microbiol. 2004, 91, 91–98. [Google Scholar] [CrossRef]
- Braga, P.C.; Ricci, D. Atomic Force Microscopy: Application to investigation of Escherichia coli morphology before and after exposure to cefodizime. Antimicrob. Agents Chemother. 1998, 42, 18–22. [Google Scholar]
- Slavik, M.F.; Kim, W.J.; Walker, J.T. Reduction of Salmonella and Campylobacter on chicken carcasses by changing scalding temperature. J. Food Prot. 1995, 58, 689–691. [Google Scholar]
- Sikkema, J.; Weber, F.J.; Heipieper, H.J.; de Bont, J.A.M. Cellular toxicity of lipophilic compounds: Mechanisms, implications, and adaptations. Biocatalysis 1994, 10, 113–122. [Google Scholar] [CrossRef]
- De Sousa, J.P.; de Araújo Torres, R.; Alves de Azerêdo, G.; Queiroz Figueiredo, B.R.C.; da Silva Vasconcelos, M.A.; Leite de Souza, E. Carvacrol and 1,8-cineole alone or in combination at sublethal concentrations induce changes in the cell morphology and membrane permeability of Pseudomonas fluorescens in a vegetable-based broth. Int. J. Food Microbiol. 2012, 158, 9–13. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial action of essential oil vapours and negative air ions against Pseudomonas fluorescens. Int. J. Food Microbiol. 2010, 143, 205–210. [Google Scholar] [CrossRef]
- Nostro, A.; Marino, A.; Blanco, A.R.; Cellini, L.; di Giulio, M.; Pizzimenti, F.; Roccaro, A.S.; Bisignano, G. In vitro activity of carvacrol against staphylococcal preformed biofilm by liquid and vapour contact. J. Med. Microbiol. 2009, 58, 791–797. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- Meincken, M.; Holroyd, D.L.; Rautenbach, M. Atomic force microscopy study of the effect of antimicrobial peptides on the cell envelope of Escherichia coli. Antimicrob. Agents Chemother. 2005, 10, 4085–4092. [Google Scholar] [CrossRef]
- Alakomi, H.L.; Paananen, A.; Suihko, M.L.; Helander, I.M.; Saarela, M. Weakening effect of cell permeabilizer on gram-negative bacteria causing biodeterioration. Appl. Environ. Microbiol. 2006, 72, 4695–4703. [Google Scholar] [CrossRef]
- Bassler, B.L. Small talk: Cell-to-cell communication in bacteria. Cell 2002, 109, 421–424. [Google Scholar] [CrossRef]
- Hentzer, M.; Givskov, M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Invest. 2003, 112, 1300–1307. [Google Scholar]
- Lewis, K.; Ausubel, F.M. Prospects of plant derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef]
- Kumar, V.P.; Chauhan, N.S.; Rajani, H.P.M. Search for antibacterial and antifungal agents from selected Indian medicinal plants. J. Ethnopharmacol. 2006, 107, 182–188. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum sensing and phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef]
- Faleiro, M.L. The Mode of Antibacterial Action of ESsential Oils. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Brown Walker Press: Boca Raton, FL, USA, 2011; pp. 1143–1156. [Google Scholar]
- Al-Shuneigat, J.; Cox, S.D.; Markham, J.L. Effects of a topical essential oil-containing formulation on bio-film-forming coagulase-negative staphylococci. Lett. Appl. Microbiol. 2005, 41, 52–55. [Google Scholar] [CrossRef]
- Khan, M.S.; Zahin, M.; Hasan, S.; Husain, F.M.; Ahmad, I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 2009, 49, 354–360. [Google Scholar] [CrossRef]
- Zaki, A.A.; Shaaban, M.I.; Hashish, N.E.; Amer, M.A.; Lahloub, M.F. Assessment of anti-quorum sensing activity for some ornamental and medicinal plants native to Egypt. Sci. Pharm. 2013, 81, 251–258. [Google Scholar] [CrossRef]
- Szabó, M.A.; Varga, G.Z.; Hohmann, J.; Schelz, Z.; Szegedi, E.; Amaral, L.; Molnár, J. Inhibition of quorum-sensing signals by essential oils. Phytother. Res. 2010, 24, 782–786. [Google Scholar]
- Chernin, L.S.; Winson, M.K.; Thompson, J.M.; Haran, S.; Bycroft, B.W.; Chet, I.; Williams, P.; Gordon, S.; Stewart, A.B. Chitinolytic activity in Chromobacterium violaceum: Substrate analysis and regulation by quorum sensing. J. Bacteriol. 1998, 180, 4435–4441. [Google Scholar]
- Niu, S.; Afre, S.; Gilbert, E.S. Subinhibitory concentrations of cinnamaldehyde interfere with quorum sensing. Lett. Appl. Microbiol. 2006, 43, 489–494. [Google Scholar] [CrossRef]
- Brackman, G.; Celen, S.; Hillaert, U.; Calenbergh, S.V.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Structure-activity relationship of cinnamaldehyde analogs as inhibitors of ai-2 based quorum sensing and their effect on virulence of Vibrio spp. PLoS One 2011, 6, e16084. [Google Scholar]
- Brackman, G.; Defoirdt, T.; Miyamoto, C.; Bossier, P.; Calenbergh, S.V.; Nelis, H.; Coenye, T. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008, 8, 1–14. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451-1474. https://doi.org/10.3390/ph6121451
Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals. 2013; 6(12):1451-1474. https://doi.org/10.3390/ph6121451
Chicago/Turabian StyleNazzaro, Filomena, Florinda Fratianni, Laura De Martino, Raffaele Coppola, and Vincenzo De Feo. 2013. "Effect of Essential Oils on Pathogenic Bacteria" Pharmaceuticals 6, no. 12: 1451-1474. https://doi.org/10.3390/ph6121451
APA StyleNazzaro, F., Fratianni, F., De Martino, L., Coppola, R., & De Feo, V. (2013). Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals, 6(12), 1451-1474. https://doi.org/10.3390/ph6121451







