Phytochemical Composition, Antioxidant and Xanthine Oxidase Inhibitory Activities of Amaranthus cruentus L. and Amaranthus hybridus L. Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Results
| Species | Moisture (%) | Yields (%) | ||
|---|---|---|---|---|
| HAE | ME | AE | ||
| A. cruentus | 80.86 ± 1.18 | 10.50 | 8.99 | 18.55 |
| A. hybridus | 83.45 ± 0.99 | 8.59 | 7.43 | 12.23 |
| A. cruentus | A. hybridus | ||||||||
| HAE | ME | AE | HAE | ME | AE | ||||
| - Polyphenols and tannins | + | + | + | + | + | + | |||
| - Flavonoids | + | + | - | + | + | - | |||
| - Anthracenosides | - | - | - | - | - | - | |||
| - Coumarins | - | - | - | - | - | - | |||
| - Steroids and triterpenes | + | + | + | + | + | + | |||
| - Iridoïds | + | + | + | + | + | + | |||
| - Cardenolids | + | - | - | + | - | - | |||
| - Carotenoids | + | + | - | + | + | - | |||
| - Sapononins | - | - | + | - | - | + | |||
| - Alkaloids | - | - | - | - | - | - | |||
| WAE | AE | WAE | AE | ||||||
| - Betalains | + | - | + | - | |||||
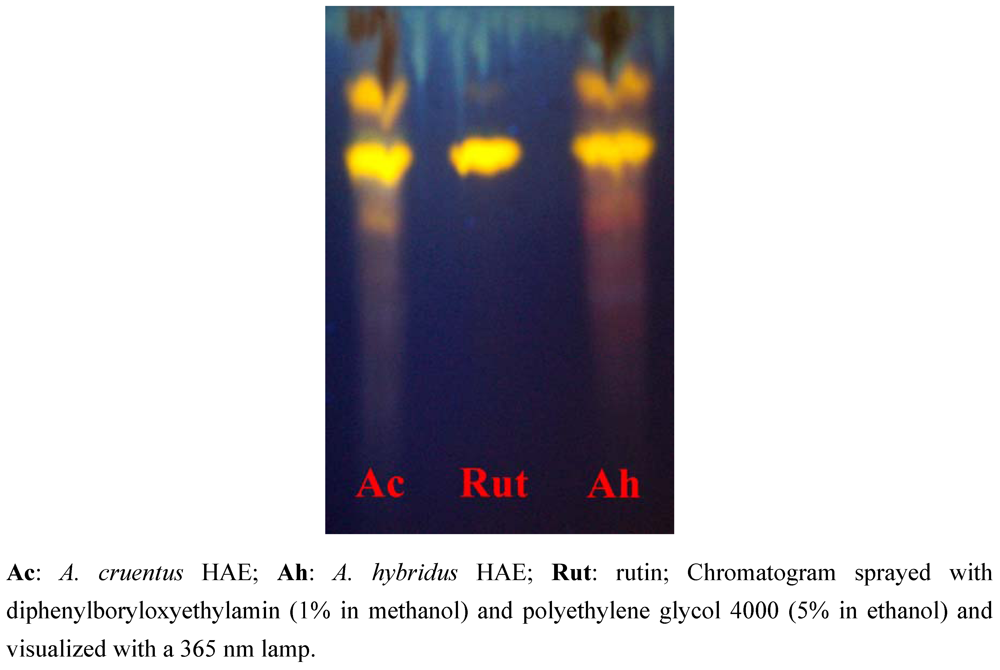
| A. cruentus | A. hybridus | |||
|---|---|---|---|---|
| Extract | λmax (nm) | Abs | λmax (nm) | Abs |
| HAE (1 mg/mL) | 218.6 | 2.468 | 256.1 | 1.755 |
| 257.6 | 1.749 | 355.1 | 1.797 | |
| 355.7 | 1.794 | 536.9 | 0.352 | |
| 386.0 | 1.682 | |||
| 536.6 | 0.312 | |||
| ME (1 mg/mL) | 216.5 | 2.765 | 216.8 | 2.608 |
| 318.5 | 1.524 | 255.5 | 1.734 | |
| 382.1 | 1.706 | 380.6 | 1.703 | |
| 536.9 | 0.327 | 537.2 | 0.361 | |
| AE (10 mg/mL) | 355.1 | 1.858 | 201.2 | 2.734 |
| 361.5 | 1.819 | 355.4 | 1.727 | |
| 372.2 | 1.803 | 366.8 | 1.766 | |
| 674.3 | 0.319 | |||
2.2. Quantification of Phytochemicals
2.2.1. Phenolic Compounds Contents
| A. cruentus | A. hybridus | ||||||
|---|---|---|---|---|---|---|---|
| HAE | ME | AE | HAE | ME | AE | ||
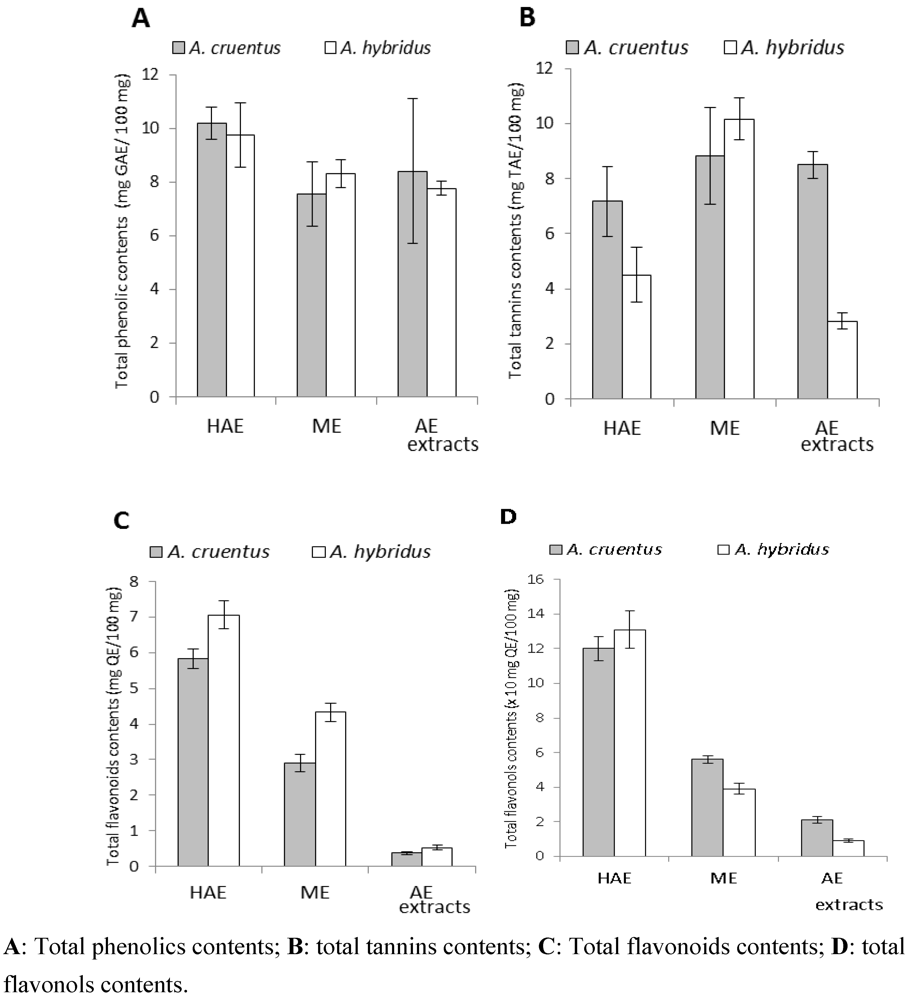
| TP | 10.18 ± 0.60 | 7.55 ± 1.18 | 8.40 ± 2.69 | 9.75 ± 1.21 | 8.30 ± 0.52 | 7.75 ± 0.26 | |
| TT | 7.17 ± 1.26 | 8.83 ± 1.76 | 8.50 ± 0.50 | 4.50 ± 1.00 | 10.17 ± 0.76 | 2.83 ± 0.29 | |
| TF | 5.83 ± 0.27 | 2.90 ± 0.25 | 0.37 ± 0.04 | 7.06 ± 0.39 | 4.33 ± 0.27 | 0.53 ± 0.06 | |
| TFV | 1.20 ± 0.07 | 0.56 ± 0.02 | 0.21 ± 0.02 | 1.31 ± 0.11 | 0.39 ± 0.03 | 0.09 ± 0.01 | |
2.2.2. Betalain Contents
2.3. Antioxidant Activities
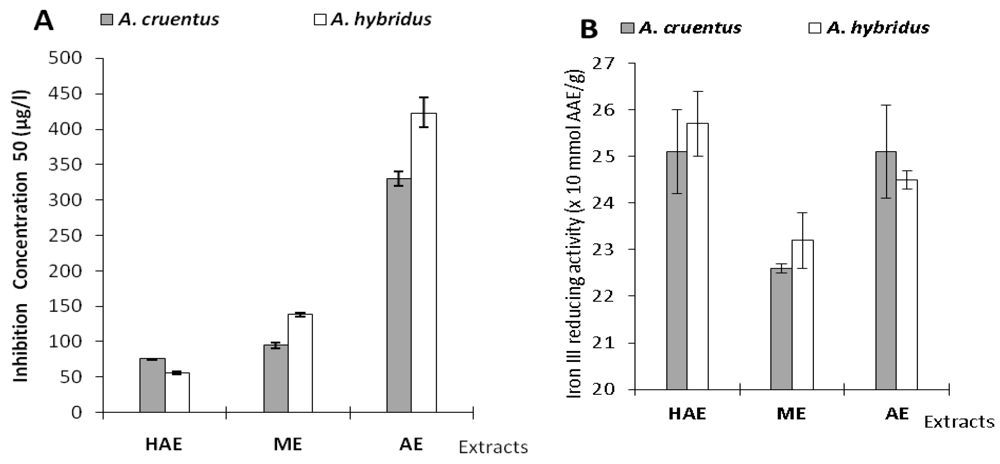
| Samples | A. cruentus | A. hybridus | |||
| DPPH | FRAP | DPPH | FRAP | ||
| HAE | 75.6 ± 0.5 | 2.51 ± 0.09 | 56 ± 2 | 2.57 ± 0.07 | |
| ME | 95 ± 4 | 2.26 ± 0.01 | 138 ± 3 | 2.32 ± 0.06 | |
| AE | 330 ± 10 | 2.51 ± 0.10 | 423 ± 21 | 2.45 ± 0.02 | |
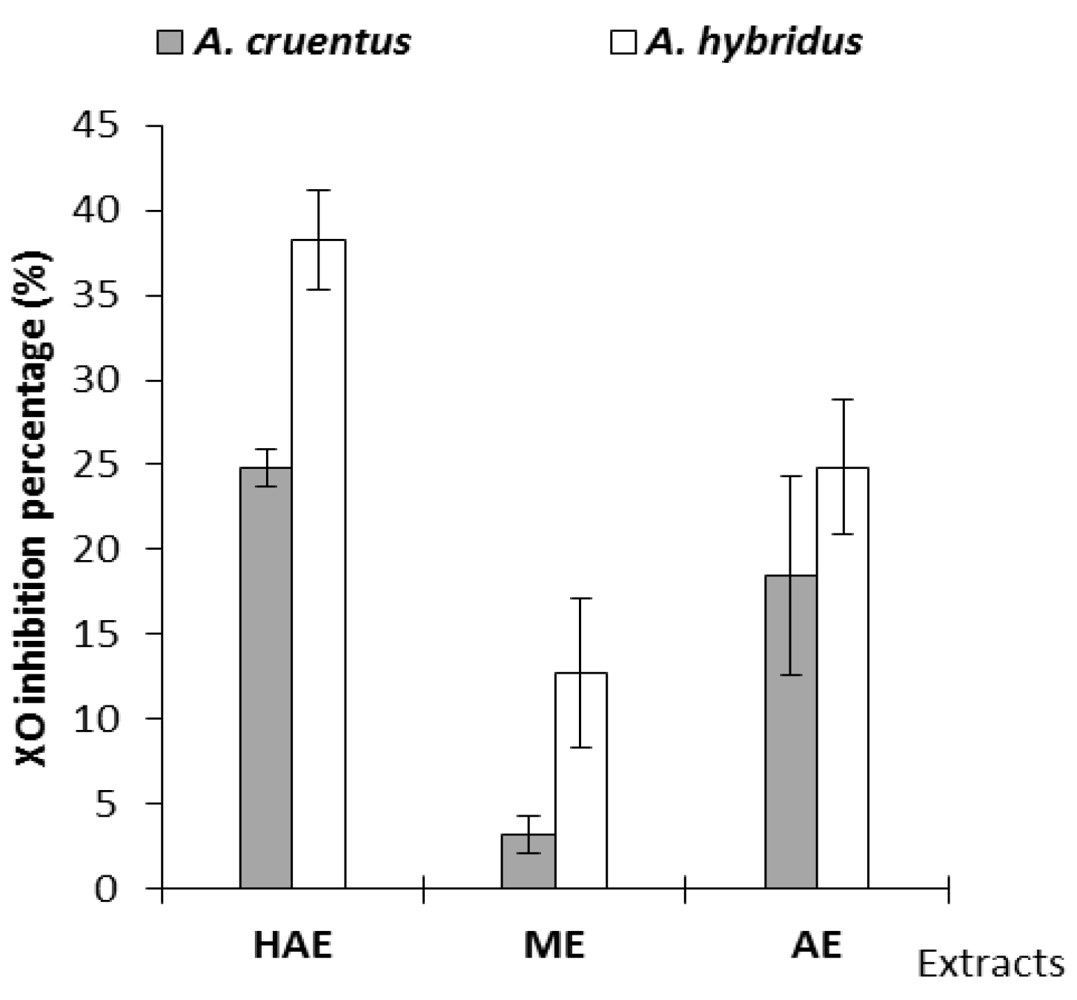
2.4. Xanthine Oxidase (XO) Inhibitory Activity
3. Conclusions
4. Experimental
4.1. Plant Materials
4.2. Extractions
4.3. Chemical Analysis
4.4. Determination of Polyphenol, Flavonoid and Flavonol Contents
4.5. Determination of Tannin Contents
4.6. Determination of Betalain Contents
4.7. Antioxidant Activity Evaluation
4.7.1. DPPH Radical Scavenging Assay
4.7.2. FRAP assay
4.8. Determination of Xanthine Oxidase (XO) Inhibitory Activity
Acknowledgements
Competing of Interests
References
- Hilou, A. Etude phytochimique et activités biologiques d’extraits de deux Caryophyllales à bétalaïnes; Amaranthus spinosus L. (Amaranthaceae) et Boerhaavia erecta (Nyctagynaceae), plantes médicinales du Burkina Faso.
- González, R.; Tosi, E.; Ré, E.; Ańón, M.C.; Pilosof, A.M.R.; Martinez, K. Amaranth starch-rich fraction properties modified by high-temperature heating. Food Chem. 2007, 103, 927–934. [Google Scholar] [CrossRef]
- Tosi, E.A.; Ré, E.; Lucero, H.; Masciarelli, R. Dietary fiber obtained from amaranth (Amaranthus cruentus) grain by differential milling. Food Chem. 2001, 73, 441–443. [Google Scholar] [CrossRef]
- Fasuyi, A.O. Bio-nutritional evaluations of three tropical leaf vegetables (Telfairia occidentalis, Amaranthus cruentus and Talinum triangulare) as sole dietary protein sources in rat assay. Food Chem. 2007, 103, 757–765. [Google Scholar] [CrossRef]
- Fasuyi, A.O. Nutritional potentials of some tropical vegetable leaf meals: chemical characterization and functional properties. Afri. J. Biotechnol. 2006, 5, 049–053. [Google Scholar]
- Odhav, B.; Beekrum, S.; Akula, U.; Baijnath, H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J. Food Comp. Anal. 2007, 20, 430–435. [Google Scholar] [CrossRef]
- Aletor, V.A.; Adeogun, O.A. Nutrient and anti-nutrient components of some tropical leafy vegetables. Food Chem. 1995, 53, 375–379. [Google Scholar] [CrossRef]
- Guerra-Matias, A.C.; Arêas, J.A.G. Glycemic and insulinemic responses in women consuming extruded amaranth (Amaranthus cruentus L.). Nutr. Res. 2005, 25, 815–822. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. Caracterization and application of betalain pigments from plants of the Amaranthaceae. Trends Food Technol. 2005, 16, 370–376. [Google Scholar] [CrossRef]
- Cai, Y.; Corke, H. Amaranthus Betacyanin pigments applied in model food systems. J. Food Sci. 1999, 64, 869–873. [Google Scholar] [CrossRef]
- Nacoulma, O.G. Plantes médicinales et pratiques médicales traditionnelles au Burkina Faso: cas du plateau central.
- Dhellot, J.R.; Matouba, E.; Maloumbi, M.G.; Nzikou, J.M.; Safou Ngoma, D.G.; Linder, M.; Desobry, S.; Parmentier, M. Extraction, chemical composition and nutrional characterization of vegetable oils: Case of Amaranthus hybridus (var 1 and 2) of Congo Brazzaville. Afri. J. Biotechnol. 2006, 5, 1095–1101. [Google Scholar]
- Sun, A.Y.; Simonyi, A.; Sun, G.Y. French paradox and beyond: neuroprotective effects of polyphénols. Free Rad. Biol. Med. 2002, 32, 314–318. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisnero-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity of guava fruit extracts. J. Food Comp. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Sage, R.F.; Sage, T.L.; Pearcy, R.W.; Borsch, T. The taxonomic distribrution of C4 photosynthesis in Amaranthaceae sensu stricto. Am. J. Botany 2007, 94, 1992–2003. [Google Scholar] [CrossRef]
- Steffensen, S.K.; Pedersen, H.A.; Labouriau, R.; Mortensen, A.G.; Laursen, B.; de Troiani, R.M.; Noellemeyer, E.J.; Janovska, D.; Stavelikova, H.; Taberner, A.; Christophersen, C.; Fomsgaard, I.S. Variation of Polyphenols and Betaines in Aerial Parts of Young, Field-Grown Amaranthus Genotypes. J. Agric. Food Chem. 2011, 59, 12073–12082. [Google Scholar]
- Pedersen, H.A.; Steffensen, S.K.; Christophersen, C.; Mortensen, A.G.; Jørgensen, L.N.; Niveyro, S.; de Troiani, R.M.; Rodríguez-Enríquez, R.J.; Barba-de la Rosa, A.P.; Fomsgaard, I.S. Synthesis and quantitation of six phenolic amides in Amaranthus spp. J. Agric. Food Chem. 2010, 58, 6306–6311. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Pagang, G. Structure-Antioxidant activity relationships flavonoïds and phenolic acids. Free Radi. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Nsimba, R.Y.; Kikuzaki, H.; Konishi, Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008, 106, 760–766. [Google Scholar] [CrossRef]
- Hilou, A. Etude de quelques colorants biologiques des plantes du Burkina Faso : cas des bétalaïnes d’ Amaranthus spinosus L. et Boerhaavia erecta L. Mémoire DEA. Université de Ouagadougou, 1997, p. 63.
- Suntornsuk, L. Capillary electrophoresis of phytochemical substances. J. Pharm. Biochem. Anal. 2002, 27, 679–698. [Google Scholar] [CrossRef]
- Schramm, D.D.; German, J.B. Potential effects of flavonoids on the etiology of vascular disease. J. Nutr. Biochem. 1998, 9, 560–566. [Google Scholar] [CrossRef]
- Lamien-Meda, A.; Lamien, C.E.; Compaoré, M.M.Y.; Meda, R.N.T.; Kiendrebeogo, M.; Zeba, B.; Millogo, J.F.; Nacoulma, O.G. Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules 2008, 13, 581–594. [Google Scholar]
- Amin, I.; Norazaidah, Y.; Hainida, K.I.E. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem. 2006, 94, 47–52. [Google Scholar] [CrossRef]
- Steffensen, S.K.; Rinnan, Å.; Mortensen, A.G.; Laursen, B.; de Troiani, R.M.; Noellemeyer, E.J.; Janovska, D.; Dusek, K.; Délano-Frier, J.; Taberner, A.; Christophersen, C.; Fomsgaard, I.S. Polyphenol Content of Seeds from Amaranth. Food Chem. 2011, 129, 131–136. [Google Scholar]
- Galvez, M.; Martin-Cordero, C.; Houghton, P.J.; Ayuso, M.J. Antioxidant activity of methanol extracts obtained from Plantago species. J. Agric. Food Chem. 2005, 53, 1927–1933. [Google Scholar]
- Bruneton, J. Pharmacognosie, phytochimie, plantes médicinales, 2nd ed; Techniques et Documentation Lavoisier, Paris, 1993; p. 217. [Google Scholar]
- Nacoulma-Ouédraogo, O.; Millogo-Rasolodimby, J. Les frotte-dents comme produits cosmétiques et médicinaux au Burkina Faso. Etudes Flor. Vég. Burkina Faso 2002, 7, 49–54. [Google Scholar]
- Sepúlveda-Jiménez, G.; Rueda-Benítez, P.; Porta, H.; Rocha-Sosa, M. Betacyanin synthesis in red beet (Beta vulgaris) leaves induced by wounding and bacterial infiltration is preceded by an oxidative burst. Physiol. Mol. Plant Pathol. 2004, 64, 125–133. [Google Scholar] [CrossRef]
- Sreekanth, D.; Arunasree, M.K.; Roy, K.R.; Reddy, T.C.; Reddy, G.V.; Reddanna, P. Betanin a betacyanin pigment purified from fruits of Opuntia ficus-indica induces apoptosis in human chronic myeloid leukaemia Cell line-K562. Phytomedicine 2007, 14, 739–746. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Hellstrøm, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxydanr activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetables oils and oil fractions using 2,2-diphhenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Samarth, R.M.; Panwar, M.; Kumar, M.; Soni, A.; Kumar, M.; Kumar, A. Evaluation of antioxidant and radical-scavenging activities of certain radioprotective plant extracts. Food Chem. 2008, 106, 868–873. [Google Scholar] [CrossRef]
- Kampkötter, A.; Nkwonkam, C.G.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 2007, 234, 113–123. [Google Scholar] [CrossRef]
- Ferraz-Filha, Z.S.; Violo, I.F.; Fietto, L.G.; Lombardi, J.A.; Saúde-Guimarães, D.A. Xanthine oxidase inhibitory activity of Lychnophora species from Brazil ("Arnica"). J. Ethnopharmacol. 2006, 107, 75–82. [Google Scholar]
- Owen, P.L.; Johns, T. Xanthine oxidase inhibitory activity of northeastern North American plant remedies used for gout. J. Ethnopharmacol. 1999, 64, 149–160. [Google Scholar] [CrossRef]
- Umamaheswari, M.; AsokKumar, K.; Somasundaram, A.; Sivashanmugam, T.; Subhadradevi, V.; Ravi, T.K. Xanthine oxidase inhibitory activity of some Indian medical plants. J. Ethnopharmacol. 2007, 109, 547–551. [Google Scholar] [CrossRef]
- Ciulei, I. Methodology for Analysis of Vegetables Drugs; Ministry of Chemical Industry: Bucarest, Roumania, 1982; p. 67. [Google Scholar]
- Steglich, W.; Strack, D. Betalains, the Alkaloids, Chemistry and Pharmacology; Academic press: Brossi, London, 1990; p. 62. [Google Scholar]
- Wagner, H.; Bladt, S. Plant Drug Analysis. A Thin Layer Chromatography Atlas, 2nd ed; Springer: Berlin, Germany, 1996; p. 384. [Google Scholar]
- Singleton, L.V.; Orthofer, R.; Lamuela-Raventos, R.R. Analysis of total phenol and other oxidation substrates and antioxidants by mean of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Arvouet-Grant, A.; Venant, B.; Pourrat, A.; Legret, P. Standardisation d’un extrait de propolis et identification des principaux constituants. J. de Pharmacie de Belgique 1994, 49, 462–468. [Google Scholar]
- Almaraz-Abarca, N.; Campos, M.G.; Reyes, J.A.A.; Jiménez, N.N.; Corral, J.H.; Valdez, S.G. Antioxydant activity of phenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis julifloral, Leguminoseae). J. Food Compos. Anal. 2007, 20, 119–124. [Google Scholar] [CrossRef]
- Méthodes de référence pour le dosage des tanins (in french). J. officiel des communautés européennes 1984, L197, 18–20.
- Cai, Y.Z.; Corke, H. Effect of postharvest treatments on Amaranthus bétacyanine degradation evaluated by Visible/Near-infrared spectroscopy. J. Food Sci. 2001, 66, 1112–1118. [Google Scholar]
- Schliemann, W.; Cai, Y.; Degenkolb, T.; Schmidt, J.; Corke, H. Betalains of Celosia argentea. Phytochemistry 2001, 58, 159–165. [Google Scholar]
- Vélazquez, E.; Tournier, H.A.; Mordujovich de Buschiazzo, P.; Saavedra, G.; Schinella, G.R. Antioxidant activity of Paraguayan plant extracts. Fitoterapia 2003, 74, 91–97. [Google Scholar] [CrossRef]
- Hinneberg, I.; Dorman, H.J.D.; Hiltunen, R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006, 92, 122–129. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nana, F.W.; Hilou, A.; Millogo, J.F.; Nacoulma, O.G. Phytochemical Composition, Antioxidant and Xanthine Oxidase Inhibitory Activities of Amaranthus cruentus L. and Amaranthus hybridus L. Extracts. Pharmaceuticals 2012, 5, 613-628. https://doi.org/10.3390/ph5060613
Nana FW, Hilou A, Millogo JF, Nacoulma OG. Phytochemical Composition, Antioxidant and Xanthine Oxidase Inhibitory Activities of Amaranthus cruentus L. and Amaranthus hybridus L. Extracts. Pharmaceuticals. 2012; 5(6):613-628. https://doi.org/10.3390/ph5060613
Chicago/Turabian StyleNana, Fernand W., Adama Hilou, Jeanne F. Millogo, and Odile G. Nacoulma. 2012. "Phytochemical Composition, Antioxidant and Xanthine Oxidase Inhibitory Activities of Amaranthus cruentus L. and Amaranthus hybridus L. Extracts" Pharmaceuticals 5, no. 6: 613-628. https://doi.org/10.3390/ph5060613
APA StyleNana, F. W., Hilou, A., Millogo, J. F., & Nacoulma, O. G. (2012). Phytochemical Composition, Antioxidant and Xanthine Oxidase Inhibitory Activities of Amaranthus cruentus L. and Amaranthus hybridus L. Extracts. Pharmaceuticals, 5(6), 613-628. https://doi.org/10.3390/ph5060613




