Intranasal Delivery of Camptothecin-Loaded Tat-Modified Nanomicells for Treatment of Intracranial Brain Tumors
Abstract
:1. Introduction
2. Results
2.1. Characterization
| Polymers | Diameter (nm) | Zeta potential (mV) |
|---|---|---|
| MPEG-PCL | 48.5 ± 3.10 | −1.77 ± 0.78 |
| MPEG-PCL-Tat | 32.1 ± 5.60 | 7.26 ± 2.25 |
| Polymers | Diameter(nm) | Zeta potential (mV) | Encapsulation efficiency of CPT (%) |
|---|---|---|---|
| MPEG-PCL | 102.8 ± 9.07 | −11.7 ± 2.72 | 56.6 ± 12.6 |
| MPEG-PCL-Tat | 88.5 ± 20.2 | 10.4 ± 2.84 | 62.5 ± 9.17 |
2.2. In Vitro Cytotoxicity
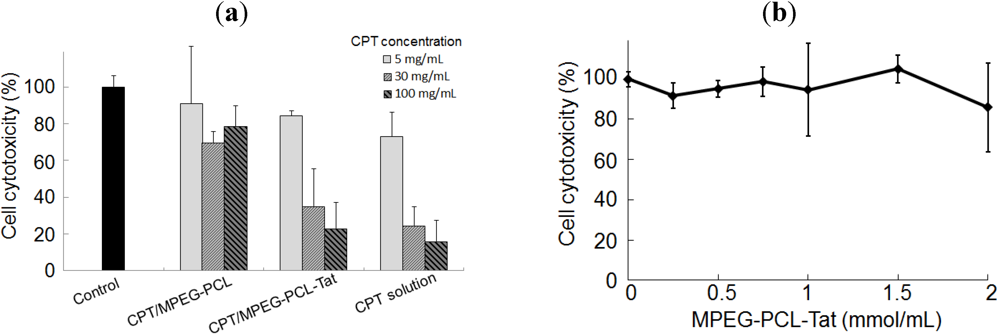
2.3. Therapeutic Effects of Brain Tumor in Glioma Model Rats
| Treatment | Number of rats | Number of long-term survivors | Average of survival period (days) | p value |
|---|---|---|---|---|
| Non-treated | 4 | 0 | 18.2 | |
| CPT solution | 4 | 0 | 23.0 | <0.05 a |
| CPT-loaded MPEG-PCL | 4 | 0 | 22.0 | <0.05 a |
| CPT-loaded MPEG-PCL-Tat | 5 | 1 (>120 days) | 32.6 | <0.01 a, <0.05 b,c |

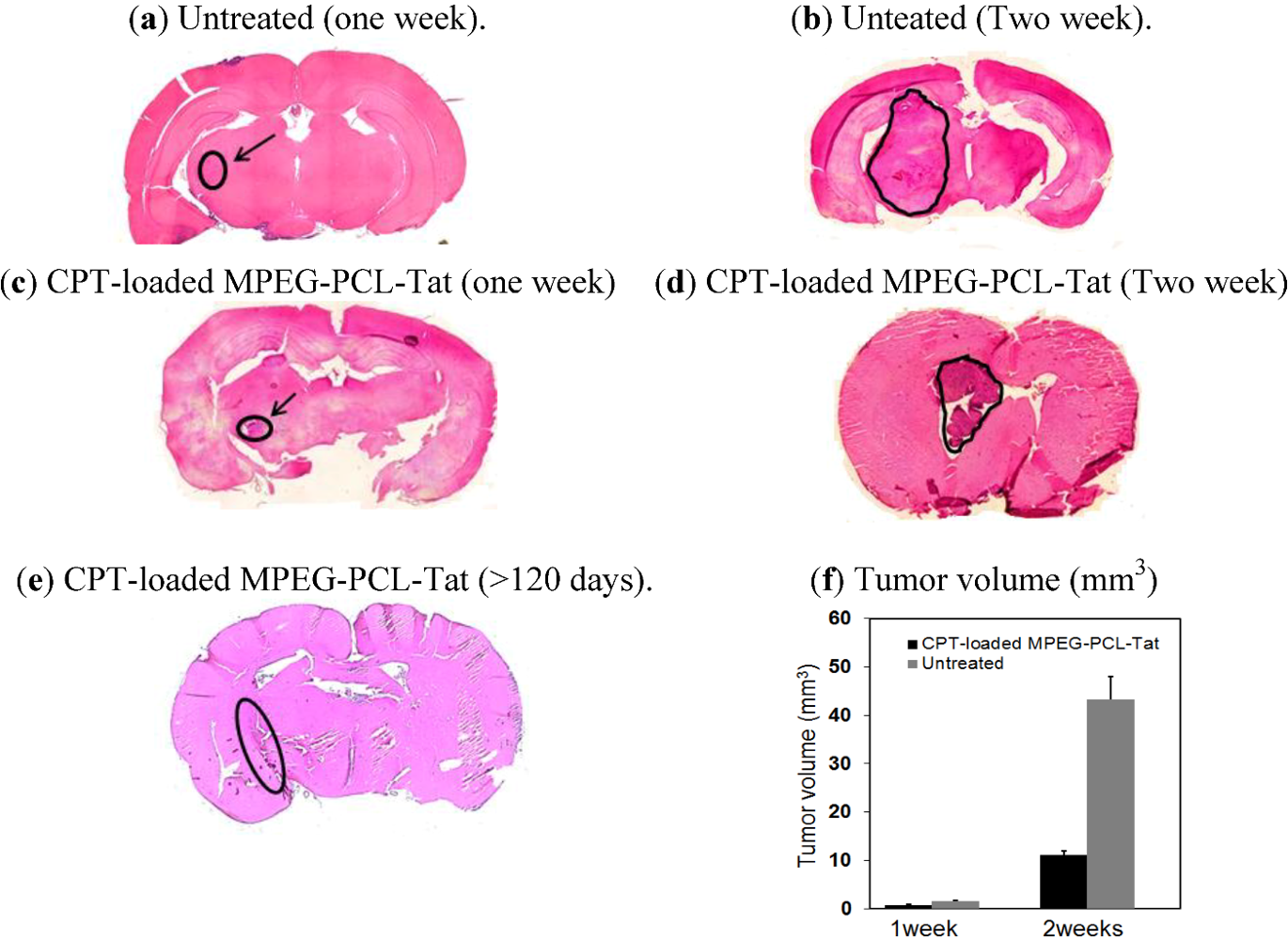
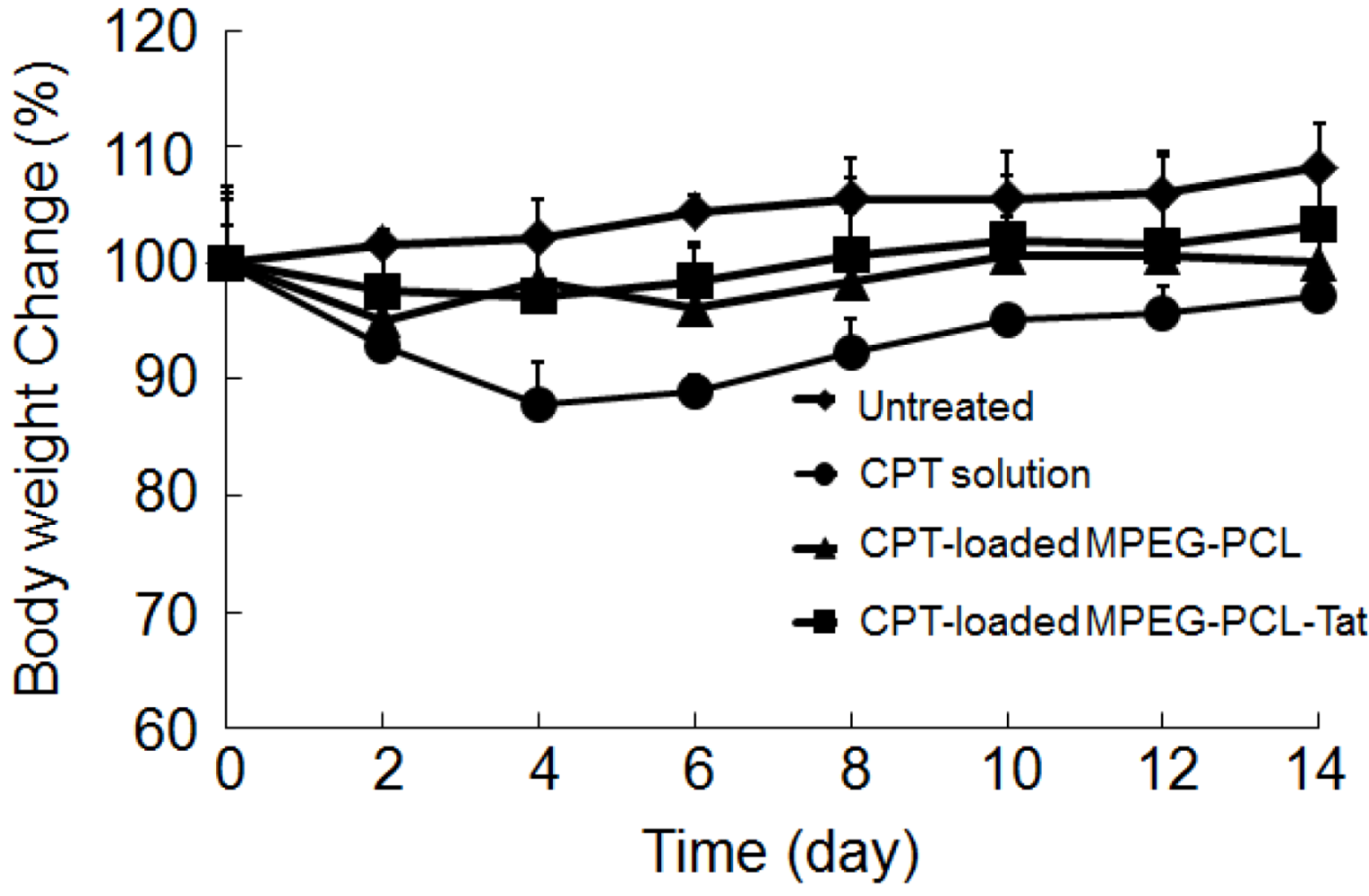
3. Discussion
4. Experimental Section
4.1. Materials, Cells and Rats
4.2. Cell Culture
4.3. Preparation of CPT-Loaded Micelles
4.4. Physiochemical Characterization
4.5. Cytotoxicity in C6 Glioma Cells Transfected with Various CPT Preparations
4.6. Intracranial Model of C6 Glioma
4.7. Therapeutic Efficacy Studies
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef]
- Attenello, F.J.; Mukherjee, D.; Datoo, G.; McGirt, M.J.; Bohan, E.; Weingart, J.D.; Olivi, A.; Quinones-Hinojosa, A.; Brem, H. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: A 10-year institutional experience. Ann. Surg. Oncol. 2009, 15, 2887–2893. [Google Scholar]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Combs, S.E.; Wagner, J.; Bischof, M.; Welzel, T.; Edler, L.; Rausch, R.; Wagner, F.; Zabel-du Bois, A.; Debus, J.; Schulz-Ertner, D. Radiochemotherapy in patients with primary glioblastoma comparing two temozolomide dose regimens. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 999–1005. [Google Scholar] [CrossRef]
- Dagıstan, Y.; Karaca, I.; Bozkurt, E.R.; Ozar, E.; Yagmurlu, K.; Toklu, A.; Bilir, A. Cmbination hyperbaric oxygen and temozolomide therapy in c6 rat glioma model. A. Acta Cir. Bras. 2012, 273, 83–87. [Google Scholar]
- Koo, Y.E.; Reddy, G.R.; Bhojani, M.; Schneider, R.; Philbert, M.A.; Rehemtulla, A.; Ross, BD.; Kopelman, R. Brain cancer diagnosis and therapy with nanoplatforms. Adv. Drug Deliv. Rev. 2006, 58, 1556–1577. [Google Scholar] [CrossRef]
- Pardridge, W.M. BBB-Genomics: Creating new openings for brain-drug targeting. Drug Discov. Today 2001, 6, 381–383. [Google Scholar] [CrossRef]
- Ke, X.Y.; Zhao, B.J.; Zhao, X.; Wang, Y.; Huang, Y.; Chen, X.M.; Zhao, B.X.; Zhao, S.S.; Zhang, X.; Zhang, Q. The therapeutic efficacy of conjugated linoleic acid-paclitaxel on glioma in the rat. Biomaterials 2010, 31, 5855–5864. [Google Scholar]
- Dhanda, D.S.; Frey, W.H., II; Leopold, D.; Kompella, U.B. Approaches for drug deposition in the human olfactory epithelium. Drug Deliv. Technol. 2005, 5, 64–72. [Google Scholar]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey, W.H., 2nd. Delivery of inslin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery-possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Thorne, R.G.; Emory, C.R.; Ala, T.A.; Frey, W.H., 2nd. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995, 692, 278–282. [Google Scholar] [CrossRef]
- Thorne, R.G.; Frey, W.H., 2nd. Delivery of neurotropic factors to the central nervous system: Pharmacokinetic considerations. Clin. Pharmacokinet. 2001, 40, 907–946. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, X.; Lu, W. Profiles of methotrexate in blood and CSF following intranasal and intravenous administration to rats. Int. J. Pharm. 2003, 263, 1–7. [Google Scholar] [CrossRef]
- Shingaki, T.; Yamashita, S.; Sezaki, H.; yoshiharu, T.; Shoubu, S. Transnasal delivery of anticancer drugs to the brain tumor: A new strategy for brain tumor chemotherapy. Deliv. System. 1999, 14, 365–371. [Google Scholar] [CrossRef]
- Sakane, T.; Yamashita, S.; Yata, N.; Sezaki, H. Transnasal delivery of 5-fluorouracil to the brain in the rat. J. Drug Target. 1999, 7, 233–240. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Y.; Yun, L. Study on brain targeting of raltitrexed following intranasal administration in rats. Cancer Chemother. Parmacol. 2006, 57, 97–104. [Google Scholar] [CrossRef]
- Kanazawa, T.; Taki, H.; Tanaka, K.; Takashima, Y.; Okada, H. Cell-penetrating peptide-modified block copolymer micelles promote direct brain delivery via intranasal administration. Pharm. Res. 2011, 28, 2130–2139. [Google Scholar] [CrossRef]
- Tanaka, K.; Kanazawa, T.; Shibata, Y.; Suda, Y.; Fukuda, T.; Takashima, Y.; Okada, H. Development of cell-penetrating peptide-modified MPEG-PCL diblock copolymeric nanoparticles for systemic gene delivery. Int. J. Pharm. 2010, 396, 229–238. [Google Scholar] [CrossRef]
- Ozeki, T.; Hashizawa, K.; Kaneko, D.; Imai, Y.; Okada, H. Treatment of rat brain tumors using sustained-release of camptothecin from poly(lactic-co-glicolic acid) microspheres in thermoreversible hydrogel. Chem. Pharm. Bull. 2010, 58, 1142–1147. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Taki, H.; Kanazawa, T.; Akiyama, F.; Takashima, Y.; Okada, H. Intranasal Delivery of Camptothecin-Loaded Tat-Modified Nanomicells for Treatment of Intracranial Brain Tumors. Pharmaceuticals 2012, 5, 1092-1102. https://doi.org/10.3390/ph5101092
Taki H, Kanazawa T, Akiyama F, Takashima Y, Okada H. Intranasal Delivery of Camptothecin-Loaded Tat-Modified Nanomicells for Treatment of Intracranial Brain Tumors. Pharmaceuticals. 2012; 5(10):1092-1102. https://doi.org/10.3390/ph5101092
Chicago/Turabian StyleTaki, Hiroyuki, Takanori Kanazawa, Fuminari Akiyama, Yuuki Takashima, and Hiroaki Okada. 2012. "Intranasal Delivery of Camptothecin-Loaded Tat-Modified Nanomicells for Treatment of Intracranial Brain Tumors" Pharmaceuticals 5, no. 10: 1092-1102. https://doi.org/10.3390/ph5101092
APA StyleTaki, H., Kanazawa, T., Akiyama, F., Takashima, Y., & Okada, H. (2012). Intranasal Delivery of Camptothecin-Loaded Tat-Modified Nanomicells for Treatment of Intracranial Brain Tumors. Pharmaceuticals, 5(10), 1092-1102. https://doi.org/10.3390/ph5101092




