The Impact of Residual Symptoms in Major Depression
Abstract
:1. Introduction
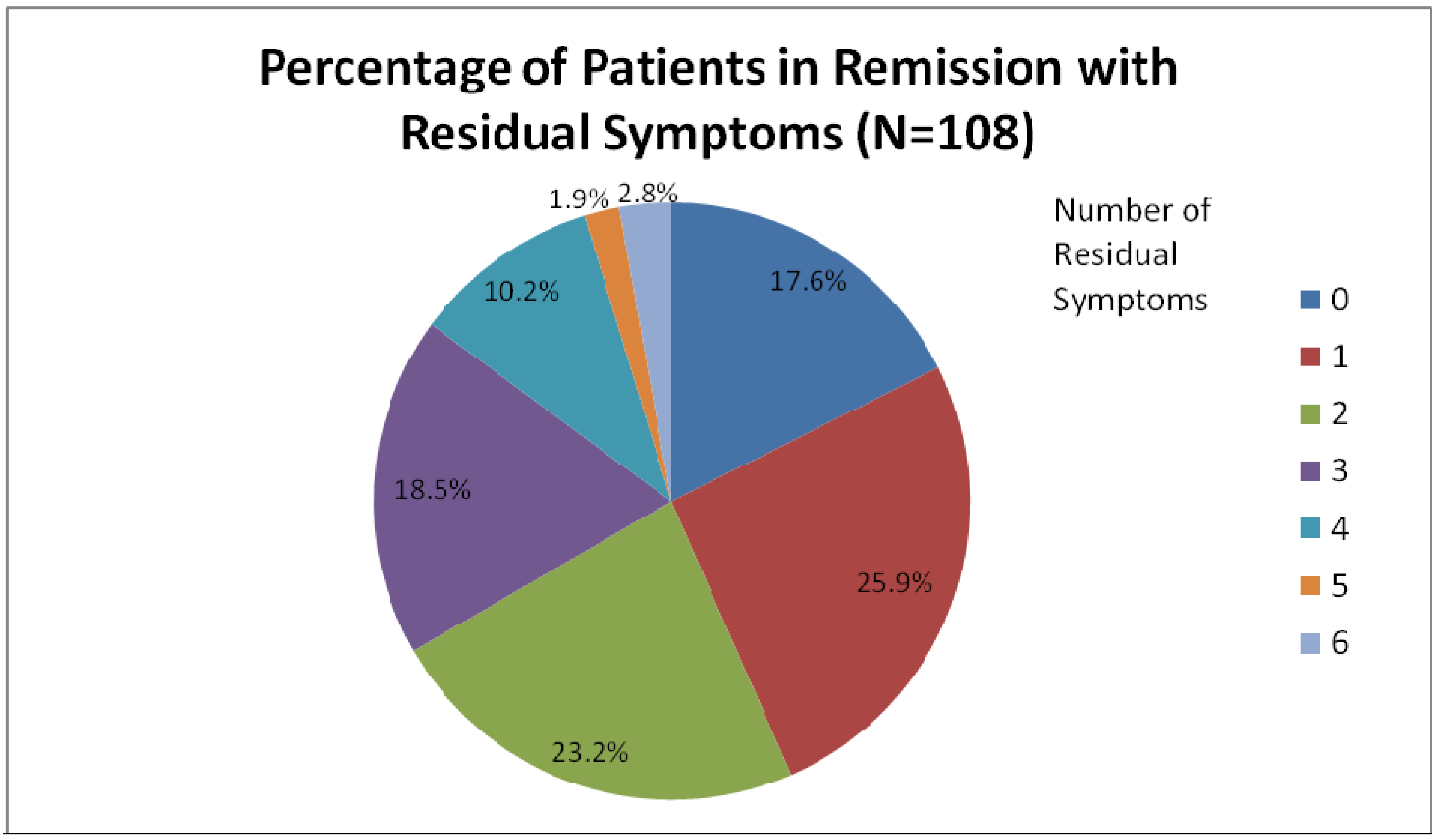
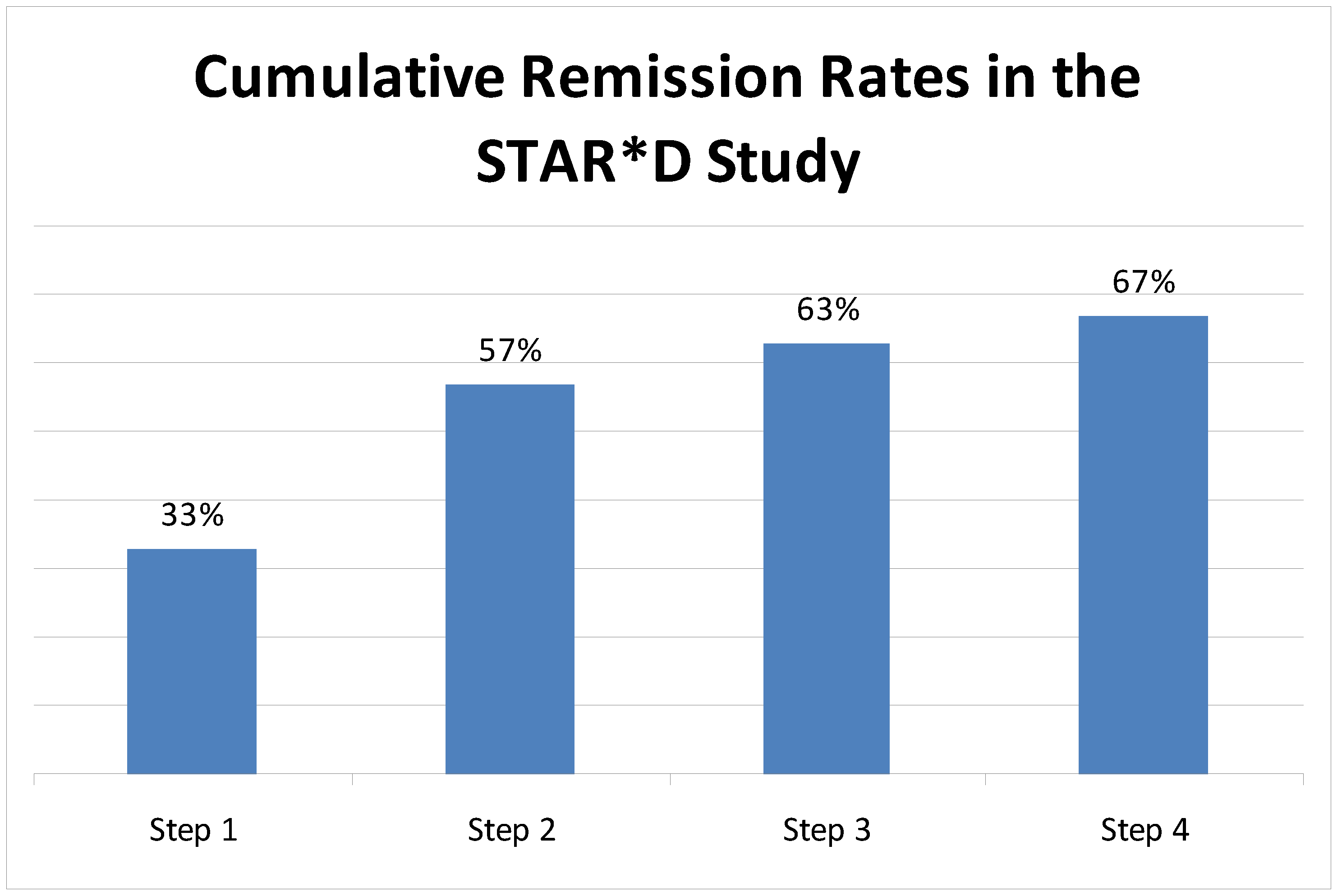
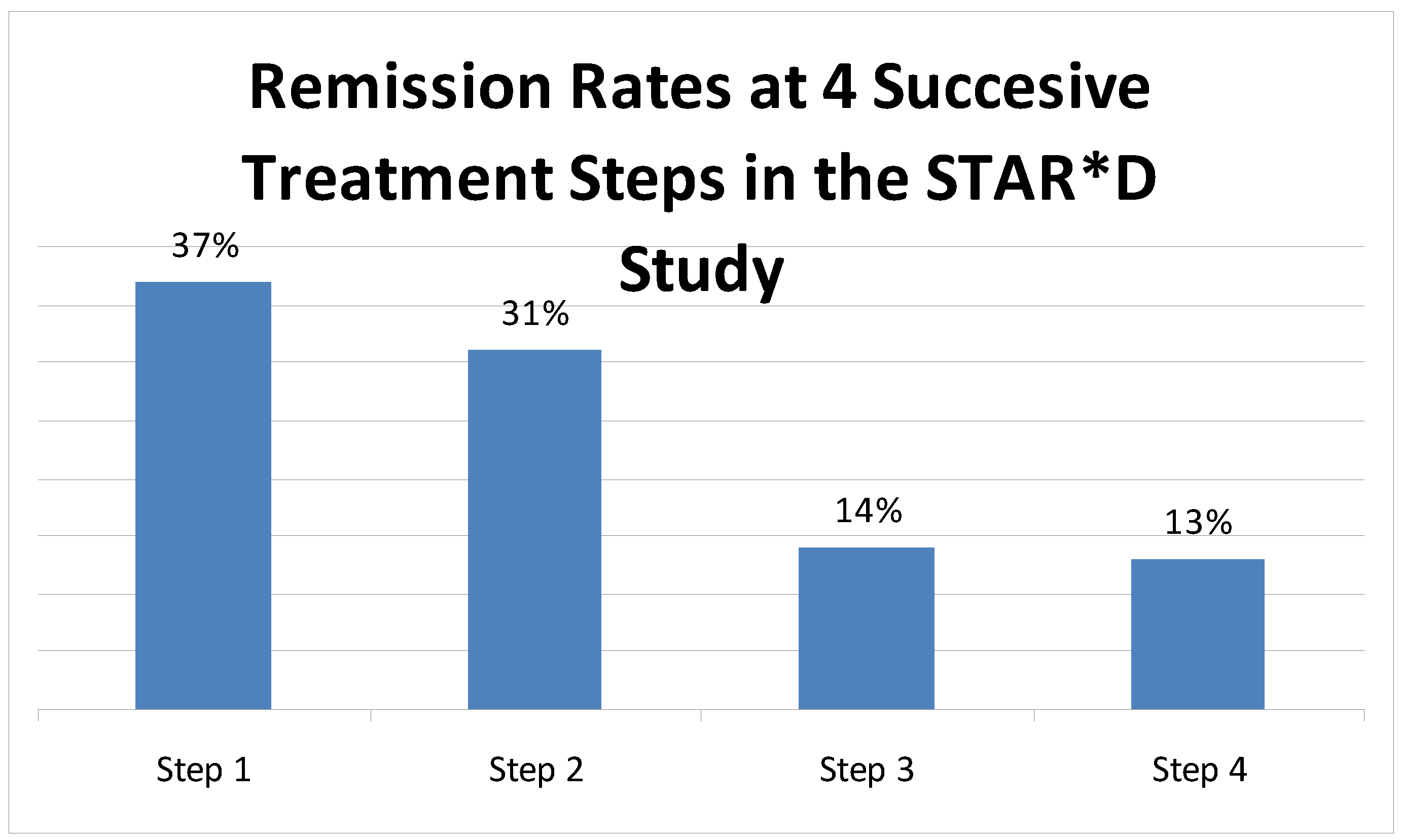
2. Common Residual Symptoms
3. Treatment of Residual Symptoms
| “Run the Axes:” Factors to Consider Before Modifying a Treatment Plan for Major Depressive Disorder | |
|---|---|
| Axis I: | Is the initial diagnosis correct? |
| Axis II: | Could there be occult substance abuse? |
| Is there are a personality disorder that is either co-morbid with, or that appears similar to, a mood disorder? | |
| Axis III: | Is there a complicating medical condition impeding treatment response? |
| Axis IV: | Does the patient have an ongoing life stressor that may limit the benefit that can be expected from pharmacology alone? |
4. Augmentation/Combination
5. Conclusions
Reference
- Trivedi, M.H.; Rush, A.J.; Wisniewski, S.R.; Nierenberg, A.A.; Warden, D.; Ritz, L.; Norquist, G.; Howland, R.H.; Lebowtiz, B.D.; McGrath, P.J.; et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D, implications for clinical practice. Am. J. Psychiatry 2006, 163, 28–40. [Google Scholar] [PubMed]
- Israel, J.A. Remission in depression, definition and initial treatment approaches. J. Psychopharmacol. 2006, 20, 5–10. [Google Scholar]
- Keller, M.B. Past, Present, and Future Directions for Defining Optimal Treatment Outcome in Depression, Remission and Beyond. JAMA 2003, 289, 3152–3160. [Google Scholar]
- Lecrubier, Y. How do you define remission? Acta Psychiatr. Scand. 2002, 415, 7–11. [Google Scholar] [CrossRef]
- Frank, E.; Prien, R.F.; Jarrett, R.B.; Keller, M.B.; Kupfer, D.J.; Lavori, P.W.; Rush, A.J.; Weissman, M.M. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch. Gen. Psychiatry 1991, 48, 851–855. [Google Scholar] [PubMed]
- Nierenberg, A.A.; DeCecco, L.M. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes, a focus on treatment-resistant depression. J. Clin. Psychiatry 2001, 62, 5–9. [Google Scholar]
- Cuffel, B.J.; Azocar, F.; Tomlin, M.; Greenfield, S.F.; Busch, A.B.; Croghan, T.W. Remission, residual symptoms, and nonresponse in the usual treatment of major depression in managed Clinical Practice. J. Clin. Psychiatry 2003, 64, 397–402. [Google Scholar]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps, a STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [PubMed]
- Rush, A.J.; Kraemer, H.C.; Sackeim, H.A.; Fava, M.; Trivedi, M.D.; Frank, E.; Ninan, P.T.; Thase, M.E.; Gelenberg, A.J.; Kupfer, D.J.; Regier, D.A.; Rosenbaum, J.F.; Ray, O.; Schatzberg, A.F. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 2006, 31, 1841–1853. [Google Scholar] [PubMed]
- Zimmerman, M.; McGlinchey, J.B.; Posternak, M.A.; Friedman, M.; Attiullah, N.; Boerescu, D. How should remission from depression be defined? The depressed patient's perspective. Am. J. Psychiatry 2006, 163, 148–50. [Google Scholar] [PubMed]
- Keller, M.B. Remission versus response, the new gold standard of antidepressant care. J Clin Psychiatry 2004, 65 (Suppl. 4), 53–9. [Google Scholar] [PubMed]
- Zimmerman, M.; Posternak, M.A.; Chelminski, I. Heterogeneity among depressed outpatients considered to be in remission. Compr. Psychiatry 2007, 48, 113–117. [Google Scholar]
- Fava, G.A.; Grandi, S.; Zielezny, M.; Canestrari, R.; Morphy, M.A. Cognitive behavioral treatment of residual symptoms in primary major depressive disorder. Am. J. Psychiatry 1994, 151, 1295–1299. [Google Scholar] [PubMed]
- Gastó, C.; Navarro, V.; Catalán, R.; Portella, M.J.; Marcos, T. Residual symptoms in elderly major depression remitters. Acta Psychiatr. Scand. 2003, 108, 15–19. [Google Scholar]
- Nierenberg, A.A.; Keefe, B.R.; Leslie, V.C.; Alpert, J.E.; Pava, J.A.; Worthington, J.J.; Rosenbaum, J.F.; Fava, M. Residual symptoms in depressed patients who respond acutely to fluoxetine. J. Clin. Psychiatry 1999, 60, 221–225. [Google Scholar]
- Quitkin, F.M.; Petkova, E.; McGrath, P.J.; Taylor, B.; Beasley, C.; Stewart, J.; Amsterdam, J.; Fava, M.; Rosenbaum, J.; Reimherr, F.; Fawcett, J.; Chen, Y.; Klein, D. When should a trial of fluoxetine for major depression be declared failed? Am. J. Psychiatry 2003, 160, 734–740. [Google Scholar] [PubMed]
- Mojtabai, R. Residual Symptoms and Impairment in Major Depression in the Community. Am. J.Psychiatry 2001, 158, 1645–1651. [Google Scholar]
- Boulenger, J.P. Residual symptoms of depression, Clinical and theoretical implications. Eur. Psychiatry 2004, 19, 209–213. [Google Scholar]
- Judd, L.L.; Akiskal, H.S.; Paulus, M.P. The role and Clnical significance of subsyndromal depressive symptoms in unipolar major depressive disorder. J. Affect. Disord. 1997, 45, 5–17. [Google Scholar]
- Pintor, L.; Torres, X.; Navarro, V.; Matrai, S.; Gastó, C. Is the type of remission after a major depressive episode an important risk factor to relapses in a 4-year follow up? J. Affect. Disord. 2004, 82, 291–296. [Google Scholar]
- Paykel, E.S.; Ramana, R.; Cooper, Z.; Hayhurst, H.; Kerr, J.; Barocka, A. Residual symptoms after partial remission, an important outcome in depression. Psychol. Med. 1995, 25, 1171–1180. [Google Scholar]
- Judd, L.L.; Akiskal, H.S.; Maser, J.D.; Zeller, P.J.; Endicott, J.; Coryell, W.; Paulus, M.P.; Kunovac, J.L.; Leon, A.C.; Mueller, T.I.; Rice, J.A.; Keller, M.B. Major depressive disorder, A prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J. Affect. Disord. 1998, 50, 97–108. [Google Scholar]
- Thase, M.E.; Simons, A.P.; McGeary, J.; Cahalane, J.F.; Hughes, C.; Harden, T.; Friedman, E. Relapse after cognitive behavior therapy of depression, potential implications for longer courses of treatment. Am. J. Psychiatry 1992, 149, 1046–52. [Google Scholar]
- Judd, L.L.; Paulus, M.J.; Schettler, P.J.; Akiskal, H.S.; Endicott, J.; Leon, A.C.; Maser, J.D.; Mueller, T.; Solomon, D.A.; Keller, M.B. Does Incomplete Recovery From First Lifetime Major Depressive Episode Herald a Chronic Course of Illness? Am. J. Psychiatry 2000, 157, 1501–1504. [Google Scholar] [PubMed]
- Carney, C.E.; Segal, Z.V.; Edinger, J.D.; Krystal, A.D. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J. Clin. Psychiatry 2007, 68, 254–260. [Google Scholar]
- Nierenberg, A.A.; Keefe, B.R.; Leslie, V.C.; Alpert, J.E.; Pava, J.A.; Worthington, J.J.; Rosenbaum, J.F.; Fava, M. Residual symptoms in depressed patients who respond acutely to fluoxetine. J. Clin. Psychiatry 1999, 60, 221–225. [Google Scholar]
- Opdyke, K.S.; Reynolds, C.F.; Frank, E.; Begley, A.E.; Buysse, D.J.; Dew, M.A.; Mulsant, B.H.; Shear, M.K.; Mazumdar, S.; Kupfer, D.J. Effect of continuation treatment on residual symptoms in late-life depression, how well is “well”? Depress. Anxiety 1996/97, 4, 312–319. [Google Scholar] [PubMed]
- Merens, W.; Booij, L.; Van Der Does, A.J. Residual cognitive impairments in remitted depressed patients. Depress. Anxiety 2007, 25, E27–36. [Google Scholar]
- Ohayon, M.M.; Roth, T. Place of chronic insomnia in the course of depressive and anxiety disorders. J. Psychiatr. Res. 2003, 37, 9–15. [Google Scholar]
- Dombrovski, A.Y.; Cyranowski, J.M.; Mulsant, B.H.; Houck, P.R.; Buysse, D.J.; Andreescu, C.; Thase, M.E.; Mallinger, A.G.; Frank, E. Which symptoms predict recurrence of depression in women treated with maintenance interpersonal psychotherapy? Depress. Anxiety 2008, 25, 1060–1066. [Google Scholar]
- Thase, M.E.; Simons, A.D.; Reynolds, C.F. Abnormal electroencephalographic sleep profiles in major depression, association with response to cognitive behavior therapy. Arch. Gen. Psychiatry 1996, 53, 99–108. [Google Scholar]
- Dombrovski, B.; Mulsant, P.; Houck, S.; Mazumdar, S.; Lenze, E.J.; Andreescu, C.; Cyranowski, J.M.; Reynolds, C.F. Residual symptoms and recurrence during maintenance treatment of late-life depression. J.Affect. Disord. 2007, 103, 77–82. [Google Scholar]
- Kupfer, D.J.; Frank, E.; McEachran, A.B.; Grochocinski, V.J. Delta sleep ratio, a biological correlate of early recurrence in unipolar affective disorder. Arch. Gen. Psychiatry 1990, 47, 1100–1105. [Google Scholar]
- Dew, M.A.; Reynolds, C.F.; Houck, P.R.; Hall, M.; Buysse, D.J.; Frank, E.; Kupfer, D.J. Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Arch. Gen. Psychiatry 1997, 54, 1016–1024. [Google Scholar] [PubMed]
- Bernert, R.A.; Joiner, T.E. Sleep disturbances and suicide risk, A review of the literature. Neuropsychiatr. Dis. Treat. 2007, 3, 737–743. [Google Scholar]
- Andreescu, C.; Lenze, E.J.; Dew, M.A.; Begley, A.E.; Mulsant, B.H.; Dombrovski, A.Y.; Pollock, B.G.; Stack, J.; Miller., M.D.; Reynolds, C.F. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression, controlled study. Br. J. Psychiatry 2007, 190, 344–349. [Google Scholar]
- Hybels, C.F.; Steffens, D.C.; McQuoid, D.R.; Rama Krishnan, K.R. Residual symptoms in older patients treated for major depression. Int. J. Geriatr. Psychiatry 2005, 20, 1196–202. [Google Scholar]
- Helmchen, H.; Linden, M. Subthreshold disorders in psychiatry, clinical reality, methodological artifact, and the double-threshold problem. Compr. Psychiatry 2000, 41, 1–7. [Google Scholar]
- Barlow, D.H.; Campbel, L.A. Mixed anxiety–depression and its implications for models of mood and anxiety disorders. Compr. Psychiatry 2000, 41, 55–60. [Google Scholar]
- Fava, G.A.; Grandi, S.; Canestrari, R.; Molnar, G. Prodromal symptoms in primary major depressive disorder. J. Affect. Disord. 1990, 19, 149–152. [Google Scholar]
- Tranter, R.; O’Donovan, C.; Chandarana, P.; Kennedy, S. Prevalence and outcome of partial remission in depression. J. Psychiatry Neurosci. 2002, 27, 241–247. [Google Scholar]
- Keller, M.B.; Hirschfeld, R.M.; Hanks, D. Double depression, a distinctive subtype of unipolar depression. J. Affect. Disord. 1997, 45, 65–73. [Google Scholar]
- Gilmer, W.S.; Gollan, J.K.; Wisniewski, S.R.; Howland, R.H.; Trivedi, M.H.; Miyahara, S.; Fleck, J.; Thase, M.E.; Alpert, J.E.; Nierenberg, A.A.; et al. Does the Duration of the Index Episode Affect the Treatment Outcome of Major Depressive Disorder? A STAR*D Report. J. Clin. Psychiatry 2007, 69, 1246–1256. [Google Scholar]
- Keller, M.B.; Boland, R.J. The implications of failing to achieve successful long–term maintenance treatment of recurrent unipolar major depression. Biol. Psychiatry 1998, 44, 348–360. [Google Scholar]
- Fava, M.; Bouffides, E.; Pava, J.A.; McCarthy, M.K.; Steingard, R.J.; Rosenbaum, J.F. Personality disorder comorbidity with major depression and response to fluoxetine treatment. Psychother. Psychosom. 1994, 62, 160–167. [Google Scholar]
- Peselow, E.D.; Sanfilipo, M.P.; Fieve, R.R.; Gulbenkian, G. Personality traits during depression and after Clinical recovery. Br. J. Psychiatry 1994, 164, 349–354. [Google Scholar]
- Williams, J.M.; Healy, D.; Teasdale, J.D.; White, W.; Paykel, E.S. Dysfunctional attitudes and vulnerability to persistent depression. Psychol. Med. 1990, 20, 375–381. [Google Scholar]
- Fava, G.A.; Rafanelli, C.; Grandi, S.; Canestrari, R.; Morphy, M.A. Six-Year Outcome for Cognitive Behavioral Treatment of Residual Symptoms in Major Depression. Am. J. Psychiatry 1998, 155, 1443–1445. [Google Scholar]
- Horsten, M.; Mittleman, M.A.; Wamala, S.P.; Schenck-Gustafsson, K.; Orth-Gomer, K. Depressive symptoms and lack of social integration in relation to prognosis of CHD in middle-aged women. The Stockholm Female Coronary Risk Study. Eur. Heart J. 2000, 21, 1072–1080. [Google Scholar]
- Jonas, B.S.; Mussolino, M.E. Symptoms of depression as a prospective risk factor for stroke. Psychosom. Med. 2000, 62, 463–471. [Google Scholar]
- Gastó, C.; Navarro, V.; Catalán, R.; Portella, M.J.; Marcos, T. Residual symptoms in elderly major depression remitters. Acta. Psychiatr. Scand. 2003, 108, 15–19. [Google Scholar]
- Mintz, J.; Mintz, L.I.; Arruda, M.J.; Hwang, S.S. Treatments of depression and the functional capacity to work. Arch. Gen. Psychiatry 1992, 49, 761–768. [Google Scholar]
- Revicki, D.A.; Brown, R.E.; Palmer, W.; Bakish, D.; Rosser, W.W.; Anton, S.F.; Feeny, D. Modeling the cost effectiveness of antidepressant treatment in primary care. Pharmacoeconomics. 1995, 8, 524–540. [Google Scholar]
- Kennedy, N.; Paykel, E.S. Residual symptoms at remission from depression, impact on long-term outcome. J. Affect. Disord. 2004, 80, 135–144. [Google Scholar]
- Hirschfeld, R.M.; Keller, M.B.; Panico, S.; Arons, B.S.; Barlow, D.; Davidoff, F.; Endicott, J.; Froom, J.; Goldstein, M.; Gorman, J.M.; et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA 1997, 277, 333–340. [Google Scholar] [PubMed]
- Sheline, Y.I.; Sanghavi, M.; Mintun, M.; Gado, M.H. Depression Duration But Not Age Predicts Hippocampal Volume Loss in Medically Healthy Women with Recurrent Major Depression. J. Neurosci. 1999, 19, 5034–5043. [Google Scholar]
- Trivedi, M.H.; Hollander, E.; Nutt, D.; Blier, P. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J. Clin. Psychiatry 2007, 69, 246–258. [Google Scholar]
- Trivedi, M.H. Major depressive disorder, remission of associated symptoms. J. Clin. Psychiatry 2006, 67, 27–32. [Google Scholar]
- Belmaker, R.H.; Agam, G. Major depressive disorder. New Engl. J. Med. 2007, 358, 55–68. [Google Scholar]
- Keitner, G.I.; Solomon, D.A.; Ryan, C.E.; STAR*D Team. Have We Learned the Right Lessons? Am. J. Psychiatry 2007, 165, 133. [Google Scholar]
- Kennedy, N.; Abbott, R.; Paykel, E.S. Remission and recurrence of depression in the maintenance era, long-term outcome in a Cambridge cohort. Psychol. Med. 2003, 33, 827–838. [Google Scholar]
- Kessing, L.V.; Hansen, M.G.; Andersen, P.K. Course of illness in depressive and bipolar disorders, naturalistic study, 1994–1999. Br. J. Psychiatry 2004, 185, 372–377. [Google Scholar]
- Moller, H.J. Outcomes in major depressive disorder: the evolving concept of remission and its implications for treatment. World J. Biol. Psychiatry 2007, 9, 102–14. [Google Scholar]
- Israel, J.A. Polypharmacy in Depression. Psychiatric Times 2007, 25, 16–20. [Google Scholar]
- Thase, M.E. Achieving remission and managing relapse in depression. J. Clin. Psychiatry 2003, 64, 3–7. [Google Scholar]
- Souery, D.; Mendlewicz, J. Compliance and therapeutic issues in resistant depression. Int. Clin. Psychopharmacol. 1998, 13, S8–S13. [Google Scholar]
- Fava, M.; Alpert, J.; Nierenberg, A.A.; Lagomasino, I.; Sonawalla, S.; Tedlow, J.; Worthington, J.; Baer, L.; Rosenbaum, J.F. Double-blind study of high-dose fluoxetine versus lithium or desipramine augmentation of fluoxetine in partial responders and nonresponders to fluoxetine. J. Clin. Psychopharmacol. 2002, 22, 379–387. [Google Scholar]
- Kingston, T.; Dooley, B.; Bates, A.; Lawlor, A.; Malone, K.E. Mindfulness-based cognitive therapy for residual depressive symptoms. Psychol. Psychother. Theory Res. Pract. 2007, 80, 193–203. [Google Scholar]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Stewart, J.W.; Nierenberg, A.A.; Thase, M.E.; Ritz, L.; Biggs, M.M.; Warden, D.; Luther, J.F.; Shores-Wilson, K.; et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. New Engl. J. Med. 2006, 354, 1231–1242. [Google Scholar]
- Fava, M.; Rush, A.J. Current status of augmentation and combination treatments for major depressive disorder, a literature review and a proposal for a novel approach to improve practice. Psychother. Pychosom. 2006, 75, 139–153. [Google Scholar]
- Heninger, G.R.; Charney, D.S.; Sternberg, D.E. Lithium carbonate augmentation of antidepressant action, An effective prescription for treatment-refractory depression. Arch. Gen. Psychiatry 1983, 40, 1335–1342. [Google Scholar] [PubMed]
- Bauer, M.; Dopfmer, S. Lithium augmentation in treatment-resistant depression, meta-analysis of placebo-controlled studies. J. Clin. Psychopharmacol. 1999, 19, 427–434. [Google Scholar]
- Nierenberg, A.A.; Fava, M.; Trivedi, M.H.; Wisniewski, S.R.; Thase, M.E.; McGrath, P.J.; Alpert, J.E.; Warden, D.; Luther, J.F.; Niederehe, G.; et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression, a STAR*D report. Am. J. Psychiatry 2006, 163, 1519–1530. [Google Scholar] [PubMed]
- Joffe, R.T.; Singer, W. A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res. 1990, 32, 241–251. [Google Scholar]
- Valenstein, M.; McCarthy, J.F.; Austin, K.L.; Greden, J.F.; Young, E.A.; Blow, F.C. What happened to lithium? Antidepressant augmentation in clinical settings. Am. J. Psychiatry 2006, 163, 1219–1225. [Google Scholar]
- Trivedi, M.H.; Fava, M.; Wisniewski, S.R.; Thase, M.E.; Quitkin, F.; Warden, D.; Ritz, L.; Nierenberg, A.A.; Lebowitz, B.D.; Biggs, M.M.; Luther, J.F. Medication augmentation after the failure of SSRIs for depression. New Eegl. J. Med. 2006, 354, 1243–1252. [Google Scholar]
- Zimmerman, M.; Posternak, M.A.; Attiullah, N.; Friedman, M.; Boland, R.J.; Baymiller, S.; Berlowitz, S.L.; Rahman, S.; Uy, K.K.; Singer, S.; Chelminski, I. Why isn't bupropion the most frequently prescribed antidepressant? J. Clin. Psychiatry 2005, 66, 603–610. [Google Scholar] [PubMed]
- DeBattista, C.; Lembke, A. Update on augmentation of antidepressant response in resistant depression. Curr. Psychiatry Rep. 2005, 7, 435–440. [Google Scholar]
- Carpenter, L.L.; Yasmin, S.; Price, L.H. A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol. Psychiatry 2002, 51, 183–188. [Google Scholar]
- Fava, M.; Rush, A.J.; Wisniewski, S.R.; Nierenberg, A.A.; Alpert, J.E.; McGrath, P.J.; Thase, M.E.; Warden, D.; Biggs, M.; Luther, J.F.; et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients, a STAR*D report. Am. J. Psychiatry 2006, 163, 1161–1172. [Google Scholar] [PubMed]
- Warden, D.; Rush, A.J.; Trivedi, M.H.; Fava, M.; Wisniewski, S.R. The STAR*D Project results: a comprehensive review of findings. Curr. Psychiatry Rep. 2007, 9, 449–459. [Google Scholar]
- McCall, W.V. What Does Star*D Tell Us about ECT? J. ECT 2007, 23, 1–2. [Google Scholar]
- Lisanby, S.H. Electroconvulsive Therapy for Depression. New Engl. J. Med. 2007, 357, 1939–1945. [Google Scholar]
- Fink, M.; Taylor, M.A. Electroconvulsive Therapy, Evidence and Challenges. JAMA 2007, 298, 330–332. [Google Scholar]
- Carpenter, L.L. Neurostimulation in resistant depression. J. Psychopharmacol. 2006, 20, 35–40. [Google Scholar]
- DeBattista, C.; Doghramji, K.; Menza, M.A.; Rosenthal, M.H.; Fieve, R.R. Adjunct modafinil for the short-term treatment of fatigue and sleepiness in patients with major depressive disorder, a preliminary double-blind, placebo-controlled study. J. Clin. Psychiatry 2003, 64, 1057–1064. [Google Scholar]
- Fava, M. Augmenting antidepressants with folate, a clinical perspective. J. Clin. Psychiatry 2007, 68, 4–7. [Google Scholar]
- Papakostas, G.I.; Petersen, T.J.; Kinrys, G.; Burns, A.M.; Worthington, J.J.; Alpert, J.E.; Fava, M.; Nierenberg, A.A. Aripiprazole augmentation of selective serotonin reuptake inhibitors for treatment-resistant major depressive disorder. J. Clin. Psychiatry 2005, 66, 1326–1330. [Google Scholar]
- Papakostas, G.I.; Petersen, T.J.; Nierenberg, A.A.; Murakami, J.L.; Alpert, J.E.; Rosenbaum, J.F.; Fava, M. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J. Clin. Psychiatry 2004, 65, 217–221. [Google Scholar]
- Henderson, D.C.; Doraiswamy, P.M. Prolactin-related and metabolic adverse effects of atypical antipsychotic agents. J. Clin. Psychiatry 2007, 69, 32–44. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Israel, J.A. The Impact of Residual Symptoms in Major Depression. Pharmaceuticals 2010, 3, 2426-2440. https://doi.org/10.3390/ph3082426
Israel JA. The Impact of Residual Symptoms in Major Depression. Pharmaceuticals. 2010; 3(8):2426-2440. https://doi.org/10.3390/ph3082426
Chicago/Turabian StyleIsrael, Joshua A. 2010. "The Impact of Residual Symptoms in Major Depression" Pharmaceuticals 3, no. 8: 2426-2440. https://doi.org/10.3390/ph3082426
APA StyleIsrael, J. A. (2010). The Impact of Residual Symptoms in Major Depression. Pharmaceuticals, 3(8), 2426-2440. https://doi.org/10.3390/ph3082426




