Non Steroidal Anti-Inflammatory Drugs and Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Pathogenesis of NSAIDS Induced GI Toxicity
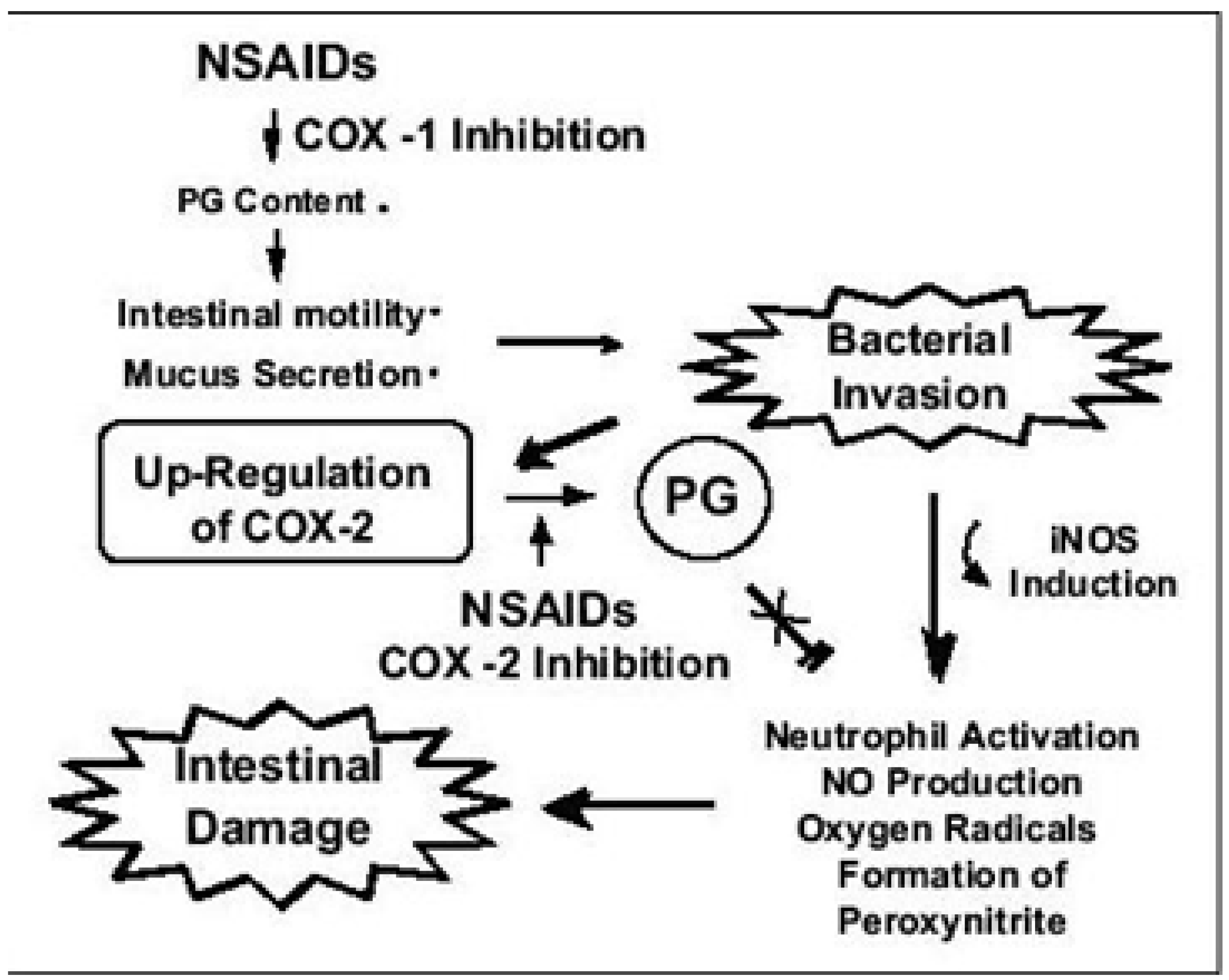
| Mechanism of action |
|---|
Prostaglandin synthesis reduction - Reduced COX1 and COX2 induced PGE2 and TXA2 production - Immunomodulatory and anti inflammatory role in the GI tract. Mucus phospholipid membrane interaction Effect on mitochondrial energy metabolism – ATP deficiency and increased mucosal permeability Increased enterohepatic circulation Formation of drug-enterocyte adducts COX independent GI toxicity Increased TNF-α, IL-10 and NO release Impaired mucosal microcirculatory blood flow Impaired mucus secretion and acid regulation Impaired renal blood flow Loss of vasodilatation Increased vascular permeability Delayed wound healing Increased leukocyte adherence to vascular epithelium Increase reactive oxygen metabolites |
3. Conventional NSAIDS and IBD
4. Selective COX2 Inhibitors and IBD
| Article | Type of study | Type of IBD | Type of NSAIDS | Conclusions |
|---|---|---|---|---|
| Takeuchi, K. et al. [15] | Prospective cohort | UC and CD | Non selective | NSAIDS ingestion is associated with frequent and early relapse of quiescent IBD. |
| Meyer A.M. et al. [24] | Retrospective cohort | UC and CD | Non selective | Use of NSAIDS was associated with relapse of IBD. |
| Felder J.B. et al. [25] | Case control | UC and CD | Non selective | NSAIDS provoke disease activity in both UC and CD. |
| Evans, J.M. et al. [26] | Case control | UC and CD | Non selective | NSAIDS are associated with hospitalizations for severe colitis in patient with IBD. |
| Bonner, G.F. et al. [27] | Retrospective cohort | UC and CD | Non selective | NSAIDS use was not associated with higher likelihood of active IBD. |
| Bonner, G.F. et al. [28] | Case control | UC and CD | Non selective | High dose NSAIDS were associated with higher disease activity index but no significant disease flares were observed. |
| Mahadevan, U. et al. [29] | Retrospective cohort | UC and CD | COX2 selective | COX2 inhibitors appear to be safe and beneficial in patients with IBD. |
| Sandborn, W.J. et al. [30] | Randomized placebo-controlled trial | UC | COX2 selective | Celecoxib treatment was not associated with greater relapse rates compared to placebo. |
| El Miedany, Y. et al. [31] | Randomized placebo-controlled trial | UC and CD | COX2 selective | Etoricoxib treatment was safe and beneficial in patients with IBD. It was not associated with IBD exacerbations. |
| Reinisch, W. et al. [32] | Prospective open label study | UC and CD | COX2 selective | Rofecoxib treatment was safe, beneficial and not associated with flares of IBD. |
| Xin-Pu Miao et al. [35] | Meta-analysis | UC and CD | COX2 selective | Insufficient data to determine the impact of COX2 inhibitors on IBD exacerbations. |
5. Summary and Conclusions
References
- Fordtman, S.A. Gastrointestinal and Liver diseases, 8th ed.; Saunders Elsevier: Philadelphia PA, USA, 2006; pp. 2459–2549. [Google Scholar]
- Loftus, E.V., Jr. Inflammatory Bowel Disease extending Its Reach. Gastroenterology 2005, 129, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K. Inflammatory Bowel Disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar]
- Kefalakes, H.; Stylianides, T.J.; Amanakis, G. Exacerbation of inflammatory bowel diseases associated with the use of nonsteroidal anti-inflammatory drugs: myth or reality? Eur J Clin Pharmacol. 2009, 65, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Maiden, L.; Thjodleifsson, B.; Theodors, A.; Gonsales, J.; Bjarnason, I. A quantitative analysis of NSAIDS-induced small bowel pathology by capsule enteroscopy. Gastroenterology 2005, 128, 1172–1178. [Google Scholar]
- Cryer, B. NSAID-associated deaths: the rise and fall of NSAID-associated GI mortality. Am. J. Gastroenterol. 2005, 100, 1694–1695. [Google Scholar]
- Wolfe, M.M.; Lichtenstein, D.R.; Singh, G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 1999, 340, 1888–1899. [Google Scholar]
- Bjarnason, I.; Hayllar, J.; Macpherson, A.J.; Russell, A.S. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 1993, 104, 1832–1847. [Google Scholar] [PubMed]
- Kurahara, K.; Matsumoto, T.; Lida, M.; Honda, K.; Yao, T.; Fujishima, M. Clinical and endoscopic features of nonsteroidal anti-inflammatory drug-induced colonic ulcerations. Am. J. Gastroenterol. 2001, 96, 473–480. [Google Scholar]
- Laine, L.; Smith, R.; Min, K.; Chen, C.; Dubois, R.W. Systematic review: the lower gastrointestinal adverse effects of non-steroidal anti-inflammatory drugs. Aliment Pharmacol. Ther. 2006, 24, 751–767. [Google Scholar] [PubMed]
- Guslandi, M. Exacerbation of inflammatory bowel disease by nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors: fact or fiction? World J. Gastroenterol. 2006, 12, 1509–1510. [Google Scholar] [PubMed]
- Wallace, J.L. Prostaglandin biology in inflammatory bowel disease. Gastroenterol Clin. North Am. 2001, 30, 971–980. [Google Scholar]
- Redfern, J.S.; Feldman, M. Role of endogenous prostaglandins in preventing gastrointestinal ulceration: induction of ulcers by antibodies to prostaglandins. Gastroenterology 1989, 96 (Suppl), 596–605. [Google Scholar] [PubMed]
- Tanaka, K.; Suemasu, S.; Ishihara, T.; Tasaka, Y.; Arai, Y.; Mizushima, T. Inhibition of both COX-1 and COX-2 and resulting decrease in the level of prostaglandins E2 is responsible for non-steroidal anti-inflammatory drug (NSAID)-dependent exacerbation of colitis. Eur. J. Pharmacol. 2009, 603, 120–132. [Google Scholar]
- Takeuchi, K.; Smale, S.; Premchand, P.; Maiden, L.; Sherwood, R.; Thjodleifsson, B.; Bjornsson, E.; Bjarnason, I. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin. Gastroenterol Hepatol 2006, 4, 196–202. [Google Scholar]
- Biancone, L.; Tosti, C.; Geremia, A.; Fina, D.; Petruzziello, C.; Emerenziani, S.; Pallone, F. Rofecoxib and early relapse of inflammatory bowel disease: an open-label trial. Aliment Pharmacol. Ther. 2004, 19, 755–764. [Google Scholar]
- Singer, II; Kawka, D.W.; Schloemann, S.; Tessner, T.; Riehl, T.; Stenson, W.F. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 1998, 115, 297–306. [Google Scholar]
- Hendel, J.; Nielsen, O.H. Expression of cyclooxygenase-2 mRNA in active inflammatory bowel disease. Am. J. Gastroenterol. 1997, 92, 1170–1173. [Google Scholar]
- Tsubouchi, R.; Hayashi, S.; Aoi, Y.; Nishio, H.; Terashima, S.; Kato, S.; Takeuchi, K. Healing impairment effect of cyclooxygenase inhibitors on dextran sulfate sodium-induced colitis in rats. Digestion 2006, 74, 91–100. [Google Scholar]
- Cipolla, G.; Crema, F.; Sacco, S.; Moro, E.; De Ponti, F.; Frigi, G. Nonsteroidal anti-inflammatory drugs and inflammatory bowel disease: current perspectives. Pharmacol. Res. 2002, 46, 1–6. [Google Scholar]
- Fries, J.F.; Miller, S.R.; Spitz, P.W.; Williams, C.A.; Hubert, H.B.; Bloch, D.A. Toward an epidemiology of gastropathy associated with nonsteroidal antiinflammatory drug use. Gastroenterology 1989, 96 (Suppl.), 647–655. [Google Scholar]
- Bonner, G.F. Exacerbation of inflammatory bowel disease associated with use of celecoxib. Am. J. Gastroenterol. 2001, 96, 1306–1308. [Google Scholar]
- Singh, S.; Graff, L.A.; Bernstein, C.N. Do NSAIDs, antibiotics, infections, or stress trigger flares in IBD? Am. J. Gastroenterol. 2009, 104, 1298–1313. [Google Scholar] [CrossRef]
- Meyer, A.M.; Ramzan, N.N.; Heigh, R.I.; Leighton, J.A. Relapse of inflammatory bowel disease associated with use of nonsteroidal anti-inflammatory drugs. Dig. Dis. Sci. 2006, 51, 168–172. [Google Scholar]
- Felder, J.B.; Korelitz, B.I.; Rajapakse, R.; Schwarz, S.; Horatagis, A.P.; Gleim, G. Effects of nonsteroidal antiinflammatory drugs on inflammatory bowel disease: a case-control study. Am. J. Gastroenterol. 2000, 95, 1949–1954. [Google Scholar]
- Evans, J.M.; McMahon, A.D.; Murray, F.E.; McDevitt, D.G.; MacDonald, T.M. Non-steroidal anti-inflammatory drugs are associated with emergency admission to hospital for colitis due to inflammatory bowel disease. Gut 1997, 40, 619–622. [Google Scholar]
- Bonner, G.F.; Walczak, M.; Kitchen, L.; Bayona, M. Tolerance of nonsteroidal antiinflammatory drugs in patients with inflammatory bowel disease. Am. J. Gastroenterol 2000, 95, 1946–1948. [Google Scholar]
- Bonner, G.F.; Fakhri, A.; Vennamaneni, S.R. A long-term cohort study of nonsteroidal anti-inflammatory drug use and disease activity in outpatients with inflammatory bowel disease. Inflamm. Bowel Dis. 2004, 10, 751–757. [Google Scholar]
- Mahadevan, U.; Loftus, E.V., jr.; Tremaine, W.J.; Sandborn, W.J. Safety of selective cyclooxygenase-2 inhibitors in inflammatory bowel disease. Am. J. Gastroenterol. 2002, 97, 910–914. [Google Scholar]
- Sandborn, W.J.; Stenson, WF.; Brynskov, J.; Lorenz, RG.; Steidle, GM.; Robbins, JL.; Kent, JD.; Bloom, BJ. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo-controlled, pilot study. Clin. Gastroenterol. Hepatol. 2006, 4, 203–211. [Google Scholar]
- El-Miedany, Y.; Youssef, S.; Ahmed, I.; El Gaafary, M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am. J. Gastroenterol. 2006, 101, 311–317. [Google Scholar]
- Reinisch, W.; Miehsler, W.; Dejaco, C.; Harrer, M.; Waldhoer, T.; Lichtenberger, C.; Vogelsang, H. An open-label trial of the selective cyclo-oxygenase-2 inhibitor, rofecoxib, in inflammatory bowel disease-associated peripheral arthritis and arthralgia. Aliment Pharmacol. Ther. 2003, 17, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G.M.; Mattia, A.R. Rofecoxib and inflammatory bowel disease: clinical and pathologic observations. J. Clin. Gastroenterol. 2005, 39, 142–143. [Google Scholar]
- Gornet, J.M.; Hassani, Z.; Modigliani, R.; Lemann, M. Exacerbation of Crohn's colitis with severe colonic hemorrhage in a patient on rofecoxib. Am. J. Gastroenterol. 2002, 97, 3209–3210. [Google Scholar]
- Miao, X.P.; Ouyang, Q.; Li, H.Y.; Wen, Z.H.; Zhang, D.K.; Cui, X.Y. Role of selective cyclooxygenase-2 inhibitors in exacerbation of inflammatory bowel disease: A systematic review and meta-analysis. Curr. Therap. Res. 2008, 69, 181–191. [Google Scholar]
- Takeuchi, A.; Tanaka, A.; Ohno, R.; Yokota, A. Role of COX inhibition in pathogenesis of NSAIDS-induced small intestinal damage. J. Physiol. Pharmacol. 2003, 54 (Suppl.), 165–182. [Google Scholar] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Klein, A.; Eliakim, R. Non Steroidal Anti-Inflammatory Drugs and Inflammatory Bowel Disease. Pharmaceuticals 2010, 3, 1084-1092. https://doi.org/10.3390/ph3041084
Klein A, Eliakim R. Non Steroidal Anti-Inflammatory Drugs and Inflammatory Bowel Disease. Pharmaceuticals. 2010; 3(4):1084-1092. https://doi.org/10.3390/ph3041084
Chicago/Turabian StyleKlein, Amir, and Rami Eliakim. 2010. "Non Steroidal Anti-Inflammatory Drugs and Inflammatory Bowel Disease" Pharmaceuticals 3, no. 4: 1084-1092. https://doi.org/10.3390/ph3041084
APA StyleKlein, A., & Eliakim, R. (2010). Non Steroidal Anti-Inflammatory Drugs and Inflammatory Bowel Disease. Pharmaceuticals, 3(4), 1084-1092. https://doi.org/10.3390/ph3041084




