Hybridoma-Derived Idiotype Vaccine for Lymphoma: Approval Must Wait
Abstract
:Introduction
Overview of the market
| Institution/Sponsor | Idiotype Production | Vaccine Formulation | Disease | Biological Efficacy | Clinical Efficacy | Clinical Benefit | Reference |
|---|---|---|---|---|---|---|---|
| Stanford University | Hybridoma | Id-KLH + SAF-1 | FL | YES | N/A | N/A | [23] |
| NCI | Hybridoma | Id-KLH + GM-CSF | MCL | YES | N/A | N/A | [37] |
| NCI | Hybridoma | Id-KLH + GM-CSF | FL | YES | YES | N/A | [24] |
| Puerta de Hierro Hospital | Hybridoma | Id-KLH + GM-CSF | FL | YES | YES | N/A | [25] |
| University of Navarra | Hybridoma | Id-KLH + GM-CSF | FL | YES | N/A | YES | [26] |
| Biovest | Hybridoma/AutovaxId/BiovaxId | Id-KLH + GM-CSF | FL | pending | pending | YES | [31] |
Introduction to the Compound
Chemistry
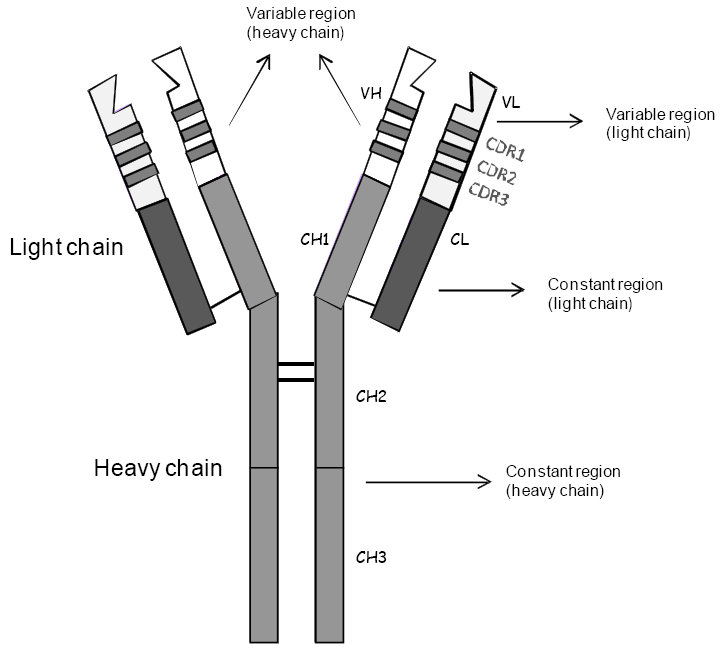
Pharmacodynamics
Pharmacokinetics and Metabolism
Clinical efficacy and benefit
| Variable | Planned | Actual |
|---|---|---|
| Accrual | 629 (later revised to 563) pts | 234 pts |
| Randomization | 375 pts | 177 (later reduced to 117 on a modified ITT basis) pts |
| Vaccinated (5 doses) | 250 pts | 76 pts (at least 1 dose) |
| Control product | 125 pts | 41 pts |
| Required statistical significance (difference in RFS) | p < 0.01 | p = 0.045 |
Safety and Tolerability
Regulatory affairs
| Pre-Vaccine Therapy | Patient Status Before Vaccination | Accrual (planned / actual) | Endpoint | Results (required / obtained) | Reference |
|---|---|---|---|---|---|
| CVP (8 cycles) | First CR or PR | 360 / 285 | PFS | p < 0.01 / p = n.s. | 34 |
| Rituximab (4 doses) | First CR or PR or SD | 342 / 345 | TTP | p < 0.01 / p = n.s. | 35 |
| PACE (90% of pts) or Rituximab-CHOP (6 cycles) | First CR | 375 / 117 (modified ITT) | DFS | p < 0.01 / p = 0.045 | 31 |
Conclusions
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.S.; Harris, N.L.; Stein, H.; Campos, E.; Pileri, S.A.; Swerdlow, S.H. Introduction and overview of the classification of the lymphoid neoplasms. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th; Swerdlow, S.H., Campos, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Vardiman, J.W., Eds.; IARC Press: Lyon, France, 2008; pp. 157–166. [Google Scholar]
- Bendandi, M. Idiotype vaccines for lymphoma: proof-of-principles and clinical trial failures. Nat. Rev. Cancer 2009, 9, 675–681. [Google Scholar]
- Bendandi, M. Anti-idiotype vaccines for human follicular lymphoma. Leukemia 2000, 14, 1333–1339. [Google Scholar]
- Inogés, S.; Rodríguez-Calvillo, M.; López-Díaz de Cerio, A.; Zabalegui, N.; Pérez-Calvo, J.; Panizo, C.; Hernandez, M.; Cuesta, B.; Rocha, E.; Bendandi, M. Feasibility of idiotype vaccination in relapsed B-cell malignancies. Haematologica 2003, 88, 1438–1440. [Google Scholar] [PubMed]
- Bendandi, M. Aiming at a curative strategy for follicular lymphoma. CA Cancer J. Clin. 2008, 58, 305–317. [Google Scholar]
- Inogés, S.; López-Díaz de Cerio, A.; Zabalegui, N.; Soria, E.; Villanueva, H.; Panizo, C.; Rodriguez-Caballero, A.; Suarez, L.; Pastor, F.; Rodriguez-Calvillo, M.; et al. Prolonged idiotypic vaccination against follicular lymphoma. Leuk Lymphoma 2009, 50, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L. Idiotype vaccination in follicular lymphoma: knocking on the doorway to cure. J. Natl. Cancer Inst. 2006, 98, 1263–1265. [Google Scholar]
- Hagenbeek, A. Monoclonal antibodies (update): CD20, rituximab. Hematol. Meeting Rep. 2008, 2, 50–51. [Google Scholar]
- Bendandi, M. Role of anti-idiotype vaccines in the modern treatment of human follicular lymphoma. Expert. Rev. Anticancer Ther. 2001, 1, 65–72. [Google Scholar]
- Bendandi, M. The role of idiotype vaccines in the treatment of human B-cell malignancies. Expert. Rev. Vaccines 2004, 3, 163–170. [Google Scholar]
- de Cerio, A.L.; Zabalegui, N.; Rodríguez-Calvillo, M.; Inoges, S.; Bendandi, M. Anti-idiotype antibodies in cancer treatment. Oncogene 2007, 26, 3594–3602. [Google Scholar]
- Harris, J.R.; Markl, J. Keyhole limpet hemocyanin: molecular structure of a potent marine immunoactivator. A review. Eur. Urology 2000, 37 (Suppl. 3), 24–33. [Google Scholar] [CrossRef]
- Kwak, L.W.; Young, H.A.; Pennington, R.W.; Weeks, S.D. Vaccinationwith syngeneic, lymphoma-derived immunoglobulin idiotypecombined with granulocyte/macrophage colony-stimulating factorprimes mice for a protective T cell response. Proc. Natl. Acad. Sci. USA 1996, 93, 10972–10977. [Google Scholar]
- Carroll, W.L.; Thielemans, K.; Dilley, J.; Levy, R. Mouse x human heterohybridomas as fusion partners with human B-cell tumors. J. Immunol. Methods 1986, 89, 61–72. [Google Scholar]
- Rodríguez-Calvillo, M.; Inogés, S.; López-Díaz de Cerio, A.; Zabalegui, N.; Villanueva, H.; Bendandi, M. Variations in “rescuability” of immunoglobulin molecules from different forms of human lymphoma: implications for antiidiotype vaccine development. Crit. Rev. Oncol. Hematol. 2004, 52, 1–7. [Google Scholar]
- De Palma, A. Vaccine manufacturing reborn. Pharmaceutical Manufacturing. 2007. Available online: http://www.pharmamanufacturing.com/articles/2007/051.html?page=full/ March 12,2007.
- Zhu, D.; McCarthy, H.; Ottensmeier, C.H.; Johnson, P.; Hamblin, T.J.; Stevenson, F.K. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood 2002, 99, 2562–2568. [Google Scholar]
- Zabalegui, N.; de Cerio, A.L.; Inogés, S.; Rodríguez-Calvillo, M.; Pérez-Calvo, J.; Hernández, M.; García-Foncillas, J.; Martín-Algarra, S.; Rocha, E.; Bendandi, M. Acquired potential N-glycosylation sites within the tumor-specific heavy chains of B-cell malignancies. Haematologica 2004, 89, 541–546. [Google Scholar]
- Radcliffe, C.M.; Arnold, J.N.; Suter, D.M.; Wormald, M.R.; Harvey, D.J.; Royle, L.; Mimura, Y.; Kimura, Y.; Sim, R.B.; Inogès, S.; et al. Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J. Biol. Chem. 2007, 282, 7405–7415. [Google Scholar] [PubMed]
- Matzinger, P. The danger model: a renewed sense of self. Science 2002, 296, 301–305. [Google Scholar]
- Kwak, L.W.; Campbell, M.J.; Czerwinski, D.K.; Hart, S.; Miller, R.A.; Levy, R. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl. J. Med. 1992, 327, 1209–1215. [Google Scholar]
- Hsu, F.J.; Caspar, C.B.; Czerwinski, D.; Kwak, L.W.; Liles, T.M.; Syrengelas, A.; Taidi-Laskowski, B.; Levy, R. Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma—long-term results of a clinical trial. Blood 1997, 89, 3129–3135. [Google Scholar]
- Bendandi, M.; Gocke, C.D.; Kobrin, C.B.; Benko, F.A.; Sternas, L.A.; Pennington, R.; Watson, T.M.; Reynolds, C.W.; Gause, B.L.; Duffey, P.L.; et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat. Med. 1999, 5, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Barrios, Y.; Cabrera, R.; Yáñez, R.; Briz, M.; Plaza, A.; Forés, R.; Fernández, MN.; Díaz-Espada, F. Anti-idiotypic vaccination in the treatment of low-grade B-cell lymphoma. Haematologica 2002, 87, 400–407. [Google Scholar] [PubMed]
- Inogès, S.; Rodrìguez-Calvillo, M.; Zabalegui, N.; Lòpez-Dìaz de Cerio, A.; Villanueva, H.; Soria, E.; Suárez, L.; Rodríguez-Caballero, A.; Pastor, F.; García-Muñóz, R.; et al. Clinical benefit associated with idiotypic vaccination in patients with follicular lymphoma. J. Natl. Cancer Inst. 2006, 98, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L. Idiotype vaccination in follicular lymphoma: knocking on the doorway to cure. J. Natl. Cancer Inst. 2006, 98, 1263–1265. [Google Scholar]
- Houot, R.; Levy, R. Vaccines for lymphomas: idiotype vaccines and beyond. Blood Rev. 2009, 23, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Foglietta, M.; Neelapu, S.S.; Kwak, L.W. Therapeutic vaccines for lymphoma: from bench to bedside. In ASCO 2009 Educational Book; American Society of Clinical Oncology: Orlando, FL, USA, 2009; pp. 495–500. [Google Scholar]
- Inogés, S.; López-Díaz de Cerio, A.; Soria, E.; Villanueva, H.; Pastor, F.; Bendandi, M. Idiotype vaccines for human B-cell malignancies. Curr. Pharm. Design 2010, 16, 300–307. [Google Scholar]
- Schuster, S.J.; Neelapu, S.S.; Gause, B.L.; Muggia, F.M.; Gockerman, J.P.; Sotomayor, E.M.; Winter, J.N.; Flowers, C.R.; Stergiou, A.M.; Kwak, L.W. Idiotype vaccine therapy (BiovaxID) in follicular lymphoma in first complete remission: Phase III clinical trial results. J. Clin. Oncol. 2009, 27, 18s. [Google Scholar]
- Bendandi, M. Clinical benefit of idiotype vaccines: too many trials for a clever demonstration? Rev. Recent Clin. Trials 2006, 1, 67–74. [Google Scholar] [CrossRef] [PubMed]
- López-Díaz de Cerio, A.; Inogés, S. Future of idiotypic vaccination for B-cell lymphoma. Expert Rev.Vaccines 2009, 8, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Robertson, M.J.; Ganjoo, K.; Leonard, J.; Vose, J.; Denney, D.J. Results of a Phase 3 trial evaluating safety and efficacy of specific immunotherapy, recombinant idiotype (Id) conjugated to KLH (Id-KLH) with GM-CSF, compared with non-specific immunotherapy, KLH with GM-CSF, in patients with follicular non-Hodgkin's lymphoma (fNHL). AACR Meeting Abstracts 2008. Abstract LB-204. [Google Scholar]
- Freedman, A.; Neelapu, S.S.; Nichols, C.; Robertson, M.J.; Djulbegovic, B.; Winter, J.N.; Bender, J.F.; Gold, D.P.; Ghalie, R.G.; Stewart, M.E.; et al. Placebo-Controlled Phase III Trial of Patient-Specific Immunotherapy With Mitumprotimut-T and Granulocyte-Macrophage Colony-Stimulating Factor After Rituximab in Patients With Follicular Lymphoma. J. Clin. Oncol. 2009, 27, 3036–3043. [Google Scholar] [PubMed]
- Bendandi, M.; Marillonnet, S.; Kandzia, R.; Thieme, F.; Nickstadt, A.; Herz, S.; Frode, R.; Inoges, S.; Lopez-Diaz de Cerio, A.; Soria, E.; et al. Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin’s lymphoma. Ann. Onc. 2010. submitted. [Google Scholar]
- Neelapu, S.S.; Kwak, L.W.; Kobrin, C.B.; Reynolds, C.W.; Janik, J.E.; Dunleavy, K.; White, T.; Harvey, L.; Pennington, R.; Stetler-Stevenson, M.; et al. Vaccine-induced tumor-specific immunity despite severe B-cell depletion in mantle cell lymphoma. Nat. Med. 2005, 11, 986–991. [Google Scholar] [PubMed]
- Bendandi, M.; Rodríguez-Calvillo, M.; Inogés, S.; López-Díaz de Cerio, A.; Pérez-Simón, J.A.; Rodríguez-Caballero, A.; García-Montero, A.; Almeida, J.; Zabalegui, N.; Giraldo, P.; et al. Combined vaccination with idiotype-pulsed allogeneic dendritic cells and soluble protein idiotype for multiple myeloma patients relapsing after reduced-intensity conditioning allogeneic stem cell transplantation. Leuk. Lymphoma 2006, 47, 29–37. [Google Scholar] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bendandi, M. Hybridoma-Derived Idiotype Vaccine for Lymphoma: Approval Must Wait. Pharmaceuticals 2010, 3, 667-678. https://doi.org/10.3390/ph3030667
Bendandi M. Hybridoma-Derived Idiotype Vaccine for Lymphoma: Approval Must Wait. Pharmaceuticals. 2010; 3(3):667-678. https://doi.org/10.3390/ph3030667
Chicago/Turabian StyleBendandi, Maurizio. 2010. "Hybridoma-Derived Idiotype Vaccine for Lymphoma: Approval Must Wait" Pharmaceuticals 3, no. 3: 667-678. https://doi.org/10.3390/ph3030667
APA StyleBendandi, M. (2010). Hybridoma-Derived Idiotype Vaccine for Lymphoma: Approval Must Wait. Pharmaceuticals, 3(3), 667-678. https://doi.org/10.3390/ph3030667




