Comparison of Functional Protein Transduction Domains Using the NEMO Binding Domain Peptide
Abstract
:1. Introduction
2. Results and Discussion
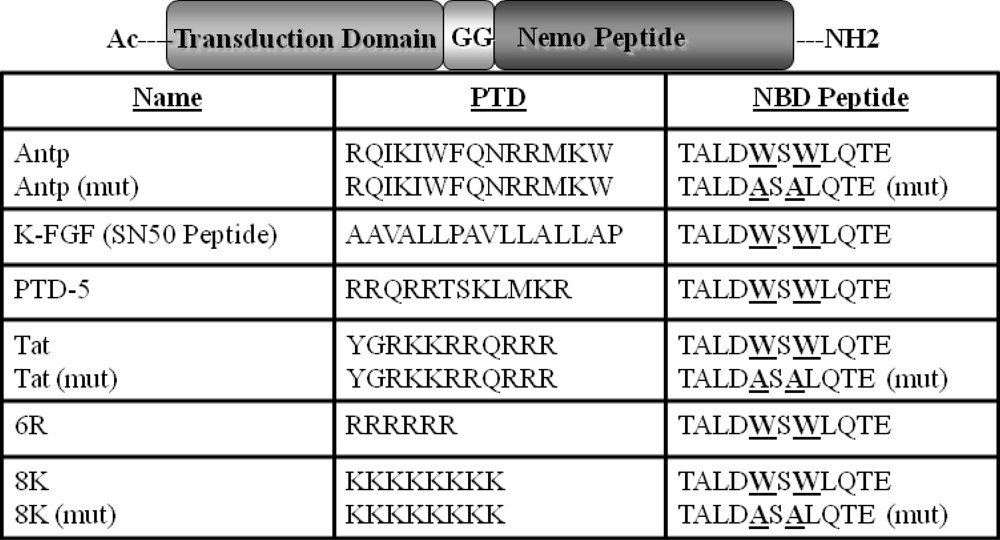
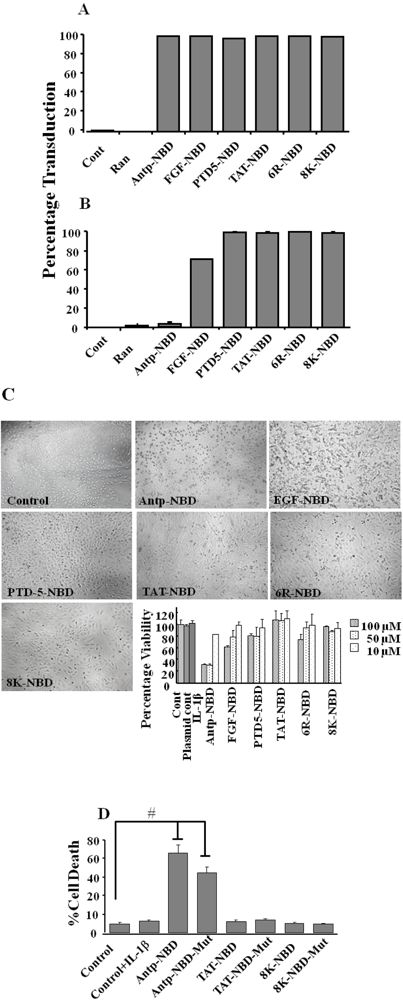
2.1. Transduction Efficiency and Toxicity of PTD-NBD Peptides in HeLa Cells
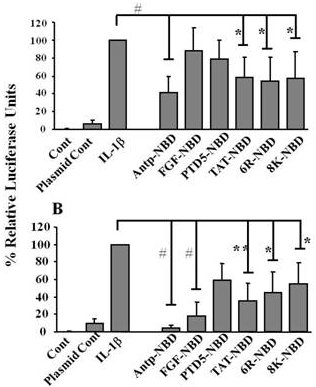
2.2. Inhibition of IL-1ß Mediated NF-κB Activation by PTD-NBD Peptides


2.3. Evaluation of Efficacy of the Different PTD-NBD Fusion Peptide on Inflammation in a Footpad Model of DTH

2.4. Discussion
3. Experimental Section
3.1. Animals
3.2. Peptide Synthesis
3.3. Flow Cytometric (FACS) Analysis of Transduction Efficiency of 6-Carboxy Fluorescien (6CF) PTD-NBD Peptides
3.4. Analysis of Cytotoxicity of PTD-NBD Peptides Using MTT Assay Protocol
3.5. Transient Transfection Assay Using NF-κB Luciferase Reporter
3.6. Immunofluorescence Staining of p65 and Confocal Microscopy
3.7. Peptide Treatment and NF-κB Nuclear Translocation Assay
3.8. Preparation of Cytosolic and Nuclear Extracts
3.9. Immunoblotting
3.10 Induction of Delayed-Type Hypersensitivity (DTH) and PTD-NBD Treatment
3.11. Measurement of DTH
3.12. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Eguchi, A.; Akuta, T.; Okuyama, H.; Senda, T.; Yokoi, H.; Inokuchi, H.; Fujita, S.; Hayakawa, T.; Takeda, K.; Hasegawa, M.; Nakanishi, M. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J. Biol. Chem. 2001, 276, 26204–26210. [Google Scholar] [CrossRef] [PubMed]
- Josephson, L.; Tung, C.H.; Moore, A.; Weissleder, R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug. Chem. 1999, 10, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.; Carlesso, N.; Tung, C.H.; Tang, X.W.; Cory, D.; Scadden, D.T.; Weissleder, R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol. 2000, 18, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Rothbard, J.B.; Garlington, S.; Lin, Q.; Kirschberg, T.; Kreider, E.; McGrane, P.L.; Wender, P.A.; Khavari, P.A. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat. Med. 2000, 6, 1253–1257. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Rammohan, R.; Weissig, V.; Levchenko, T.S. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. USA 2001, 98, 8786–8791. [Google Scholar] [CrossRef]
- Wadia, J.S.; Dowdy, S.F. Protein transduction technology. Curr. Opin. Biotechnol. 2002, 13, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.C.; Mi, Z.; Kim, S.H.; Ng, B.; Robbins, P.D. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001, 61, 7709–7712. [Google Scholar] [PubMed]
- May, M.J.; D'Acquisto, F.; Madge, L.A.; Glockner, J.; Pober, J.S.; Ghosh, S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science 2000, 289, 1550–1554. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, S.R.; Ho, A.; Vocero-Akbani, A.; Dowdy, S.F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 1999, 285, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.P.; Feng, Z.G.; Yuan, B.L.; Yu, S.Z.; Li, Q.; Qu, H.Y.; Sun, M.J. Transduced PTD-BDNF fusion protein protects against beta amyloid peptide-induced learning and memory deficits in mice. Brain Res. 2008, 1191, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Pei, W.; Ge, H.; Liang, Q.; Luo, Y.; Sharp, F.R.; Lu, A.; Ran, R.; Graham, S.H.; Chen, J. In Vivo Delivery of a Bcl-xL Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis. J. Neurosci. 2002, 22, 5423–5431. [Google Scholar] [PubMed]
- Mai, J.C.; Shen, H.; Watkins, S.C.; Cheng, T.; Robbins, P.D. Efficiency of protein transduction is cell type-dependent and is enhanced by dextran sulfate. J. Biol. Chem. 2002, 277, 30208–30218. [Google Scholar] [CrossRef] [PubMed]
- Benito, M.J.; Murphy, E.; Murphy, E.P.; van den Berg, W.B.; FitzGerald, O.; Bresnihan, B. Increased synovial tissue NF-kappa B1 expression at sites adjacent to the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 2004, 50, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Handel, M.L.; McMorrow, L.B.; Gravallese, E.M. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995, 38, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.S.; Yoon, C.S.; Zbytnuik, L.; van Rooijen, N.; Yoon, J.W. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Exp. Med. 1999, 189, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Ran, S.; Ishida, T.; Nadaf, S.; Kerr, L.; Carbone, D.P.; Gabrilovich, D.I. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J. Immunol. 1998, 160, 1224–1232. [Google Scholar] [PubMed]
- Poligone, B.; Weaver Jr., D.J.; Sen, P.; Baldwin Jr., A.S.; Tisch, R. Elevated NF-kappaB activation in nonobese diabetic mouse dendritic cells results in enhanced APC function . J. Immunol. 2002, 168, 188–196. [Google Scholar] [PubMed]
- Weaver Jr., D.J.; Poligone, B.; Bui, T.; Abdel-Motal, U.M.; Baldwin Jr., A.S.,; Tisch, R. Dendritic cells from nonobese diabetic mice exhibit a defect in NF-kappa B regulation due to a hyperactive I kappa B kinase . J. Immunol. 2001, 167, 1461–1468. [Google Scholar] [PubMed]
- Wheat, W.; Kupfer, R.; Gutches, D.G.; Rayat, G.R.; Beilke, J.; Scheinman, R.I.; Wegmann, D.R. Increased NF-kappa B activity in B cells and bone marrow-derived dendritic cells from NOD mice. Eur. J. Immunol. 2004, 34, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- DiDonato, J.A.; Hayakawa, M.; Rothwarf, D.M.; Zandi, E.; Karin, M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 1997, 388, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.K.; Bertera, S.; Bottino, R.; Balamurugan, A.N.; Mai, J.C.; Mi, Z.; Trucco, M.; Robbins, P.D. Protection of islets by in situ peptide-mediated transduction of the Ikappa B kinase inhibitor Nemo-binding domain peptide. J. Biol. Chem. 2003, 278, 9862–9868. [Google Scholar] [CrossRef] [PubMed]
- Giannoukakis, N.; Rudert, W.A.; Trucco, M.; Robbins, P.D. Protection of human islets from the effects of interleukin-1beta by adenoviral gene transfer of an Ikappa B repressor. J. Biol. Chem. 2000, 275, 36509–36513. [Google Scholar] [CrossRef] [PubMed]
- Zandi, E.; Chen, Y.; Karin, M. Direct phosphorylation of IkappaB by IKKalpha and IKKbeta: discrimination between free and NF-kappaB-bound substrate. Science 1998, 281, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Yoshikawa, T.; Mukai, Y.; Yamanada, N.; Imai, S.; Nagano, K.; Yoshida, Y.; Shibata, H.; Yoshioka, Y.; Nakagawa, S.; Kamada, H.; Tsunoda, S.I.; Tsutsumi, Y. Comparative study on transduction and toxicity of protein transduction domains. Br. J. Pharmacol. 2008, 153, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Aoki, K.; Saito, H.; D'Acquisto, F.; May, M.J.; Nakamura, I.; Sudo, T.; Kojima, T.; Okamoto, F.; Fukushima, H.; Okabe, K.; Ohya, K.; Ghosh, S. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat. Med. 2004, 10, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Tas, S.W.; Vervoordeldonk, M.J.; Hajji, N.; May, M.J.; Ghosh, S.; Tak, P.P. Local treatment with the selective IkappaB kinase beta inhibitor NEMO-binding domain peptide ameliorates synovial inflammation . Arthritis Res. Ther. 2006, 8, R86. [Google Scholar] [CrossRef] [PubMed]
- di Meglio, P.; Ianaro, A.; Ghosh, S. Amelioration of acute inflammation by systemic administration of a cell-permeable peptide inhibitor of NF-kappaB activation. Arthritis Rheum. 2005, 52, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.H.; Tilstra, J.S.; Matsuoka, K.; Li, F.; Karrasch, T.; Uno, J.K.; Sepulveda, A.R.; Jobin, C.; Baldwin Jr., A.S.; Robbins, P.D.; Plevy, S.E. Amelioration of chronic murine colitis by peptide-mediated transduction of the IkappaB kinase inhibitor NEMO binding domain peptide. J. Immunol. 2007, 179, 7852–7859. [Google Scholar] [PubMed]
- Acharyya, S.; Villalta, S.A.; Bakkar, N.; Bupha-Intr, T.; Janssen, P.M.; Carathers, M.; Li, Z.W.; Beg, A.A.; Ghosh, S.; Sahenk, Z.; Weinstein, M.; Gardner, K.L.; Rafael-Fortney, J.A.; Karin, M.; Tidball, J.G.; Baldwin, A.S.; Guttridge, D.C. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J. Clin. Invest. 2007, 117, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Bianco, N.R.; Kim, S.H.; Ruffner, M.A.; Robbins, P.D. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 2009, 60, 380–389. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khaja, K.; Robbins, P. Comparison of Functional Protein Transduction Domains Using the NEMO Binding Domain Peptide. Pharmaceuticals 2010, 3, 110-124. https://doi.org/10.3390/ph3010110
Khaja K, Robbins P. Comparison of Functional Protein Transduction Domains Using the NEMO Binding Domain Peptide. Pharmaceuticals. 2010; 3(1):110-124. https://doi.org/10.3390/ph3010110
Chicago/Turabian StyleKhaja, Khaleel, and Paul Robbins. 2010. "Comparison of Functional Protein Transduction Domains Using the NEMO Binding Domain Peptide" Pharmaceuticals 3, no. 1: 110-124. https://doi.org/10.3390/ph3010110
APA StyleKhaja, K., & Robbins, P. (2010). Comparison of Functional Protein Transduction Domains Using the NEMO Binding Domain Peptide. Pharmaceuticals, 3(1), 110-124. https://doi.org/10.3390/ph3010110