Malaria-Infected Mice Are Cured by a Single Low Dose of a New Silylamide Trioxane Plus Mefloquine
Abstract
:1. Introduction
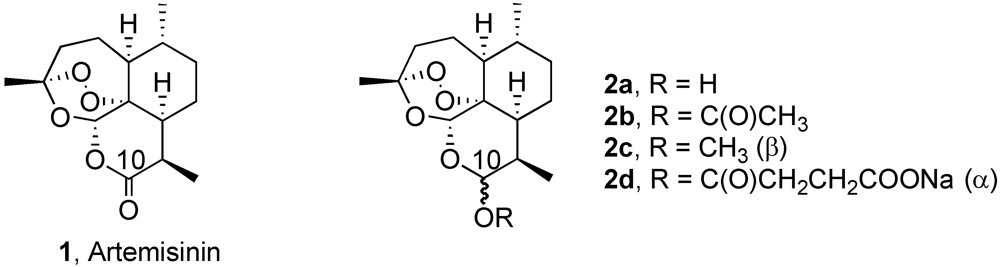
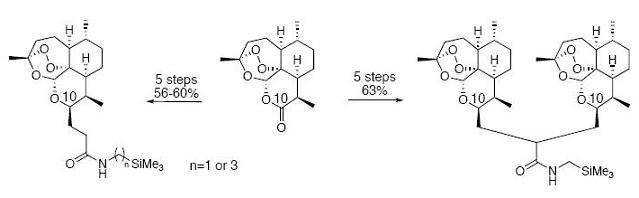
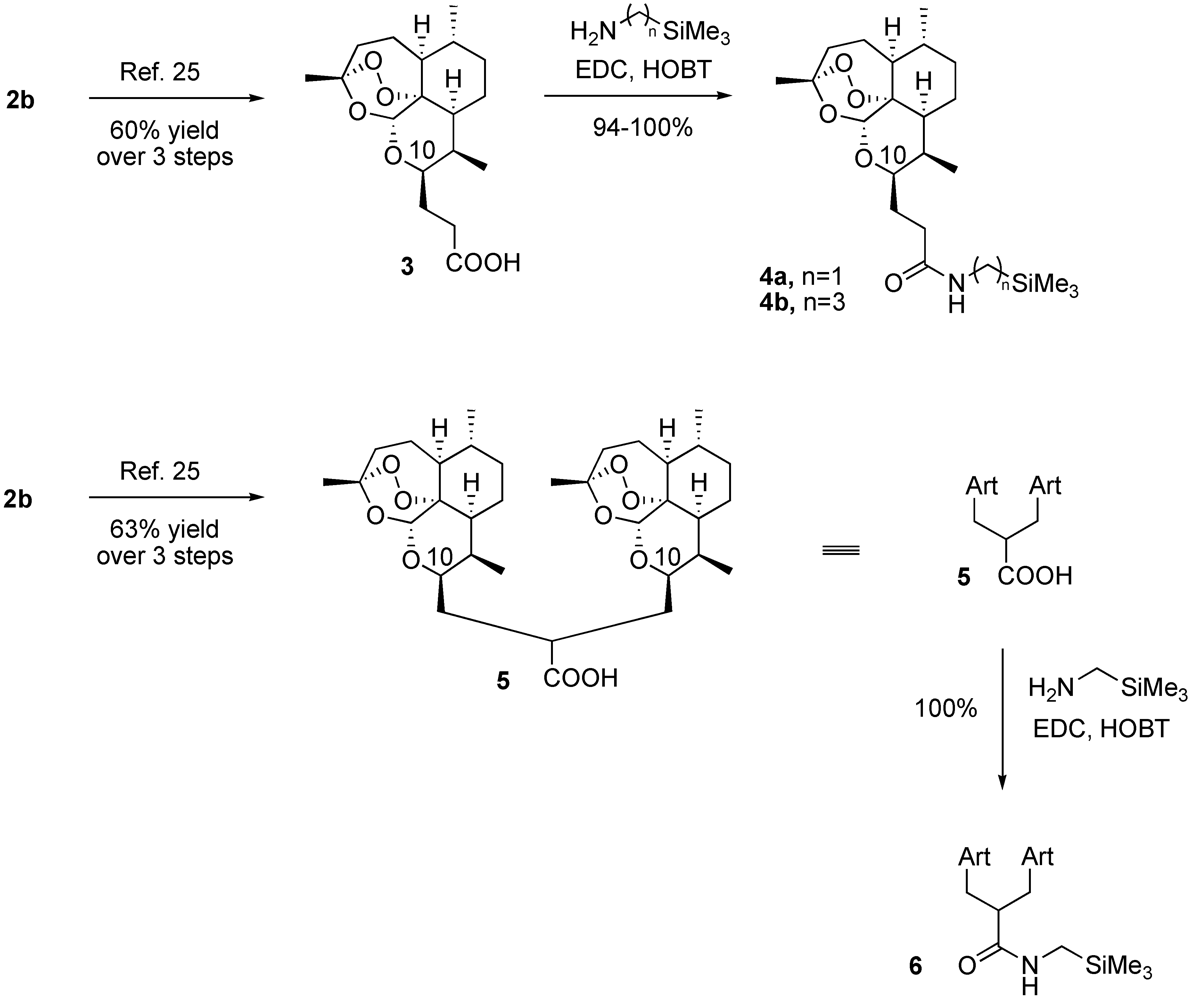
2. Results and Discussion
2.1. Chemistry
2.2. Biology
| Trioxane | Average Survival (days) after Infection | % Suppression of Parasitemia (on day 3 post infection) |
|---|---|---|
| 2c | 23 | > 99.5 |
| 4a | > 57 | > 99.5 |
| 4b | 44a | > 99.5 |
| 6 | > 57 | > 99.5 |
3. Experimental
4. Conclusions
Acknowledgments
References
- Ridley, R.G. Medical Need, Scientific Opportunity, and the Drive for Antimalarial Drugs. Nature 2002, 415, 686–693. [Google Scholar]
- LeBlanc, R.; Vasquez, Y.; Hannaman, D.; Kumar, N. Markedly Enhanced Immunogenicity of a Pfs25 DNA Based Malaria Transmission Blocking Vaccine by in Vivo Electroporation. Vaccine 2008, 26, 185–192. [Google Scholar] [PubMed]
- Troye-Blomberg, M.; Berzins, K. Rational Vaccine Development against Malaria. Microbes Infect. 2007, 9, 749–750. [Google Scholar]
- Olliaro, P.L.; Boland, P.B. Clinical Public Health Implications of Antimalarial Drug Resistance. In Antimalarial Chemotherapy: Mechanisms of Action, Resistance, and New Directions in Drug Discovry; Rosenthal, P.J., Ed.; Humana Press: Totowa, NJ, USA, 2002; pp. 65–83. [Google Scholar]
- Hof, F.; Schütz, A.; Fäh, C.; Meyer, S.; Bur, D.; Liu, J.; Goldberg, D.E.; Diederich, F. Starving the Malaria Parasite: Inhibitors Active Against the Aspartic Proteases Plasmepsins I, II, and IV. Angew. Chem. Int. Ed. 2006, 45, 2138–2141. [Google Scholar]
- Pandey, K.C.; Sijwall, P.S.; Singh, A.; Na, B.-K.; Rosenthal, P.J. Independent Intramolecular Mediators of Folding, Activity, and Inhibition for the Plasmodium falciparum Cysteine Protease Falcipain-2. J. Biol. Chem. 2004, 279, 3484–3491. [Google Scholar] [PubMed]
- Kelly, J.X.; Smilkstein, M.J.; Brun, R.; Wittlin, S.; Cooper, R.A.; Lane, K.D.; Janowsky, A.; Johnson, R.A.; Dodean, R.A.; Winter, R.; Hinrichs, D.J.; Riscoe, M.K. Discovery of dual function acridones as a new antimalarial chemotype. Nature 2009, 459, 270–273. [Google Scholar]
- Yearick, K.; Ekoue-Kovi, K.; Iwaniuk, D.P.; Natarajan, J.K.; Alumasa, J.; de Dios, A.C.; Roepe, P.D.; Wolf, C. Overcoming Drug Resistance to Heme-targeted Antimalarials by Systematic Side Chain Variation of 7-Chloro-4-aminoquinolines. J. Med. Chem. 2008, 51, 1995–1998. [Google Scholar]
- Ramanathan-Girish, S.; Catz, P.; Creek, M.R.; Wu, B.; Thomas, D.; Krogstad, D.J.; De, D; Mirsalis, J.C.; Green, C.E. Pharmacokinetics of the Antimalarial Drug, AQ-13, in Rats and Cynomolgus Macaques. Int. J. Toxicol. 2004, 23, 179–189. [Google Scholar] [PubMed]
- Klayman, D.L. Qinghaosu (Artemisinin): An Antimalarial Drug from China. Science 1985, 228, 1049–1055. [Google Scholar]
- O’Neill, P.M.; Posner, G.H. A Medicinal Chemistry Perspective on Artemisinin and Related Endoperoxides. J. Med. Chem. 2004, 47, 2945–2964. [Google Scholar]
- Tang, Y.; Dong, Y.; Vennerstrom, J.L. Synthetic Peroxides as Antimalarials. Med. Res. Rev. 2004, 24, 425–448. [Google Scholar]
- Jefford, C.W. Synthetic Peroxides as Antimalarials. Curr. Opin. Vest. Drugs (Thomson Sci.) 2004, 5, 866–872. [Google Scholar]
- Haynes, R.K. From Artemisinin to New Artemisinin Antimalarials: Biosynthesis, Extraction, Old and New Derivatives, Stereochemistry and Medicinal Chemistry Requirements. Curr. Top. Med. Chem. 2006, 6, 509–537. [Google Scholar] [CrossRef] [PubMed]
- Bégué, J.-P.; Bonnet-Delpon, D. Fluoroartemisinins: Metabolically More Stable Antimalarial Artemisinin Derivatives. ChemMedChem 2007, 2, 608–624. [Google Scholar]
- Gelb, M.H. Drug discovery for malaria: A very challenging endeavor. Curr. Opin. Chem. Biol. 2007, 11, 440–445. [Google Scholar]
- World Health Organization. Guidelines for the Treatment of Malaria; WHO: Geneva, Switzerland, 2006.
- Ashley, E.A.; White, N.J. Artemisinin-based combinations. Curr. Opin. Infect. Dis. 2005, 18, 531–536. [Google Scholar]
- Adjuik, M.; Babiker, A.; Garner, P.; Olliaro, P.; Taylor, W.; White, N. Artesunate combinations for treatment of malaria: meta-analysis. Lancet 2004, 363, 9–17. [Google Scholar]
- Guthmann, J.-P.; Cohuet, S.; Rigutto, C.; Fortes, F.; Saraiva, N.; Kiguli, J.; Kyomuhendo, J.; Francis, M.; Noel, F.; Mulemba, M.; Balkan, S. High efficacy of two artemisinin-based combinations (artesunate + amodiaquine and artemether + lumefantrine) in Caala, Central Angola. Am. J. Trop. Med. Hyg. 2006, 75, 143–145. [Google Scholar]
- Myint, H.Y.; Ashley, E.A.; Day, N.P.J.; Nosten, F.; White, N.J. Efficacy and safety of dihydroartemisinin-piperaquine. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 858–866. [Google Scholar]
- Fanello, C.I.; Karema, C.; van Doren, W.; Van Overmeir, C.; Ngamije, D.; D’Alessandro, U. A randomised trial to assess the safety and efficacy of artemether-lumefantrine (Coartem®) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwanda. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Posner, G.H.; Paik, I.-H.; Chang, W.; Borstnik, K.; Sinishtaj, S.; Rosenthal, A.S.; Shapiro, T.A. Malaria-infected mice are cured by a single dose of novel artemisinin derivatives. J. Med. Chem. 2007, 50, 2516–2519. [Google Scholar]
- Posner, G.H.; Chang, W.; Hess, L.; Woodard, L.; Sinishtaj, S.; Usera, A.R.; Maio, W.; Rosenthal, A.S.; Kalinda, A.S.; D’Angelo, J.G.; Petersen, K.S.; Stohler, R.; Chollet, J.; Santo-Tomas, J.; Snyder, C.; Rottmann, M.; Wittlin, S.; Brun, R.; Shapiro, T.A. Malaria-infected mice are cured by oral administration of new artemisinin derivatives. J. Med. Chem. 2008, 51, 1035–1042. [Google Scholar]
- Rosenthal, A.S.; Chen, X.; Liu, J.O.; West, D.C.; Hergenrother, P.J.; Shapiro, T.A.; Posner, G.H. Malaria-Infected Mice Are Cured by a Single Oral Dose of New Dimeric Trioxane Sulfones Which Are Also Selectively and Powerfully Cytotoxic to Cancer Cells. J. Med. Chem. 2009, 52, 1198–1203. [Google Scholar]
- Woodard, L.E.; Chang, W.; Chem, X.; Liu, J.O.; Shapiro, T.A.; Posner, G.H. Malaria-Infected Mice Live until at Least Day 30 after a New Monomeric Trioxane Combined with Mefloquine are Administered Together in a Single Low Dose. J. Med. Chem. 2009, 52. in press. [Google Scholar]
- Moon, D.K.; Singhal, V.; Kumar, N.; Shapiro, T.A.; Posner, G.H. Antimalarial Preclinical Drug Development: A Single Oral Dose of A 5-Carbon-linked Trioxane Dimer Plus Mefloquine Cures Malaria-Infected Mice. Drug Dev. Res. 2009. in press.
- Gately, S.; West, R. Novel Therapeutics With Enhanced Biological Activity Generated by the Strategic Introduction of Silicon Isosteres into Known Drug Scaffolds. Drug Dev. Res. 2007, 68, 156–163. [Google Scholar]
- Sagara, I.; Rulisa, S.; Mbacham, W.; Adam, I.; Sissoko, K.; Maiga, H.; Traore, O.B.; Dara, N.; Dicko, Y.T.; Dicko, A.; Djimde, A.; Jansen, F.H.; Doumbo, O.K. Efficacy and Safety of a Fixed Dose Artesunate-sulphamethoxypyrazine-pyrimethamine Compared to Artemether-lumefantrine for the Treatment of Uncomplicated falciparum malaria across Africa: A Randomized Multi-centre trial. Malaria J. 2009, 8, 63. [Google Scholar]
- Gautam, A.; Ahmed, T.; Batra, V.; Paliwal, J. Pharmacokinetics and Pharmacodynamics of Endoperoxide Antimalarials. Curr. Drug. Metab. 2009, 10, 289–306. [Google Scholar]
- Arinaitwe, E.; Sandison, T.G.; Wanzira, H.; Kakuru, A.; Homsy, J.; Kalamya, J.; Kamya, M.R.; Vora, N.; Greenhouse, B.; Rosenthal, P.J.; Tappero, J.; Dorsey, G. Artemether-Lumefantrine versus Dihydroartemisinin-Piperaquine for Falciparum Malaria: A Longitudinal, Randomized Trial in Young Ugandan Children. Clin. Infect. Dis. 2009, 49, 1629–1637. [Google Scholar]
- Eastman, R.T.; Fidock, D.A. Artemisinin-based Combination Therapies: A Vital Tool in Efforts to Eliminate Malaria. Nat. Rev. Micro. 2009, 7, 864–874. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Woodard, L.E.; Mott, B.T.; Singhal, V.; Kumar, N.; Shapiro, T.A.; Posner, G.H. Malaria-Infected Mice Are Cured by a Single Low Dose of a New Silylamide Trioxane Plus Mefloquine. Pharmaceuticals 2009, 2, 228-235. https://doi.org/10.3390/ph2030228
Woodard LE, Mott BT, Singhal V, Kumar N, Shapiro TA, Posner GH. Malaria-Infected Mice Are Cured by a Single Low Dose of a New Silylamide Trioxane Plus Mefloquine. Pharmaceuticals. 2009; 2(3):228-235. https://doi.org/10.3390/ph2030228
Chicago/Turabian StyleWoodard, Lauren E., Bryan T. Mott, Vandana Singhal, Nirbhay Kumar, Theresa A. Shapiro, and Gary H. Posner. 2009. "Malaria-Infected Mice Are Cured by a Single Low Dose of a New Silylamide Trioxane Plus Mefloquine" Pharmaceuticals 2, no. 3: 228-235. https://doi.org/10.3390/ph2030228
APA StyleWoodard, L. E., Mott, B. T., Singhal, V., Kumar, N., Shapiro, T. A., & Posner, G. H. (2009). Malaria-Infected Mice Are Cured by a Single Low Dose of a New Silylamide Trioxane Plus Mefloquine. Pharmaceuticals, 2(3), 228-235. https://doi.org/10.3390/ph2030228