Synthesis, Characterization, Antimicrobial and Anticancer Evaluation of Novel Heterocyclic Diazene Compounds Derived from 8-Quinolinol
Abstract
1. Introduction
2. Results
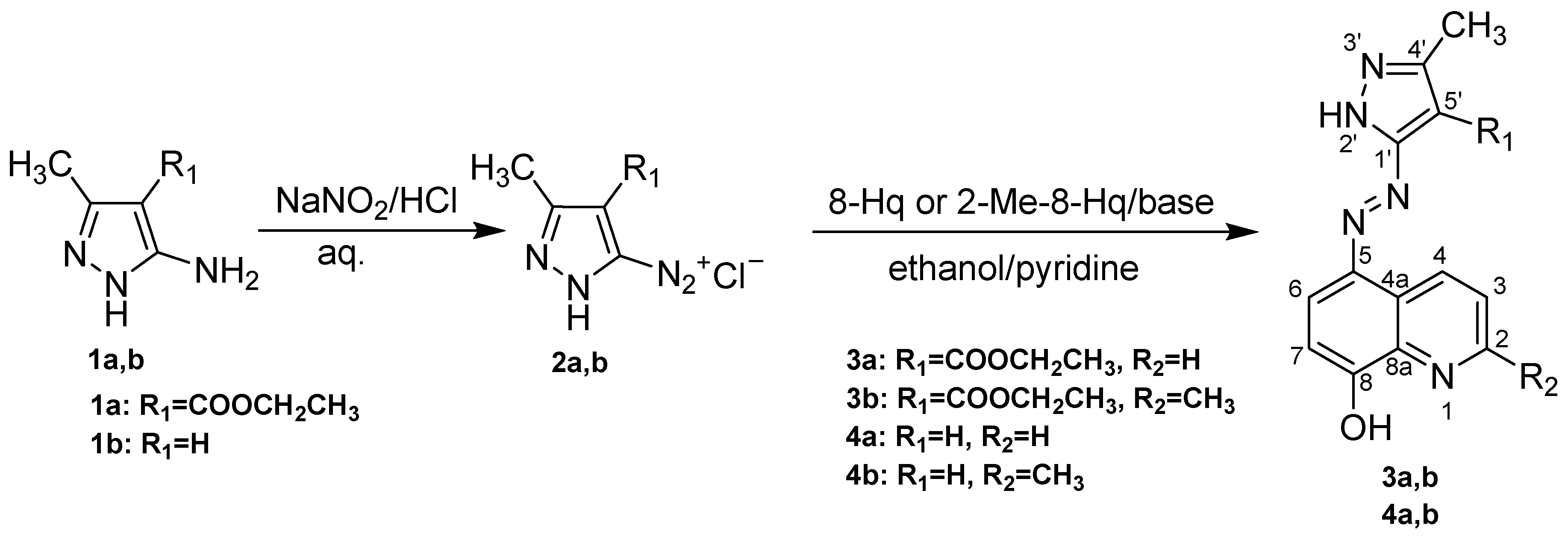
2.1. Chemical Synthesis and Characterization of 8-Quinolinol Derivatives with Pyrazole Moieties
2.2. Screening of the Biological Activities
3. Discussion
Biological Activity Evaluation
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Spectroscopy and Chromatographic Analysis
4.2.2. General Procedure for the Synthesis of Diazonium Salts (2a) and (2b)
4.2.3. General Procedure of the Diazo Coupling and Hydrolysis Reactions for the Synthesis of Compounds (3a), (3b), (4a), and (4b)
4.2.4. Evaluation of the Biological Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abah, L.O.; Egu, S.A.; Omale, A.; Ocholi, S.S.; Omale, J.A.; Onoja, A.O.; Kazeem, S.; Abdulrazak, A.; Okpanachi, C.B.; Amlabu, E. Highlights of the Chemistry and Pharmacological Potential of 8-Hydroxyquinoline: A Review. Discov. Chem. 2025, 2, 174. [Google Scholar] [CrossRef]
- Yadav, V.; Reang, J.; Sharma, V.; Majeed, J.; Sharma, P.C.; Sharma, K.; Giri, N.; Kumar, A.; Tonk, R.K. Quinoline-Derivatives as Privileged Scaffolds for Medicinal and Pharmaceutical Chemists: A Comprehensive Review. Chem. Biol. Drug Des. 2022, 100, 389–418. [Google Scholar] [CrossRef]
- Maity, A.; Rajak, K.K. Photo Induced Trans→Cis Isomerism Studies of Heteroleptic Iridium Complex with 8-Quinolinol-5-phenylazo Ligand: Photophysical and Electrochemical Studies and Its Theoretical Investigations. J. Mol. Struct. 2019, 1181, 38–47. [Google Scholar] [CrossRef]
- Kaulage, M.H.; Maji, B.; Pasadi, S.; Bhattacharya, S.; Muniyappa, K. Novel Ruthenium Azo-Quinoline Complexes with Enhanced Photonuclease Activity in Human Cancer Cells. Eur. J. Med. Chem. 2017, 139, 1016–1029. [Google Scholar] [CrossRef]
- Shervedani, R.K.; Rezvaninia, Z.; Sabzyan, H.; Zali-Boeini, H. Characterization of Gold-Thiol-8-hydroxyquinoline Self-Assembled Monolayers for Selective Recognition of Aluminum Ion Using Voltammetry and Electrochemical Impedance Spectroscopy. Anal. Chim. Acta 2014, 825, 34–41. [Google Scholar] [CrossRef]
- Abu-Zuhri, A.Z.; Rady, M. Spectrophotometric Studies and Analytical Application of Gold(III), Yttrium(III) and Palladium(II) Chelates with 7-(2-Pyridylazo)-5-chloro-8-hydroxyquinoline. J. Chem. Technol. Biotechnol. 1992, 54, 39–42. [Google Scholar] [CrossRef]
- Huang, H.; Chikushi, H.; Nakamura, M.; Kai, F. Selectivity of Coordination Sites in Ni(II), Zn(II), and Cd(II) Complexes with 7-[(3,5-Dichloro(or bromo)-2-pyridyl)azo]-8-hydroxyquinoline-5-sulfonic Acid. Bull. Chem. Soc. Jpn. 1990, 63, 1985–1993. [Google Scholar] [CrossRef]
- Bhagwat, A.; Butts, A.; Greve, E.; Cheung, Y.; Melief, E.; Gomez, J.; Hung, D.T.; Parish, T. 8-Hydroxyquinoline Series Exerts Bactericidal Activity against Mycobacterium Tuberculosis Via Copper-Mediated Toxicity. ACS Infect. Dis. 2024, 10, 3692–3698. [Google Scholar] [CrossRef]
- Zhou, S.-H.; Liao, W.-H.; Yang, Y.; Li, W.; Wu, Y.; Wu, T.-T.; Deng, S.-H.; Zhou, J.; Li, Z.; Zhao, Q.-H.; et al. (8-Hydroxyquinoline) Gallium(III) Complex with High Antineoplastic Efficacy for Treating Colon Cancer via Multiple Mechanisms. ACS Omega 2023, 8, 6945–6958. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.; Bulut, I.; Sergi, B.; Pósa, V.; Spengler, G.; Sciortino, G.; André, V.; Ferreira, L.P.; Biver, T.; Ugone, V.; et al. Promising Anticancer Agents Based on 8-Hydroxyquinoline Hydrazone Copper(II) Complexes. Front. Chem. 2023, 11, 1106349. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Sweidan, K.A.; Mubarak, M.S. Recent Advances in the Synthesis and Biological Activity of 8-Hydroxyquinolines. Molecules 2020, 25, 4321. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Zhang, N.; Fan, R.; Ye, Y.; Xu, J. Recent Advances in the Development of Pyrazole Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 12724. [Google Scholar] [CrossRef]
- Hosamani, K.R.; K, H.; Pal, R.; Matada, G.S.P.; B, K.; I, A.; Aishwarya, N.V.S.S. Pyrazole, Pyrazoline, and Fused Pyrazole Derivatives: New Horizons in EGFR-Targeted Anticancer Agents. Chem. Biodivers. 2024, 21, e202400880. [Google Scholar] [CrossRef] [PubMed]
- Alnufaie, R.; Raj KC, H.; Alsup, N.; Whitt, J.; Andrew Chambers, S.; Gilmore, D.; Alam, M.A. Synthesis and Antimicrobial Studies of Coumarin-Substituted Pyrazole Derivatives as Potent Anti-Staphylococcus Aureus Agents. Molecules 2020, 25, 2758. [Google Scholar] [CrossRef]
- Ragab, A.; Fouad, S.A.; Ammar, Y.A.; Aboul-Magd, D.S.; Abusaif, M.S. Antibiofilm and Anti-Quorum-Sensing Activities of Novel Pyrazole and Pyrazolo [1,5-a]Pyrimidine Derivatives as Carbonic Anhydrase I and II Inhibitors: Design, Synthesis, Radiosterilization, and Molecular Docking Studies. Antibiotics 2023, 12, 128. [Google Scholar] [CrossRef]
- Masih, A.; Agnihotri, A.K.; Srivastava, J.K.; Pandey, N.; Bhat, H.R.; Singh, U.P. Discovery of Novel Pyrazole Derivatives as a Potent Anti-Inflammatory Agent in RAW264.7 Cells via Inhibition of NF-ĸB for Possible Benefit against SARS-CoV-2. J. Biochem. Mol. Toxicol. 2021, 35, e22656. [Google Scholar] [CrossRef]
- Mantzanidou, M.; Pontiki, E.; Hadjipavlou-Litina, D. Pyrazoles and Pyrazolines as Anti-Inflammatory Agents. Molecules 2021, 26, 3439. [Google Scholar] [CrossRef]
- Pape, V.F.S.; Palkó, R.; Tóth, S.; Szabó, M.J.; Sessler, J.; Dormán, G.; Enyedy, É.A.; Soós, T.; Szatmári, I.; Szakács, G. Structure–Activity Relationships of 8-Hydroxyquinoline-Derived Mannich Bases with Tertiary Amines Targeting Multidrug-Resistant Cancer. J. Med. Chem. 2022, 65, 7729–7745. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; Al-Hussain, S.A. Design, Synthesis, Antimicrobial Activity, and Molecular Docking of Novel Thiazoles, Pyrazoles, 1,3-Thiazepinones, and 1,2,4-Triazolopyrimidines Derived from Quinoline-Pyrido[2,3-d] Pyrimidinones. Pharmaceuticals 2024, 17, 1632. [Google Scholar] [CrossRef] [PubMed]
- El-Ghamry, H.A.; Gaber, M.; Napolion, M.S.; Atta, A.; Mohamed, T.M.; El-Wakiel, N.A. Synthesis, Characterization and Theoretical Studies of Nitroxoline Azo Dye Metal Complexes and Their Role in Mitigation of Rheumatoid Arthritis. Sci. Rep. 2025, 15, 20213. [Google Scholar] [CrossRef]
- Wen, J.; Charan Dash, R.; Zaino, A.M.; Harrahill, N.J.; Calhoun, J.T.; Dusek, C.O.; Morel, S.R.; Russolillo, M.; Kyle Hadden, M. 8-Hydroxyquinoline Derivatives Suppress GLI1-Mediated Transcription through Multiple Mechanisms. Bioorg. Chem. 2023, 132, 106387. [Google Scholar] [CrossRef]
- Saeed, A.; Madkhli, A.Y.; Pashameah, R.A.; Bataweel, N.M.; Razvi, M.A.; Salah, N. Antibacterial Activity of the Micro and Nanostructures of the Optical Material Tris(8-Hydroxyquinoline)Aluminum and Its Application as an Antimicrobial Coating. RSC Adv. 2022, 12, 27131–27144. [Google Scholar] [CrossRef]
- Burcă, I.; Badea, V.; Deleanu, C.; Bercean, V.-N. 5-((8-Hydroxyquinolin-5-Yl)Diazenyl)-3-Methyl-1H-Pyrazole-4-Carboxylic Acid. Molbank 2021, 2021, M1238. [Google Scholar] [CrossRef]
- Burcă, I.; Diaconescu, A.-M.; Badea, V.; Péter, F. 5-((4-(-Phenyldiazenyl)Phenyl)Diazenyl)Quinolin-8-Ol. Molbank 2023, 2023, M1701. [Google Scholar] [CrossRef]
- Brennecke, P.; Rasina, D.; Aubi, O.; Herzog, K.; Landskron, J.; Cautain, B.; Vicente, F.; Quintana, J.; Mestres, J.; Stechmann, B.; et al. EU-OPENSCREEN: A Novel Collaborative Approach to Facilitate Chemical Biology. SLAS Discov. 2019, 24, 398–413. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side Effects and Toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Holcombe, L.J.; O′Gara, F.; Morrissey, J.P. Implications of Interspecies Signaling for Virulence of Bacterial and Fungal Pathogens. Future Microbiol. 2011, 6, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida Auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J. The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- CLSI M100 Performance Standards for Antimicrobial Susceptibility Testing. Available online: https://clsi.org/shop/standards/m100/ (accessed on 3 September 2025).
- Staicu, M.; Brundige, M.L.; Datta, S.; Laguio-Vila, M. Safety of Stopping Antibiotics Prescribed “Just in Case”—Comparison of Mortality, Readmissions and Clostridium Difficile in Patients with Accepted Stewardship Interventions Compared with Declined. Open Forum Infect. Dis. 2017, 4, S482. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of Action and Resistance of Older and Newer Fluoroquinolones. Clin. Infect. Dis. 2000, 31, S24–S28. [Google Scholar] [CrossRef]
- Ayati, A.; Esmaeili, R.; Moghimi, S.; Bakhshaiesh, T.O.; Eslami-S, Z.; Majidzadeh-A, K.; Safavi, M.; Emami, S.; Foroumadi, A. Synthesis and Biological Evaluation of 4-Amino-5-Cinnamoylthiazoles as Chalcone-like Anticancer Agents. Eur. J. Med. Chem. 2018, 145, 404–412. [Google Scholar] [CrossRef]
- Butler, R.N. Diazotization of Heterocyclic Primary Amines. Chem. Rev. 1975, 75, 241–257. [Google Scholar] [CrossRef]
- Sidunets, Y.A.; Melekhina, V.G.; Fershtat, L.L. Tandem Diazotization/Cyclization Approach for the Synthesis of a Fused 1,2,3-Triazinone-Furazan/Furoxan Heterocyclic System. Beilstein J. Org. Chem. 2024, 20, 2342–2348. [Google Scholar] [CrossRef]
- Mezgebe, K.; Mulugeta, E. Synthesis and Pharmacological Activities of Azo Dye Derivatives Incorporating Heterocyclic Scaffolds: A Review. RSC Adv. 2022, 12, 25932–25946. [Google Scholar] [CrossRef]
- Çolak, N.; Erten, G.; İzancı, A.; Sancur, U. Monoazo Dyes: Coupling Reactions between Indanone Compounds and Heterocyclic Aminothiophene-Containing Spiro Group. Hitit J. Sci. 2024, 1, 1–7. [Google Scholar]
- Ayon, N.J. High-Throughput Screening of Natural Product and Synthetic Molecule Libraries for Antibacterial Drug Discovery. Metabolites 2023, 13, 625. [Google Scholar] [CrossRef] [PubMed]
- Blay, V.; Tolani, B.; Ho, S.P.; Arkin, M.R. High-Throughput Screening: Today’s Biochemical and Cell-Based Approaches. Drug Discov. Today 2020, 25, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Fiala, J.; Schöbel, H.; Vrabl, P.; Dietrich, D.; Hammerle, F.; Artmann, D.J.; Stärz, R.; Peintner, U.; Siewert, B. A New High-Throughput-Screening-Assay for Photoantimicrobials Based on EUCAST Revealed Unknown Photoantimicrobials in Cortinariaceae. Front. Microbiol. 2021, 12, 703544. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, L.M.; Sensbach, J.; Pipp, F.; Werkmann, D.; Hewitt, P. Increasing Sustainability and Reproducibility of in Vitro Toxicology Applications: Serum-Free Cultivation of HepG2 Cells. Front. Toxicol. 2024, 6, 1439031. [Google Scholar] [CrossRef]
- Sokal, A.; Wrzalik, R.; Latocha, M.; Kadela-Tomanek, M. The 8-Hydroxyquinoline Derivatives of 1,4-Naphthoquinone: Synthesis, Computational Analysis, and Anticancer Activity. Int. J. Mol. Sci. 2025, 26, 5331. [Google Scholar] [CrossRef] [PubMed]
- Zieba, A.; Pindjakova, D.; Latocha, M.; Plonka-Czerw, J.; Kusmierz, D.; Cizek, A.; Jampilek, J. Design, Synthesis, and Anticancer and Antibacterial Activities of Quinoline-5-Sulfonamides. Molecules 2024, 29, 4044. [Google Scholar] [CrossRef]
- Rbaa, M.; Haida, S.; Tuzun, B.; Hichar, A.; El Hassane, A.; Kribii, A.; Lakhrissi, Y.; Ben Hadda, T.; Zarrouk, A.; Lakhrissi, B.; et al. Synthesis, Characterization and Bioactivity of Novel 8-Hydroxyquinoline Derivatives: Experimental, Molecular Docking, DFT and POM Analyses. J. Mol. Struct. 2022, 1258, 132688. [Google Scholar] [CrossRef]
- Beyer, H.; Wolter, G. Über Thiazole, XXIX. Mitteil.: Über die Kondensationsprodukte von Thiosemicarbazid mit α- Chloracetessigester und eine neuartige Ringverengung des 2-Amino-5- methyl-6-carbäthoxy −1.3.4-thiodiazins zum 3-Methyl-4- carbäthoxy-5-amino-pyrazol. Chem. Ber. 1956, 89, 1652–1658. [Google Scholar] [CrossRef]
- Ege, G.; Heck, R.; Gilbert, K.; Irngartinger, H.; Huber-Patz, U.; Rodewald, H. Reactions with Diazoazoles. Part VI. Unequivocal Synthesis of 3-Methyl-3h-Azolotetrazoles. Correction of the Formerly Described 3-Methylazolotetrazoles in Favour of Mesoionic 2-Methylazolotetrazoles. J. Heterocyc. Chem. 1983, 20, 1629–1639. [Google Scholar] [CrossRef]


| HSQC 1H-13C | HMBC 1H-13C | HMBC 1H-15N | COSY 1H-1H |
|---|---|---|---|
| 9.45→133.3 (4-H) (4-C) | 4.47→164.8 (-CH2-) (-C=O) | 8.97→298.8 (2-H) (1-N) | 9.45→7.47 (4-H) (3-H) |
| 8.97→149.6 (2-H) (2-C) | 8.49→160.6 (6-H) (8-C) | 7.52→298.8 (7-H) (1-N) | 8.97→7.47 (2-H) (3-H) |
| 8.49→118.9 (6-H) (6-C) | 7.52→160.6 (7-H) (8-C) | 8.56→7.52 (6-H) (7-H) | |
| 7.52→113.0 (7-H) (7-C) | 9.45→149.6 (4-H) (2-C) | ||
| 7.47→123.6 (3-H) (3-C) | 7.47→149.6 (3-H) (2-C) | ||
| 8.49→140.8 (6-H) (8a-C) | |||
| 7.52→140.8 (7-H) (8a-C) | |||
| 9.45→139.7 (4-H) (4a-C) | |||
| 8.97→139.7 (2-H) (4a-C) | |||
| 7.52→139.7 (7-H) (4a-C) | |||
| 8.97→133.3 (2-H) (4-C) | |||
| 8.49→133.3 (6-H) (4-C) | |||
| 8.97→128.9 (2-H) (5-C) | |||
| 8.49→128.9 (6-H) (5-C) | |||
| 7.47→128.9 (3-H) (5-C) | |||
| 2.73→159.4 (-CH3) (4′-C) |
| HSQC 1H-13C | HMBC 1H-13C | HMBC 1H-15N | COSY 1H-1H |
|---|---|---|---|
| 9.36→133.1 (4-H) (4-C) | 9.36→138.6 (4-H) (8a-C) | 7.50→291.1 (7-H) (1-N) | 9.36→7.33 (4-H) (3-H) |
| 8.42→117.8 (6-H) (6-C) | 9.36→157.9 (4-H) (2-C) | 7.33→291.1 (3-H) (1-N) | 8.42→7.50 (6-H) (7-H) |
| 7.50→112.3 (7-H) (7-C) | 8.42→126.1 (6-H) (4a-C) | 2.56→291.1 (2-C-CH3) (1-N) | |
| 7.33→124.3 (3-H) (3-C) | 8.42→159.3 (6-H) (8-C) | ||
| 2.74→13.1 (4′-C-CH3) (4′-C-CH3) | 7.50→138.6 (7-H) (8a-C) | ||
| 2.56→24.6 (2-C-CH3) (2-C-CH3) | 7.50→159.3 (7-H) (8-C) | ||
| 7.33→126.1 (3-H) (4a-C) |
| HSQC 1H-13C | HMBC 1H-13C | HMBC 1H-15N | COSY 1H-1H |
|---|---|---|---|
| 9.25→131.7 (4-H) (4-C) | 9.25→138.7 (4-H) (8a-C) | 9.00→299.0 (2-H) (1-N) | 9.25→7.75 (4-H) (3-H) |
| 9.00→148.8 (2-H) (2-C) | 9.25→148.8 (4-H) (2-C) | 7.75→299.0 (3-H) (1-N) | 9.00→7.75 (2-H) (3-H) |
| 7.86→114.2 (6-H) (6-C) | 9.00→122.9 (2-H) (3-C) | 2.33→209.2 (CH3) (3′-N) | 7.86→7.24 (6-H) (7-H) |
| 7.75→122.9 (3-H) (3-C) | 9.00→131.7 (2-H) (4-C) | ||
| 7.24→111.6 (7-H) (7-C) | 9.00→137.8 (2-H) (4a-C) | ||
| 6.52→92.5 (5′-H) (5′-C) | 7.86→127.1 (6-H) (5-C) | ||
| 2.33→15.1 (CH3) (CH3) | 7.86→137.8 (6-H) (4a-C) | ||
| 7.75→127.1 (3-H) (5-C) | |||
| 7.75→148.8 (3-H) (2-C) | |||
| 7.24→138.7 (7-H) (8a-C) | |||
| 2.33→140.1 (CH3) (4′-C) | |||
| 2.33→92.5 (CH3) (5′-C) |
| HSQC 1H-13C | HMBC 1H-13C | HMBC 1H-15N | COSY 1H-1H |
|---|---|---|---|
| 9.15→157.5 (4-H) (2-C) | 7.87→125.4 (6-H) (4a-C) | 2.76→292.8 (2-C-CH3) (1-N) | 9.15→7.62 (4-H) (3-H) |
| 7.87→113.2 (6-H) (6-C) | 7.87→139.2 (6-H) (5-C) | 2.33→208.9 (4′-C-CH3) (2′-N) | 7.87→7.20 (6-H) (7-H) |
| 7.62→123.7 (3-H) (3-C) | 7.87→156.2 (6-H) (8-C) | ||
| 7.20→111.4 (7-H) (7-C) | 7.62→125.4 (3-H) (4a-C) | ||
| 6.49→92.5 (5′-H) (5′-C) | 7.62→157.5 (3-H) (2-C) | ||
| 2.76→24.6 (2-C-CH3) (2-C-CH3) | 7.20→137.2 (7-H) (8a-C) | ||
| 2.33→10.6 (4′-C-CH3) (4′-C-CH3) | 7.20→139.2 (7-H) (5-C) | ||
| 7.20→156.2 (7-H) (8-C) | |||
| 2.76→123.7 (2-C-CH3) (3-C) | |||
| 2.76→157.5 (2-C-CH3) (2-C) | |||
| 2.33→92.5 (4′-C-CH3) (5′-C) | |||
| 2.33→140.2 (4′-C-CH3) (4′-C) |
| HSQC 1H-13C | HMBC 1H-13C | HMBC 1H-15N | COSY 1H-1H |
|---|---|---|---|
| 9.15→131.3 (2-H) (2-C) | 9.15→132.9 (2-H) (8a-C) | n.d. | 9.15→7.55 (2-H) (3-H) |
| 8.69→146.5 (4-H) (4-C) | 9.15→141.0 (2-H) (8-C) | 7.95→6.74 (7-H) (6-H) | |
| 7.95→117.3 (7-H) (7-C) | 8.69→131.3 (4-H) (2-C) | ||
| 7.55→122.3 (3-H) (3-C) | 8.69→122.3 (4-H) (3-C) | ||
| 6.74→113.9 (6-H) (6-C) | 7.55→129.2 (3-H) (4a-C) | ||
| 2.26→11.5 (CH3) (CH3) | 6.74→129.2 (6-H) (4a-C) | ||
| 2.26→93.0 (CH3) (5′-C) |
| HSQC 1H-13C | HMBC 1H-13C | HMBC 1H-15N | COSY 1H-1H |
|---|---|---|---|
| 9.37→132.8 (4-H) (4-C) | 9.37→138.5 (4-H) (4a-C) | 2.60→291.1 (2-C-CH3) (1-N) | 9.37→7.47 (4-H) (3-H) |
| 8.33→114.7 (6-H) (6-C) | 8.33→126.4 (4-H) (5-C) | 8.33→7.47 (6-H) (7-H) | |
| 7.47→123.8 (7-H) (7-C) | 7.47→138.5 (3-H) (4a-C) | ||
| 7.47→112.0 (3-H) (3-C) | 2.60→157.8 (2-C-CH3) (2-C) | ||
| 2.60→24.7 (2-C-CH3) (2-C-CH3) | 2.41→94.6 (4′-C-CH3) (5′-C) |
| Microorganism | Compound Inhibition [%] | ||||||
|---|---|---|---|---|---|---|---|
| Amphotericin B [26,27,28] | (3a) | (3b) | (4a) | (4b) | (5a) | (5b) | |
| C. albicans | 95 ± 3% | 94.63 | 82.35 | 96.71 | 96.84 | 94.90 | 94.45 |
| C. auris | 90 ± 4% | 99.10 | 97.01 | 98.37 | 98.74 | 97.92 | 99.48 |
| A. fumigatus | 85 ± 4% | 102.07 | 13.71 | 102.14 | 69.65 | 102.54 | 45.10 |
| Microorganism | Compound Inhibition [%] | ||||||
|---|---|---|---|---|---|---|---|
| Vancomycin [29,30] | (3a) | (3b) | (4a) | (4b) | (5a) | (5b) | |
| E. faecalis | 95 ± 3% | 4.77 | 1.34 | −0.97 | 15.51 | 3.97 | 2.05 |
| S. aureus | 90 ± 4% | 9.35 | 16.50 | 30.99 | 23.42 | 51.29 | 15.20 |
| Microorganism | Compound Inhibition [%] | ||||||
|---|---|---|---|---|---|---|---|
| Ciprofloxacin [31,32] | (3a) | (3b) | (4a) | (4b) | (5a) | (5b) | |
| P. aeruginosa | 60 ± 6% | −0.27 | −13.20 | 7.62 | 1.46 | 6.04 | −21.90 |
| E.coli | 80 ± 5% | −7.90 | −12.16 | −0.27 | −2.39 | −0.71 | −12.16 |
| K. pneumoniae | 65 ± 6% | −6.40 | −26.00 | 37.00 | −24.00 | 36.00 | 7.40 |
| A. baumannii | 40 ± 7% | −22.85 | −22.10 | −15.72 | −11.24 | −7.34 | −5.02 |
| Cell Line | Compound Inhibition [%] | ||||||
|---|---|---|---|---|---|---|---|
| Etoposide [33] | (3a) | (3b) | (4a) | (4b) | (5a) | (5b) | |
| Cell line permanent Hep-G2 | 61.7 ± 6% | 65.70 | 46.80 | 69.60 | 59.60 | 71.80 | 52.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Burcă, I.; Diaconescu, A.-M.; Badea, V.; Péter, F. Synthesis, Characterization, Antimicrobial and Anticancer Evaluation of Novel Heterocyclic Diazene Compounds Derived from 8-Quinolinol. Pharmaceuticals 2026, 19, 4. https://doi.org/10.3390/ph19010004
Burcă I, Diaconescu A-M, Badea V, Péter F. Synthesis, Characterization, Antimicrobial and Anticancer Evaluation of Novel Heterocyclic Diazene Compounds Derived from 8-Quinolinol. Pharmaceuticals. 2026; 19(1):4. https://doi.org/10.3390/ph19010004
Chicago/Turabian StyleBurcă, Ion, Alexandra-Mihaela Diaconescu, Valentin Badea, and Francisc Péter. 2026. "Synthesis, Characterization, Antimicrobial and Anticancer Evaluation of Novel Heterocyclic Diazene Compounds Derived from 8-Quinolinol" Pharmaceuticals 19, no. 1: 4. https://doi.org/10.3390/ph19010004
APA StyleBurcă, I., Diaconescu, A.-M., Badea, V., & Péter, F. (2026). Synthesis, Characterization, Antimicrobial and Anticancer Evaluation of Novel Heterocyclic Diazene Compounds Derived from 8-Quinolinol. Pharmaceuticals, 19(1), 4. https://doi.org/10.3390/ph19010004








