Comparative Study of Ferrocene- and Indene-Based Tamoxifen Derivatives of Different Molecular Flexibility on High-Mortality Cancer Cell Lines
Abstract
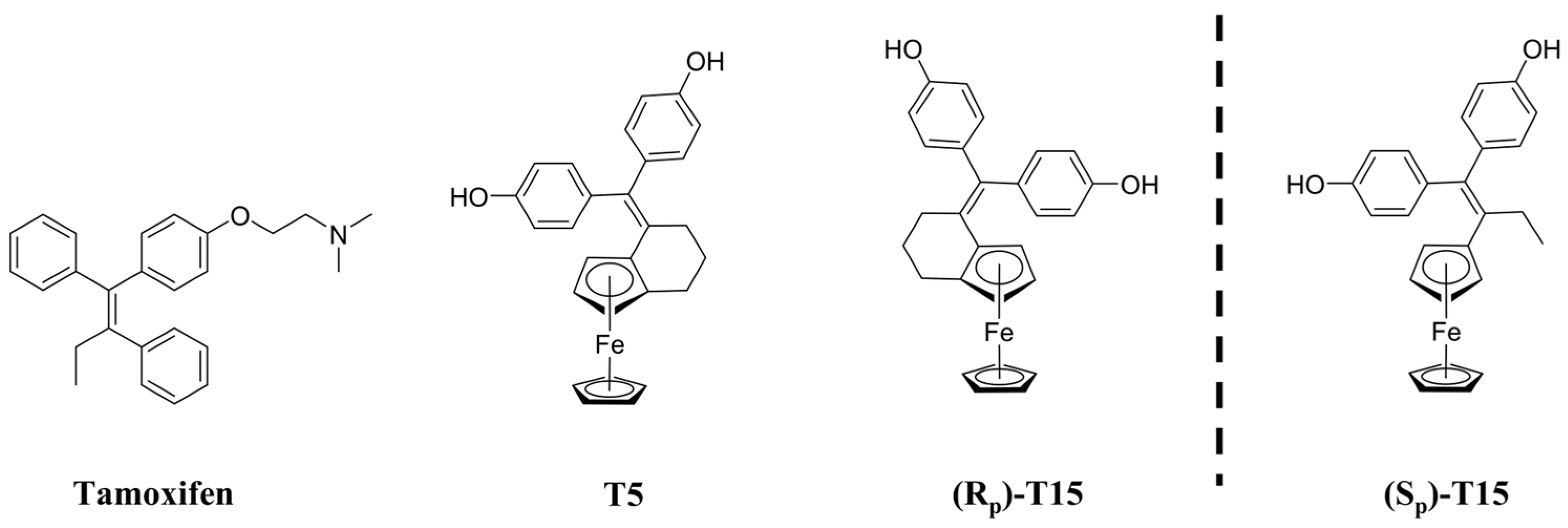
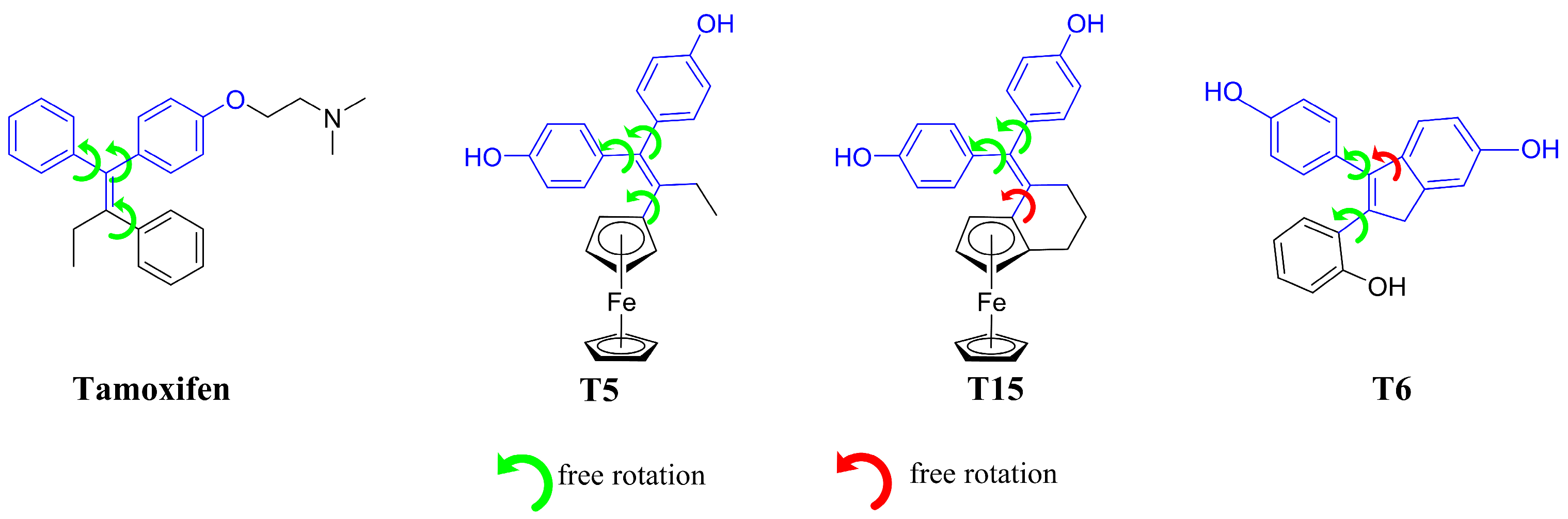
1. Introduction
2. Results
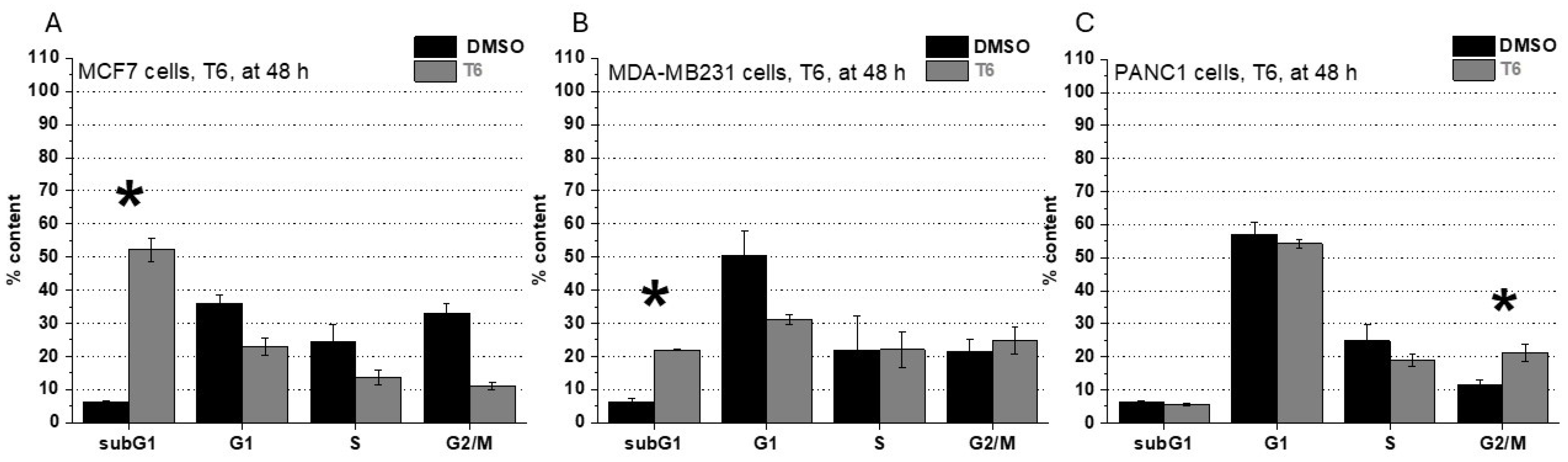
2.1. Cell Viability, Cell Cycle Analysis and ROS Production After T6 Treatment
2.2. Apoptosis Induction
2.3. Cell Cycle Regulator Expression Profile
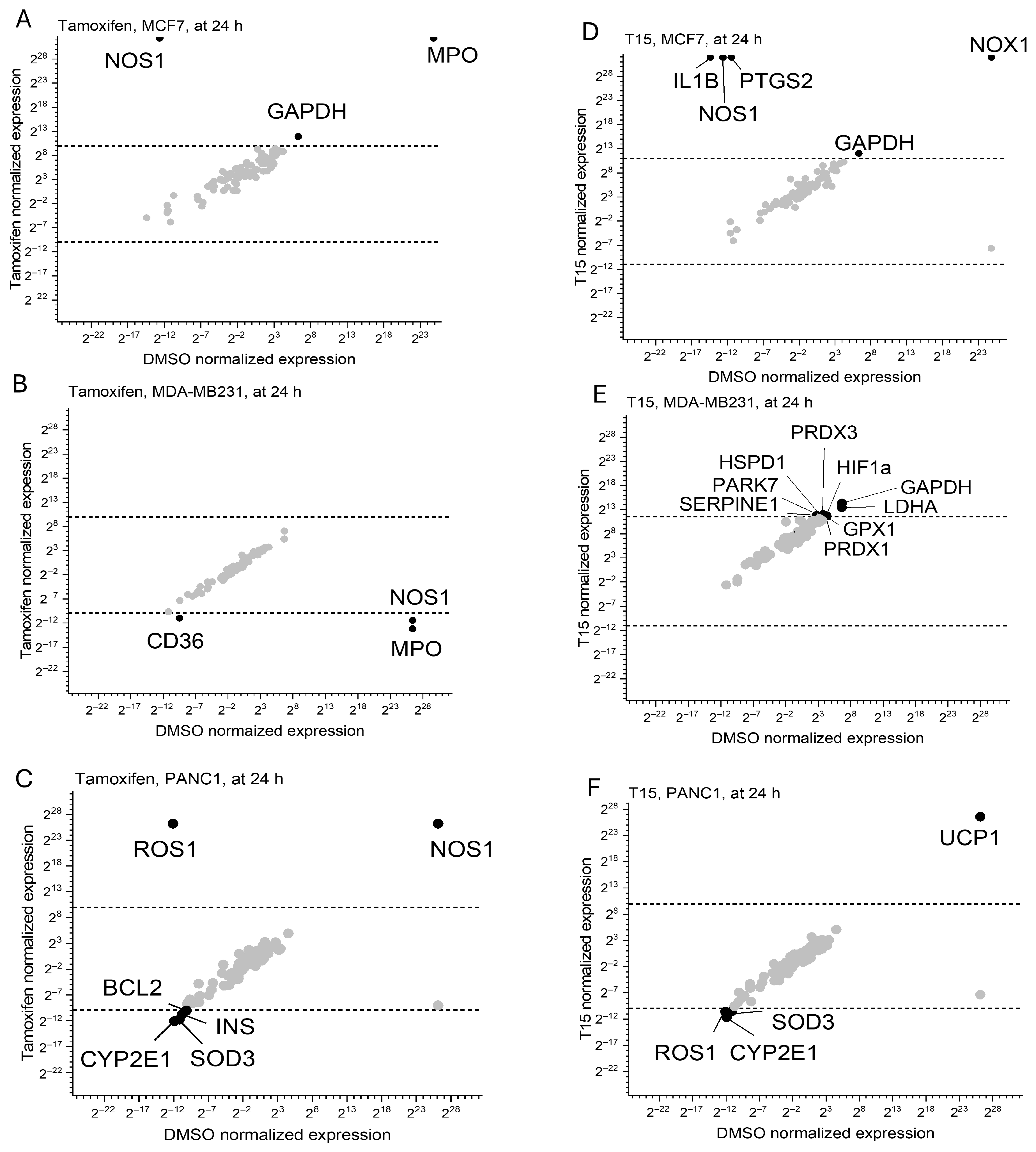
2.4. Oxidative Stress Pathway Regulator Expression Profile
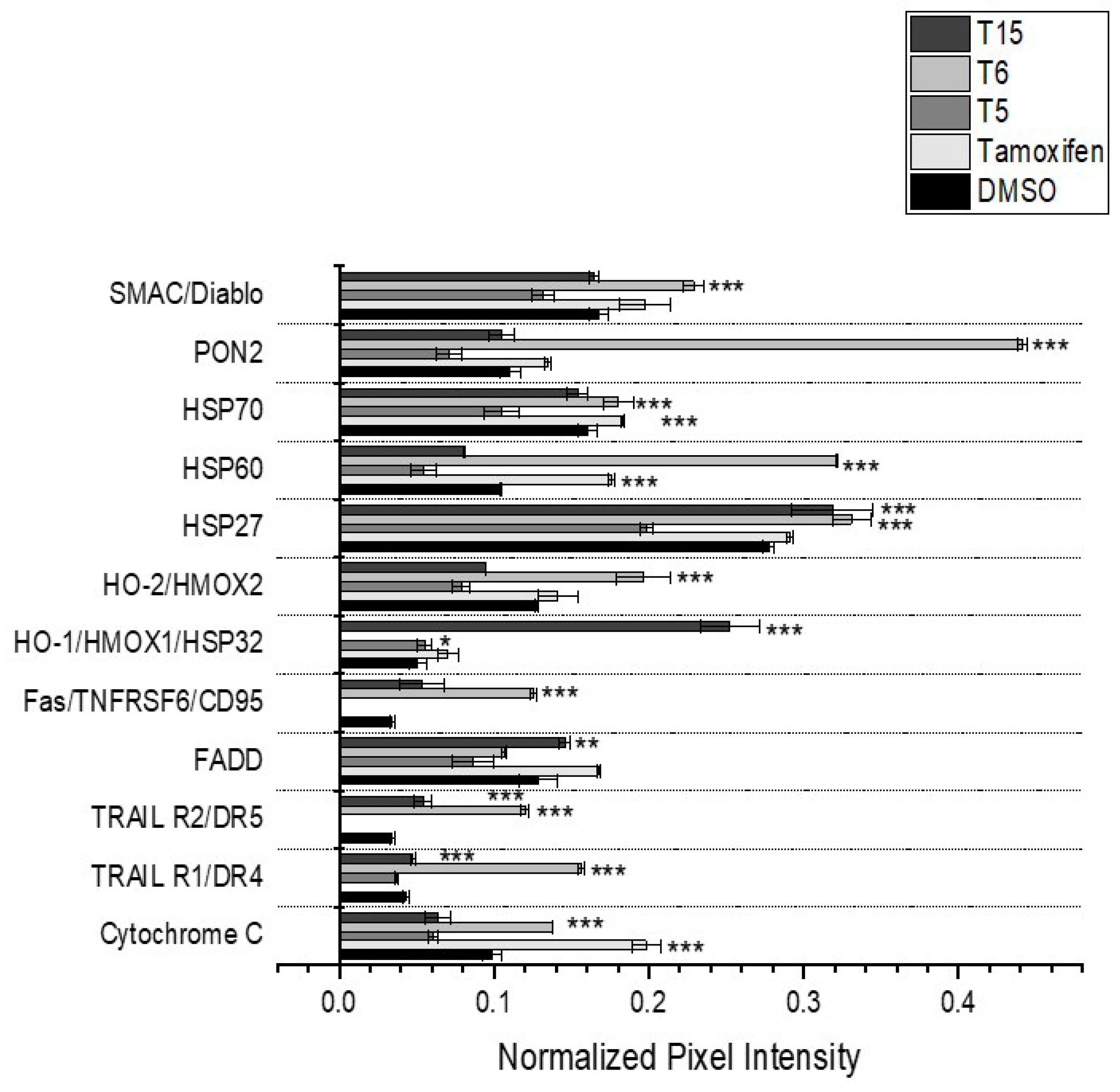
2.5. Apoptotic Regulator Protein Levels
3. Discussion
4. Materials and Methods
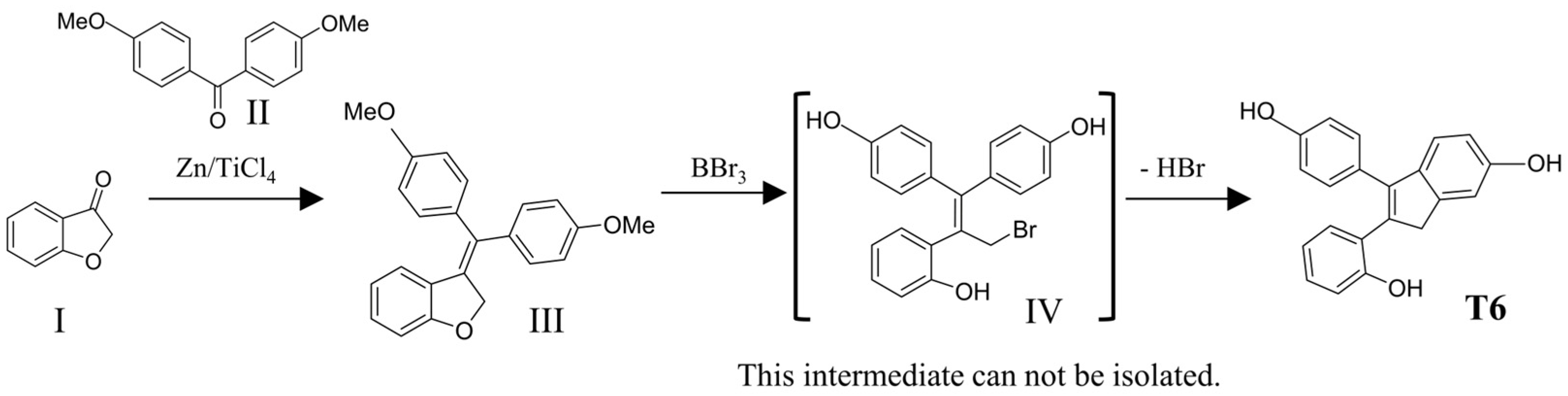
4.1. Synthesis of the Novel Tamoxifen Derivatives
4.2. Cell Lines and Culturing
4.3. Cell Viability Assays
4.4. Cell Cycle Analysis
4.5. ROSGlo Assay
4.6. Measuring Apoptosis Induction
4.7. Characterizing the Expression of Apoptosis Regulating Proteins
4.8. Measuring the Expression of Cell Cycle and Oxidative Stress Regulating Factors
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCL2 | B-cell lymphoma 2 |
| Bort | Bortezomib |
| CCNA1 | Cyclin A1 |
| CCNA2 | Cyclin A2 |
| CCNB2 | Cyclin B2 |
| CCND1 | Cyclin D1 |
| CCND2 | Cyclin D2 |
| CCND3 | Cyclin D3 |
| CCNE1 | Cyclin E1 |
| CD36 | Cluster of Differentiation 36 |
| CDC25 | Cell Division Cycle 25 |
| CDC25A | Cell Division Cycle 25A |
| CDC25B | Cell Division Cycle 25B |
| CDC25C | Cell Division Cycle 25C |
| CDK1 | Cyclin-dependent kinase 1 |
| CDK2 | Cyclin-dependent kinase 2 |
| CDK4 | Cyclin-dependent kinase 4 |
| CDK6 | Cyclin-dependent kinase 6 |
| cDNA | Complementary DNA |
| CYP2E1 | Cytochrome P450 2E1 |
| CytC | Cytochrome c |
| DMSO | Dimethyl sulfoxide |
| E2F1 | E2F Transcription Factor 1 |
| E2F2 | E2F Transcription Factor 2 |
| E2F3 | E2F Transcription Factor 3 |
| E2F5 | E2F Transcription Factor 5 |
| EC80 | Effective Concentration 80% |
| ER | Estrogen Receptor |
| FADD | Fas-Associated Death Domain |
| Fas/TNFRSF6/CD95 | Fas cell surface death receptor |
| GADH | Glutamate Dehydrogenase |
| GPX1 | Glutathione Peroxidase 1 |
| HGPRT | Hypoxanthine-guanine phosphoribosyltransferase |
| HIF1a | Hypoxia-Inducible Factor 1-alpha |
| HO1/HMOX1/HSP32 | Heme Oxygenase 1 |
| HO2/HMOX2 | Heme Oxygenase 2 |
| HSP27 | Heat Shock Protein 27 |
| HSP60 | Heat Shock Protein 60 |
| HSP70 | Heat Shock Protein 70 |
| HSPD1 | Heat Shock Protein Family D Member 1 |
| IC50 | Half Maximal Inhibitory Concentration |
| IL1B | Interleukin-1 Beta |
| INS | Insulin |
| LDHA | Lactate Dehydrogenase A |
| MPO | Myeloperoxidase |
| mRNA | Messenger RNA |
| NOS1 | Nitric Oxide Synthase 1 |
| NOX1 | NADPH Oxidase 1 |
| PARK7 | Parkinsonism-associated Deglycase |
| PON2 | Paraoxonase 2 |
| PRDX1 | Peroxiredoxin 1 |
| PRDX3 | Peroxiredoxin 3 |
| PTGS2 | Prostaglandin-Endoperoxide Synthase 2 (also known as COX-2) |
| RB1 | Retinoblastoma Protein |
| RBL1 | Retinoblastoma-like Protein 1 |
| RBL2 | Retinoblastoma-like Protein 2 |
| ROS | Reactive Oxygen Species |
| ROS1 | ROS Proto-Oncogene 1 |
| SERM | Selective Estrogen Receptor Modulator |
| SERPINE1 | Serpin Family E Member 1 |
| SMAC/Diablo | Second Mitochondria-derived Activator of Caspases |
| SOD3 | Superoxide Dismutase 3 |
| TBP | TATA-Binding Protein |
| TFDP1 | Transcription Factor Dp-1 |
| TRAIL R2/DR5 | TNF-Related Apoptosis-Inducing Ligand Receptor 2/Death Receptor 5 |
| TRAILR1/DR4 | TNF-Related Apoptosis-Inducing Ligand Receptor 1/Death Receptor 4 |
| UCP1 | Uncoupling Protein 1 |
References
- Jordan, V.C. Effect of tamoxifen (ICI 46,474) on initiation and growth of DMBA-induced rat mammary carcinomata. Eur. J. Cancer 1965 1976, 12, 419–424. [Google Scholar] [CrossRef]
- Plazuk, D.; Top, S.; Vessières, A.; Plamont, M.-A.; Huché, M.; Zakrzewski, J.; Makal, A.; Woźniak, K.; Jaouen, G. Organometallic cyclic polyphenols derived from 1,2-(α-keto tri or tetra methylene) ferrocene show strong antiproliferative activity on hormone-independent breast cancer cells. Dalton Trans. 2010, 39, 7444–7450. [Google Scholar] [CrossRef]
- Meegan, M.J.; Carr, M.; Knox, A.J.; Zisterer, D.M.; Lloyd, D.G. Beta-lactam type molecular scaffolds for antiproliferative activity: Synthesis and cytotoxic effects in breast cancer cells. J. Enzym. Inhib. Med. Chem. 2008, 23, 668–685. [Google Scholar] [CrossRef]
- Kalabay, M.; Szász, Z.; Láng, O.; Lajkó, E.; Pállinger, É.; Duró, C.; Jernei, T.; Csámpai, A.; Takács, A.; Kőhidai, L. Investigation of the Antitumor Effects of Tamoxifen and Its Ferrocene-Linked Derivatives on Pancreatic and Breast Cancer Cell Lines. Pharmaceuticals 2022, 15, 314. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Levin, E.R. Nature of functional estrogen receptors at the plasma membrane. Mol. Endocrinol. 2006, 20, 1996–2009. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Filardo, E.J.; Dong, J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2005, 146, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Duró, C.; Jernei, T.; Szekeres, K.J.; Láng, G.G.; Oláh-Szabó, R.; Bősze, S.; Szabó, I.; Hudecz, F.; Csámpai, A. Synthesis and SAR Analysis of Novel 4-Hydroxytamoxifen Analogues Based on Their Cytotoxic Activity and Electron-Donor Character. Molecules 2022, 27, 6758. [Google Scholar] [CrossRef] [PubMed]
- Scalcon, V.; Bonsignore, R.; Aupič, J.; Thomas, S.R.; Folda, A.; Heidecker, A.A.; Pöthig, A.; Magistrato, A.; Casini, A.; Rigobello, M.P. Exploring the Anticancer Activity of Tamoxifen-Based Metal Complexes Targeting Mitochondria. J. Med. Chem. 2023, 66, 9823–9841. [Google Scholar] [CrossRef]
- Tsantili-Kakoulidou, A.; Demopoulos, V.J. Drug-like Properties and Fraction Lipophilicity Index as a combined metric. ADMET DMPK 2021, 9, 177–190. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M. Medicinal Chemistry of Indane and Its Analogues: A Mini Review. ChemistrySelect 2021, 6, 2658–2677. [Google Scholar] [CrossRef]
- Sogbein, O.; Paul, P.; Umar, M.; Chaari, A.; Batuman, V.; Upadhyay, R. Bortezomib in cancer therapy: Mechanisms, side effects, and future proteasome inhibitors. Life Sci. 2024, 358, 123125. [Google Scholar] [CrossRef]
- Hancock, G.R.; Gertz, J.; Jeselsohn, R.; Fanning, S.W. Estrogen Receptor Alpha Mutations, Truncations, Heterodimers, and Therapies. Endocrinology 2024, 165, bqae051. [Google Scholar] [CrossRef]
- Yao, J.; Tao, Y.; Hu, Z.; Li, J.; Xue, Z.; Zhang, Y.; Lei, Y. Optimization of small molecule degraders and antagonists for targeting estrogen receptor based on breast cancer: Current status and future. Front. Pharmacol. 2023, 14, 1225951. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Chen, K.J.; Wu, T.H.; Chang, H.K.; Tsuchido, Y.; Sei, Y.; Chen, P.L.; Horie, M. Ring rotation of ferrocene in interlocked molecules in single crystals. Chem. Sci. 2021, 12, 3871–3875. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.; Wieske, L.H.E.; Diehl, C.J.; Ciulli, A. Rational Design of PROTAC Linkers Featuring Ferrocene as a Molecular Hinge to Enable Dynamic Conformational Changes. J. Am. Chem. Soc. 2025, 147, 13328–13344. [Google Scholar] [CrossRef]
- Shani, J.; Gazit, A.; Livshitz, T.; Biran, S. Synthesis and receptor-binding affinity of fluorotamoxifen, a possible estrogen-receptor imaging agent. J. Med. Chem. 1985, 28, 1504–1511. [Google Scholar] [CrossRef]
- Patel, H.K.; Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef]
- Carmona-Negron, J.A.; Santana, A.; Rheingold, A.L.; Melendez, E. Synthesis, structure, docking and cytotoxic studies of ferrocene-hormone conjugates for hormone-dependent breast cancer application. Dalton Trans. 2019, 48, 5952–5964. [Google Scholar] [CrossRef] [PubMed]
- Knoblich, J.A.; Sauer, K.; Jones, L.; Richardson, H.; Saint, R.; Lehner, C.F. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 1994, 77, 107–120. [Google Scholar] [CrossRef]
- Ishteyaque, S.; Yadav, K.S.; Verma, S.; Washimkar, K.R.; Mugale, M.N. CYP2E1 triggered GRP78/ATF6/CHOP signaling axis inhibit apoptosis and promotes progression of hepatocellular carcinoma. Arch. Biochem. Biophys. 2023, 745, 109701. [Google Scholar] [CrossRef]
- Emons, G.; Mustea, A.; Tempfer, C. Tamoxifen and Endometrial Cancer: A Janus-Headed Drug. Cancers 2020, 12, 2535. [Google Scholar] [CrossRef] [PubMed]
- Torres-López, L.; Maycotte, P.; Liñán-Rico, A.; Liñán-Rico, L.; Donis-Maturano, L.; Delgado-Enciso, I.; Meza-Robles, C.; Vásquez-Jiménez, C.; Hernández-Cruz, A.; Dobrovinskaya, O. Tamoxifen induces toxicity, causes autophagy, and partially reverses dexamethasone resistance in Jurkat T cells. J. Leukoc. Biol. 2019, 105, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Ertugrul, B.; Aytatli, A.; Karatas, O.F.; Saracoglu, N. Design, synthesis, and biological evaluation of indole-modified tamoxifen relatives as potent anticancer agents. RSC Med. Chem. 2023, 14, 1362–1376. [Google Scholar] [CrossRef]
- Zafonte, B.T.; Hulit, J.; Amanatullah, D.F.; Albanese, C.; Wang, C.; Rosen, E.; Reutens, A.; Sparano, J.A.; Lisanti, M.P.; Pestell, R.G. Cell-cycle dysregulation in breast cancer: Breast cancer therapies targeting the cell cycle. Front. Biosci. J. Virtual Libr. 2000, 5, D938–D961. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Frasor, J.M.; Dai, Y. An integrated bioinformatics approach identifies elevated cyclin E2 expression and E2F activity as distinct features of tamoxifen resistant breast tumors. PLoS ONE 2011, 6, e22274. [Google Scholar] [CrossRef]
- Gao, T.; Han, Y.; Yu, L.; Ao, S.; Li, Z.; Ji, J. CCNA2 is a prognostic biomarker for ER+ breast cancer and tamoxifen resistance. PLoS ONE 2014, 9, e91771. [Google Scholar] [CrossRef]
- Niu, D.; Wang, G.; Wang, X. Up-regulation of cyclin E in breast cancer via estrogen receptor pathway. Int. J. Clin. Exp. Med. 2015, 8, 910–915. [Google Scholar] [PubMed]
- Jacenik, D.; Beswick, E.J.; Krajewska, W.M.; Prossnitz, E.R. G protein-coupled estrogen receptor in colon function, immune regulation and carcinogenesis. World J. Gastroenterol. 2019, 25, 4092–4104. [Google Scholar] [CrossRef]
- Mori, T.; Doi, R.; Toyoda, E.; Koizumi, M.; Ito, D.; Kami, K.; Kida, A.; Masui, T.; Kawaguchi, Y.; Fujimoto, K. Regulation of the resistance to TRAIL-induced apoptosis as a new strategy for pancreatic cancer. Surgery 2005, 138, 71–77. [Google Scholar] [CrossRef]
- Tarnow, P.; Tralau, T.; Luch, A. G protein-coupled receptor 30 ligand G-1 increases aryl hydrocarbon receptor signalling by inhibition of tubulin assembly and cell cycle arrest in human MCF-7 cells. Arch. Toxicol. 2016, 90, 1939–1948. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, R.; Xia, M.; Sun, C.; Zhong, B.; Yu, J.; Ai, N.; Lu, J.J.; Ge, W.; Liu, B.; et al. Nannocystin ax, an eEF1A inhibitor, induces G1 cell cycle arrest and caspase-independent apoptosis through cyclin D1 downregulation in colon cancer in vivo. Pharmacol. Res. 2021, 173, 105870. [Google Scholar] [CrossRef]
- Zhao, R.; Song, N.; Ning, X.; Chen, X.; Ma, R. ZNF146 regulates cell cycle progression via TFDP1 and DEPDC1B in ovarian cancer cells. Reproduction 2024, 168, e230484. [Google Scholar] [CrossRef]
- Shen, C.; Huang, Y.; Liu, Y.; Wang, G.; Zhao, Y.; Wang, Z.; Teng, M.; Wang, Y.; Flockhart, D.A.; Skaar, T.C.; et al. A modulated empirical Bayes model for identifying topological and temporal estrogen receptor α regulatory networks in breast cancer. BMC Syst. Biol. 2011, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Urano, T.; Horie, K.; Ikeda, K.; Tsukui, T.; Fukuoka, H.; Tsutsumi, O.; Ouchi, Y.; Inoue, S. Estrogen activates cyclin-dependent kinases 4 and 6 through induction of cyclin D in rat primary osteoblasts. Biochem. Biophys. Res. Commun. 2002, 299, 222–228. [Google Scholar] [CrossRef]
- Bois, C.; Delalande, C.; Bouraïma-Lelong, H.; Durand, P.; Carreau, S. 17β-Estradiol regulates cyclin A1 and cyclin B1 gene expression in adult rat seminiferous tubules. J. Mol. Endocrinol. 2012, 48, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.M.; Dickler, M.N. Estrogen Receptor-Positive Breast Cancer: Exploiting Signaling Pathways Implicated in Endocrine Resistance. Oncologist 2018, 23, 528–539. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.I.; Edwards, C.D.; Rollins, B.J. The physiology of p16(INK4A)-mediated G1 proliferative arrest. Cell Biochem. Biophys. 2000, 33, 189–197. [Google Scholar] [CrossRef]
- Sun, C.; Wang, G.; Wrighton, K.H.; Lin, H.; Songyang, Z.; Feng, X.H.; Lin, X. Regulation of p27(Kip1) phosphorylation and G1 cell cycle progression by protein phosphatase PPM1G. Am. J. Cancer Res. 2016, 6, 2207–2220. [Google Scholar]
- Hamel, P.A.; Gallie, B.L.; Phillips, R.A. The retinoblastoma protein and cell cycle regulation. Trends Genet. TIG 1992, 8, 180–185. [Google Scholar] [CrossRef]
- Meek, D.W. The p53 response to DNA damage. DNA Repair 2004, 3, 1049–1056. [Google Scholar] [CrossRef]
- Tomar, V.; Kumar, P.; Sharma, D.; Joshi, R.K.; Nemiwal, M. Anticancer potential of ferrocene-containing derivatives: Current and future prospective. J. Mol. Struct. 2025, 1319, 139589. [Google Scholar] [CrossRef]
- Li, Y.; Ma, H.L.; Han, L.; Liu, W.Y.; Zhao, B.X.; Zhang, S.L.; Miao, J.Y. Novel ferrocenyl derivatives exert anti-cancer effect in human lung cancer cells in vitro via inducing G1-phase arrest and senescence. Acta Pharmacol. Sin. 2013, 34, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.; Hoppe, J.; Cea-Medina, P.; Bruch, P.M.; Krings, K.S.; Lechtenberg, I.; Drießen, D.; Peter, C.; Bhatia, S.; Dietrich, S.; et al. Novel meriolin derivatives potently inhibit cell cycle progression and transcription in leukemia and lymphoma cells via inhibition of cyclin-dependent kinases (CDKs). Cell Death Discov. 2024, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Abdelhamid, A.M.; Saber, S.; Elmorsy, E.A.; Hamad, R.S.; Abdel-Reheim, M.A.; Yahya, G.; Salama, M.M. Cell cycle machinery in oncology: A comprehensive review of therapeutic targets. FASEB J. 2024, 38, e23734. [Google Scholar] [CrossRef]
- Sun, W.J.; Huang, H.; He, B.; Hu, D.H.; Li, P.H.; Yu, Y.J.; Zhou, X.H.; Lv, Z.; Zhou, L.; Hu, T.Y.; et al. Romidepsin induces G2/M phase arrest via Erk/cdc25C/cdc2/cyclinB pathway and apoptosis induction through JNK/c-Jun/caspase3 pathway in hepatocellular carcinoma cells. Biochem. Pharmacol. 2017, 127, 90–100. [Google Scholar] [CrossRef]
- Roy, D.; Cai, Q.; Felty, Q.; Narayan, S. Estrogen-induced generation of reactive oxygen and nitrogen species, gene damage, and estrogen-dependent cancers. J. Toxicol. Environ. Health B Crit. Rev. 2007, 10, 235–257. [Google Scholar] [CrossRef]
- Scordalakes, E.M.; Shetty, S.J.; Rissman, E.F. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. J. Comp. Neurol. 2002, 453, 336–344. [Google Scholar] [CrossRef]
- Zhuo, Y.; Yang, L.; Li, D.; Zhang, L.; Zhang, Q.; Zhang, S.; Li, C.; Cui, L.; Hao, J.; Li, J.; et al. Syringaresinol Resisted Sepsis-Induced Acute Lung Injury by Suppressing Pyroptosis Via the Oestrogen Receptor-β Signalling Pathway. Inflammation 2022, 45, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, S.; Krukoff, T.L. Estrogen modulates endothelial and neuronal nitric oxide synthase expression via an estrogen receptor beta-dependent mechanism in hypothalamic slice cultures. Endocrinology 2005, 146, 2933–2941. [Google Scholar] [CrossRef]
- Chu, R.; van Hasselt, A.; Vlantis, A.C.; Ng, E.K.; Liu, S.Y.; Fan, M.D.; Ng, S.K.; Chan, A.B.; Liu, Z.; Li, X.Y.; et al. The cross-talk between estrogen receptor and peroxisome proliferator-activated receptor gamma in thyroid cancer. Cancer 2014, 120, 142–153. [Google Scholar] [CrossRef]
- Yu, M.; Jiang, M.; Chen, Y.; Zhang, S.; Zhang, W.; Yang, X.; Li, X.; Li, Y.; Duan, S.; Han, J.; et al. Inhibition of Macrophage CD36 Expression and Cellular Oxidized Low Density Lipoprotein (oxLDL) Accumulation by Tamoxifen: A PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR (PPAR)γ-DEPENDENT MECHANISM. J. Biol. Chem. 2016, 291, 16977–16989. [Google Scholar] [CrossRef]
- Ayoub, N.M.; Ibrahim, D.R.; Alkhalifa, A.E.; Al-Husein, B.A. Crizotinib induced antitumor activity and synergized with chemotherapy and hormonal drugs in breast cancer cells via downregulating MET and estrogen receptor levels. Investig. New Drugs 2021, 39, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, N.; Fang, J.; Huang, J.; Tian, F.; Li, C.; Xie, F. Role of PKC-ERK signaling in tamoxifen-induced apoptosis and tamoxifen resistance in human breast cancer cells. Oncol. Rep. 2012, 27, 1879–1886. [Google Scholar]
- Chong, S.J.; Low, I.C.; Pervaiz, S. Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion 2014, 19 Pt A, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K. The role of cytochrome P4502E1 in the pathogenesis of alcoholic liver disease and carcinogenesis. Chem.-Biol. Interact. 2020, 316, 108918. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Favaron, C.; Gabano, E.; Zanellato, I.; Gaiaschi, L.; Casali, C.; Bottone, M.G.; Ravera, M. Effects of Ferrocene and Ferrocenium on MCF-7 Breast Cancer Cells and Interconnection with Regulated Cell Death Pathways. Molecules 2023, 28, 6469. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yang, H.; Zhang, Y.; Zou, F.; He, H.; Xie, W.; Zou, Z.; Liu, R.; Xu, Q.; Zhang, J.; et al. Ferrocene-Based Polymeric Nanoparticles Carrying Doxorubicin for Oncotherapeutic Combination of Chemotherapy and Ferroptosis. Small 2023, 19, e2205024. [Google Scholar] [CrossRef]
- Luo, J.; Li, Y.; Li, Y.; Chen, X.; Du, P.; Wang, Z.; Tian, A.; Zhao, Y. Reversing Ferroptosis Resistance in Breast Cancer via Tailored Lipid and Iron Presentation. ACS Nano 2023, 17, 25257–25268. [Google Scholar] [CrossRef]
- Wang, H.; Mao, W.; Zhang, Y.; Feng, W.; Bai, B.; Ji, B.; Chen, J.; Cheng, B.; Yan, F. NOX1 triggers ferroptosis and ferritinophagy, contributes to Parkinson’s disease. Free Radic. Biol. Med. 2024, 222, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Ling, Y.Y.; Zhong, Y.M.; Li, Z.Y.; Tan, C.P.; Mao, Z.W. Ferroptosis-Enhanced Cancer Immunity by a Ferrocene-Appended Iridium(III) Diphosphine Complex. Angew. Chem. Int. Ed. Engl. 2022, 61, e202115247. [Google Scholar] [CrossRef]
- Chen, X.; Huang, J.; Yu, C.; Liu, J.; Gao, W.; Li, J.; Song, X.; Zhou, Z.; Li, C.; Xie, Y.; et al. A noncanonical function of EIF4E limits ALDH1B1 activity and increases susceptibility to ferroptosis. Nat. Commun. 2022, 13, 6318. [Google Scholar] [CrossRef]
- Olivo, E.; La Chimia, M.; Ceramella, J.; Catalano, A.; Chiaradonna, F.; Sinicropi, M.S.; Cuda, G.; Iacopetta, D.; Scumaci, D. Moving beyond the Tip of the Iceberg: DJ-1 Implications in Cancer Metabolism. Cells 2022, 11, 1432. [Google Scholar] [CrossRef]
- Kulikov, A.V.; Shilov, E.S.; Mufazalov, I.A.; Gogvadze, V.; Nedospasov, S.A.; Zhivotovsky, B. Cytochrome c: The Achilles’ heel in apoptosis. Cell. Mol. Life Sci. CMLS 2012, 69, 1787–1797. [Google Scholar] [CrossRef]
- Yang, D.L.; Xu, J.W.; Zhu, J.G.; Zhang, Y.L.; Xu, J.B.; Sun, Q.; Cao, X.N.; Zuo, W.L.; Xu, R.S.; Huang, J.H.; et al. Role of GPR30 in estrogen-induced prostate epithelial apoptosis and benign prostatic hyperplasia. Biochem. Biophys. Res. Commun. 2017, 487, 517–524. [Google Scholar] [CrossRef]
- Lanneau, D.; de Thonel, A.; Maurel, S.; Didelot, C.; Garrido, C. Apoptosis versus cell differentiation: Role of heat shock proteins HSP90, HSP70 and HSP27. Prion 2007, 1, 53–60. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflammation. Cells 2021, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636. [Google Scholar] [CrossRef]
- Lavrik, I.; Golks, A.; Krammer, P.H. Death receptor signaling. J. Cell Sci. 2005, 118, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Elledge, R.M.; Lock-Lim, S.; Allred, D.C.; Hilsenbeck, S.G.; Cordner, L. p53 mutation and tamoxifen resistance in breast cancer. Clin. Cancer Res. 1995, 1, 1203–1208. [Google Scholar]
- Pavitra, E.; Kancharla, J.; Gupta, V.K.; Prasad, K.; Sung, J.Y.; Kim, J.; Tej, M.B.; Choi, R.; Lee, J.-H.; Han, Y.-K.; et al. The role of NF-κB in breast cancer initiation, growth, metastasis, and resistance to chemotherapy. Biomed. Pharmacother. 2023, 163, 114822. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Wang, H.G. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004, 279, 45495–45502. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, C.; Adriaenssens, E.; Toillon, R.A.; Chopin, V.; Romon, R.; Van Coppenolle, F.; Hondermarck, H.; Le Bourhis, X. Tamoxifen and TRAIL synergistically induce apoptosis in breast cancer cells. Oncogene 2008, 27, 1472–1477. [Google Scholar] [CrossRef]
- Arpino, G.; Nair Krishnan, M.; Doval Dinesh, C.; Bardou, V.J.; Clark, G.M.; Elledge, R.M. Idoxifene versus tamoxifen: A randomized comparison in postmenopausal patients with metastatic breast cancer. Ann. Oncol. 2003, 14, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Hazra, S.; Thareja, S. Selective Estrogen receptor degraders (SERDs) for the treatment of breast cancer: An overview. Eur. J. Med. Chem. 2023, 256, 115422. [Google Scholar] [CrossRef]
- Scott, J.S.; Klinowska, T.C.M. Selective estrogen receptor degraders (SERDs) and covalent antagonists (SERCAs): A patent review (July 2021-December 2023). Expert Opin. Ther. Pat. 2024, 34, 333–350. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Vessières, A.; Corbet, C.; Heldt, J.M.; Lories, N.; Jouy, N.; Laïos, I.; Leclercq, G.; Jaouen, G.; Toillon, R.A. A ferrocenyl derivative of hydroxytamoxifen elicits an estrogen receptor-independent mechanism of action in breast cancer cell lines. J. Inorg. Biochem. 2010, 104, 503–511. [Google Scholar] [CrossRef]
- Top, S.; Vessières, A.; Leclercq, G.; Quivy, J.; Tang, J.; Vaissermann, J.; Huché, M.; Jaouen, G. Synthesis, biochemical properties and molecular modelling studies of organometallic specific estrogen receptor modulators (SERMs), the ferrocifens and hydroxyferrocifens: Evidence for an antiproliferative effect of hydroxyferrocifens on both hormone-dependent and hormone-independent breast cancer cell lines. Chemistry 2003, 9, 5223–5236. [Google Scholar]
- Buriez, O.; Heldt, J.M.; Labbé, E.; Vessières, A.; Jaouen, G.; Amatore, C. Reactivity and Antiproliferative Activity of Ferrocenyl–Tamoxifen Adducts with Cyclodextrins against Hormone-Independent Breast-Cancer Cell Lines. Chem.–Eur. J. 2008, 14, 8195–8203. [Google Scholar] [CrossRef] [PubMed]
- Lainé, A.L.; Adriaenssens, E.; Vessières, A.; Jaouen, G.; Corbet, C.; Desruelles, E.; Pigeon, P.; Toillon, R.A.; Passirani, C. The in vivo performance of ferrocenyl tamoxifen lipid nanocapsules in xenografted triple negative breast cancer. Biomaterials 2013, 34, 6949–6956. [Google Scholar] [CrossRef]
- Allard, E.; Jarnet, D.; Vessières, A.; Vinchon-Petit, S.; Jaouen, G.; Benoit, J.P.; Passirani, C. Local delivery of ferrociphenol lipid nanocapsules followed by external radiotherapy as a synergistic treatment against intracranial 9L glioma xenograft. Pharm. Res. 2010, 27, 56–64. [Google Scholar] [CrossRef]
- Laine, A.-L.; Huynh, N.T.; Clavreul, A.; Balzeau, J.; Béjaud, J.; Vessieres, A.; Benoit, J.-P.; Eyer, J.; Passirani, C. Brain tumour targeting strategies via coated ferrociphenol lipid nanocapsules. Eur. J. Pharm. Biopharm. 2012, 81, 690–693. [Google Scholar] [CrossRef]
- Topin-Ruiz, S.; Mellinger, A.; Lepeltier, E.; Bourreau, C.; Fouillet, J.; Riou, J.; Jaouen, G.; Martin, L.; Passirani, C.; Clere, N. p722 ferrocifen loaded lipid nanocapsules improve survival of murine xenografted-melanoma via a potentiation of apoptosis and an activation of CD8(+) T lymphocytes. Int. J. Pharm. 2021, 593, 120111. [Google Scholar] [CrossRef]
- Idlas, P.; Ladaycia, A.; Némati, F.; Lepeltier, E.; Pigeon, P.; Jaouen, G.; Decaudin, D.; Passirani, C. Ferrocifen stealth LNCs and conventional chemotherapy: A promising combination against multidrug-resistant ovarian adenocarcinoma. Int. J. Pharm. 2022, 626, 122164. [Google Scholar] [CrossRef]
- Wang, Y.; Dansette, P.M.; Pigeon, P.; Top, S.; McGlinchey, M.J.; Mansuy, D.; Jaouen, G. A new generation of ferrociphenols leads to a great diversity of reactive metabolites, and exhibits remarkable antiproliferative properties. Chem. Sci. 2018, 9, 70–78. [Google Scholar] [CrossRef]
- Citta, A.; Folda, A.; Bindoli, A.; Pigeon, P.; Top, S.; Vessières, A.; Salmain, M.; Jaouen, G.; Rigobello, M.P. Evidence for targeting thioredoxin reductases with ferrocenyl quinone methides. A possible molecular basis for the antiproliferative effect of hydroxyferrocifens on cancer cells. J. Med. Chem. 2014, 57, 8849–8859. [Google Scholar] [CrossRef]
- Mombelli, E. Evaluation of the OECD (Q)SAR Application Toolbox for the profiling of estrogen receptor binding affinities. SAR QSAR Environ. Res. 2012, 23, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Clegg, N.J.; Paruthiyil, S.; Leitman, D.C.; Scanlan, T.S. Differential response of estrogen receptor subtypes to 1,3-diarylindene and 2,3-diarylindene ligands. J. Med. Chem. 2005, 48, 5989–6003. [Google Scholar] [CrossRef] [PubMed]
- Kirkiacharian, S.; Koutsourakis, P.G.; Philibert, D.; Bouchoux, F.; Van de Velde, P. Synthesis and relative binding affinity to steroid receptors and antiproliferative activity on MCF-7 cells of 2,3-disubstituted indenes. Il Farmaco 1999, 54, 678–683. [Google Scholar] [CrossRef] [PubMed]
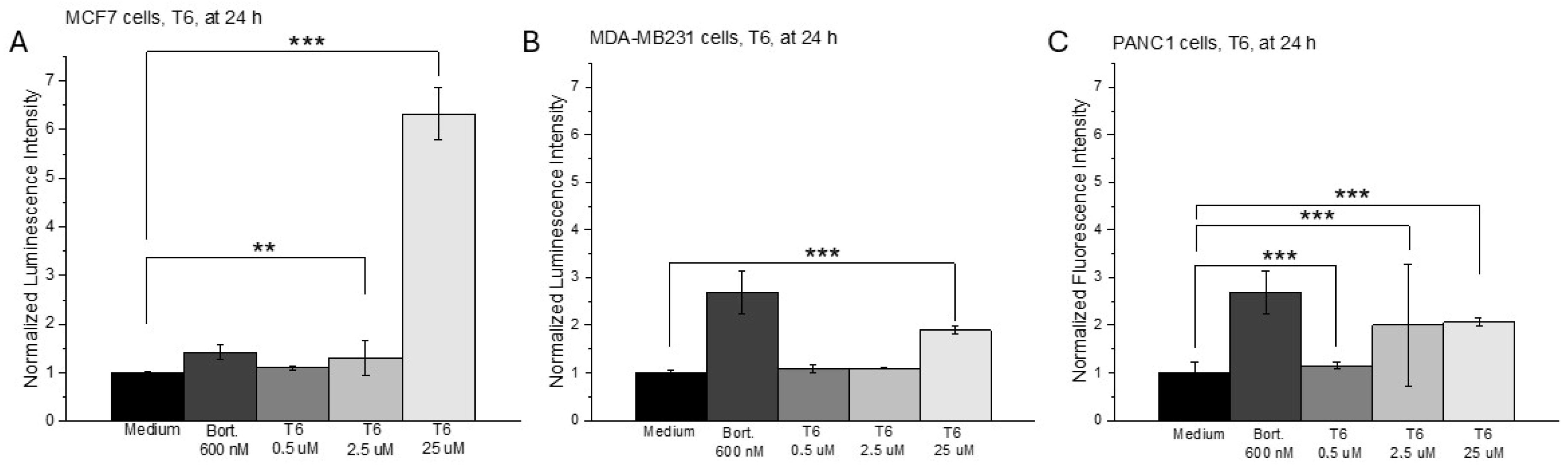
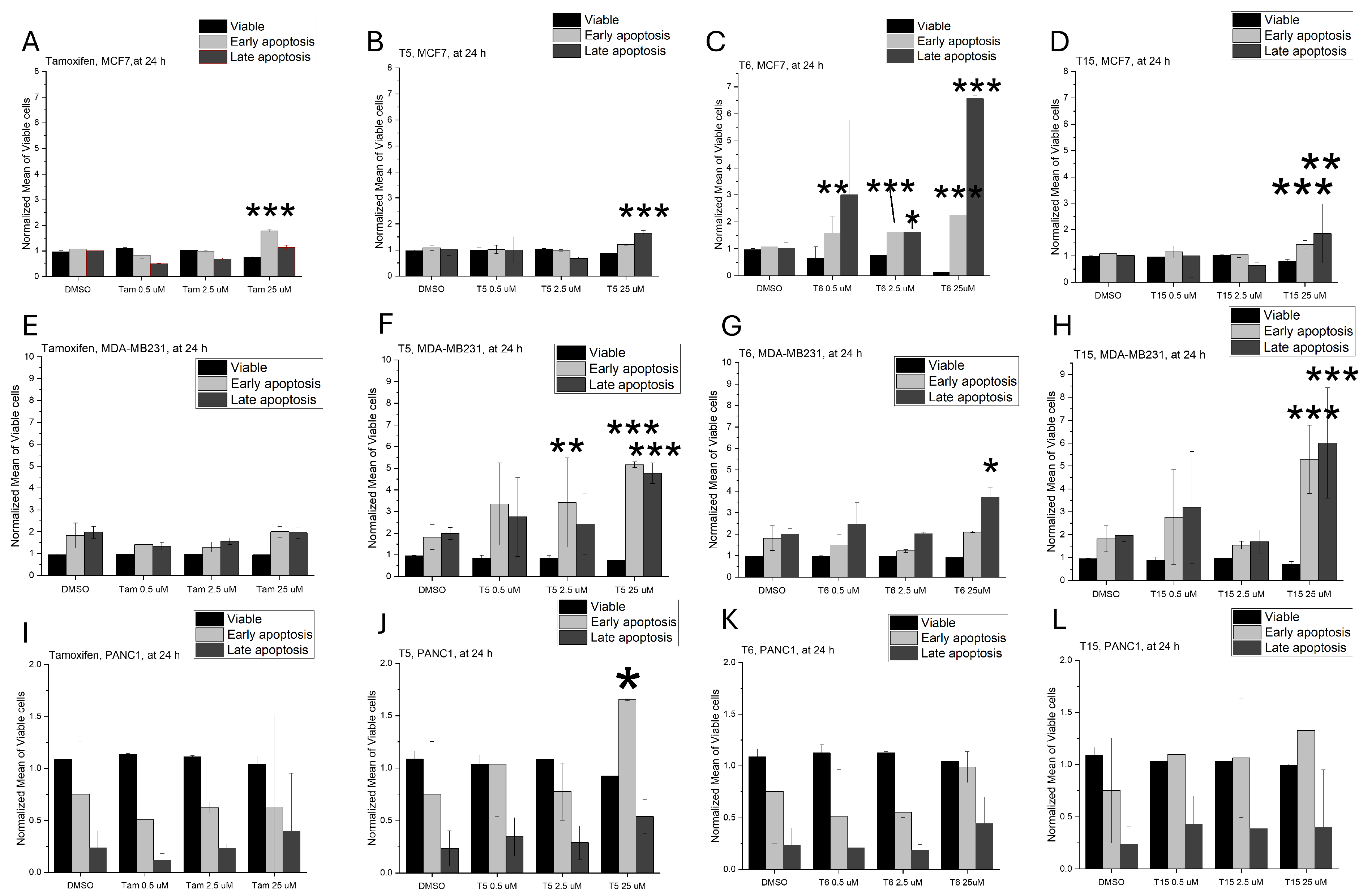
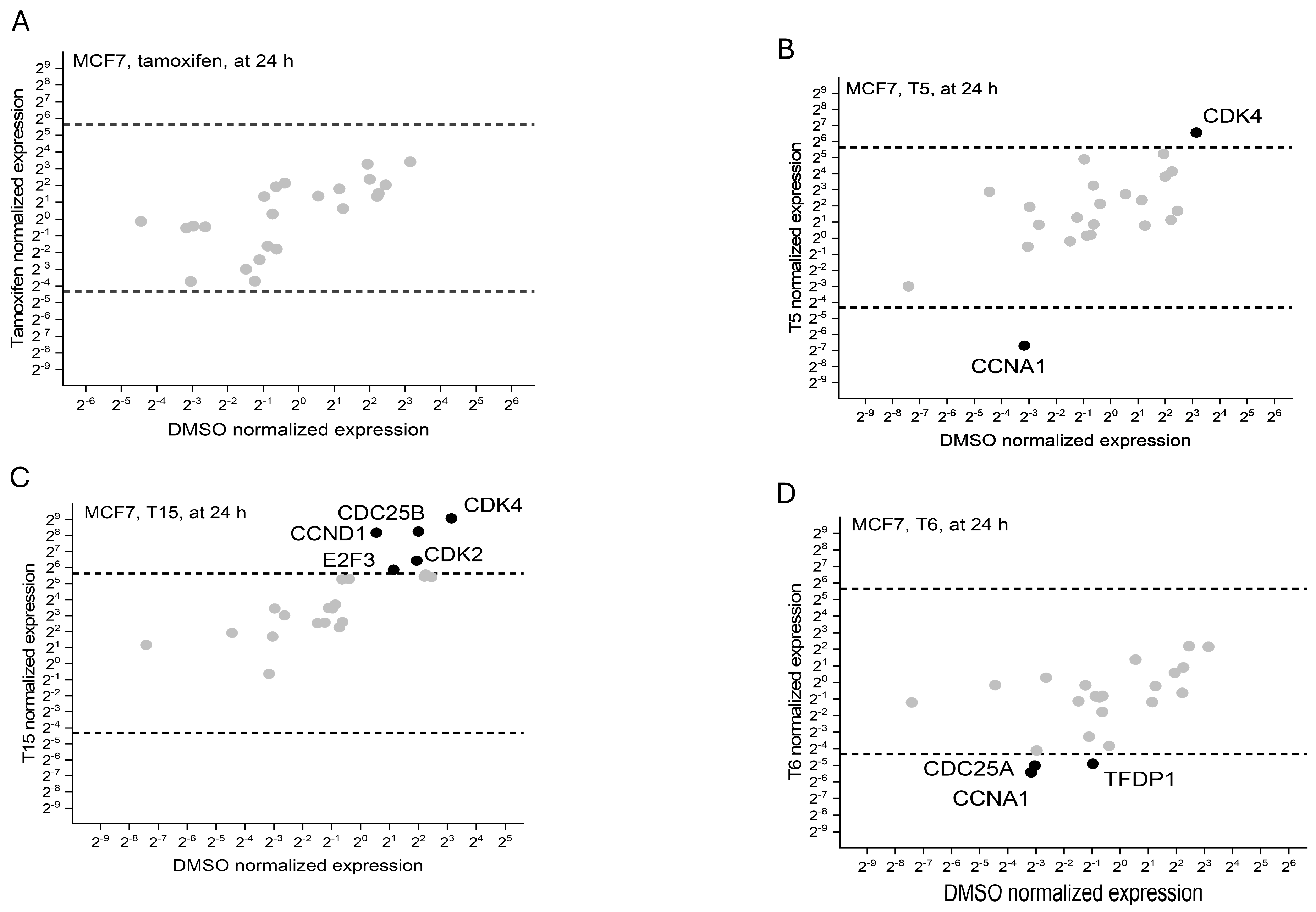

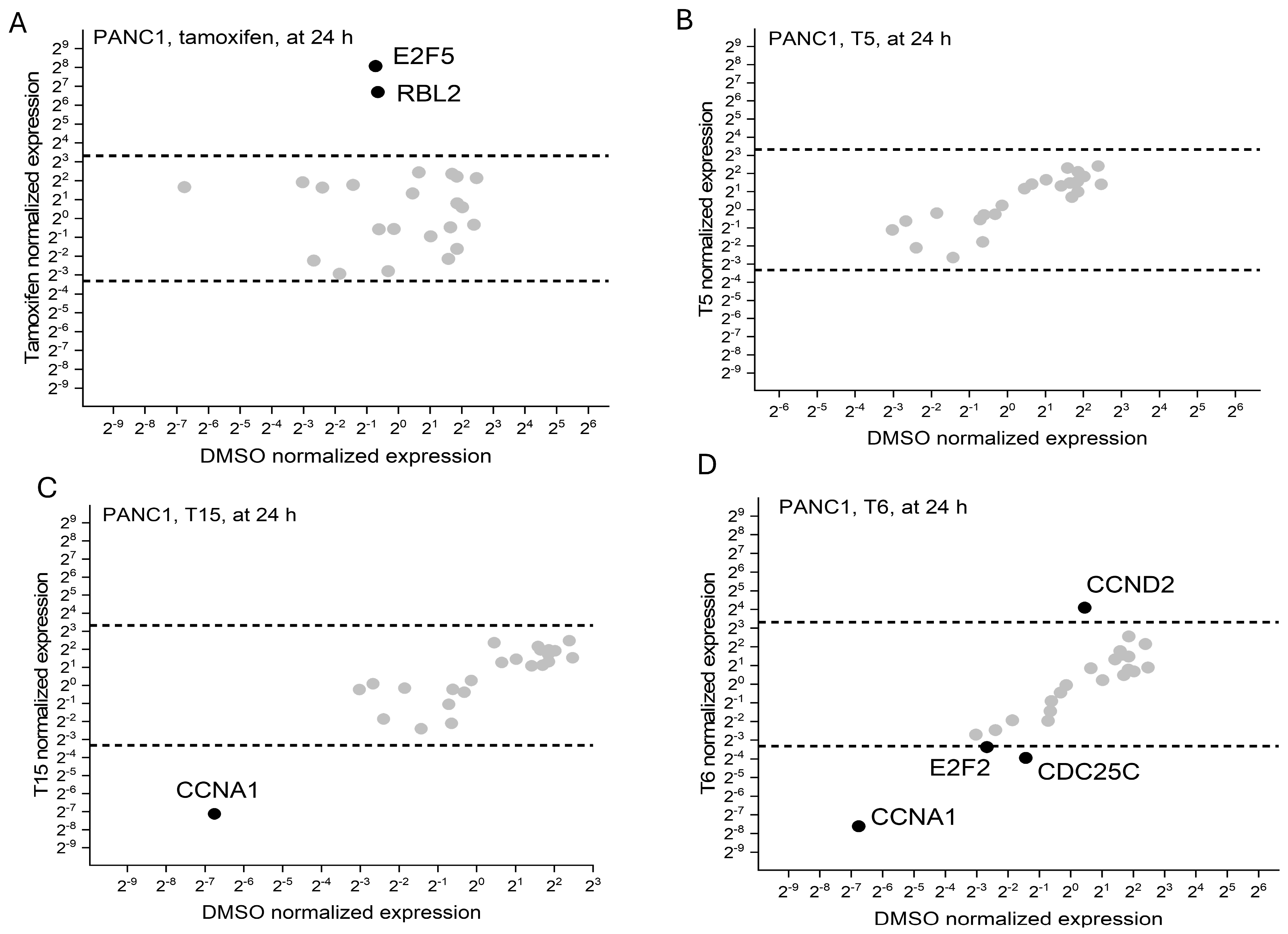
| MCF7 | MDA-MB231 | PANC1 | NHDF | |
|---|---|---|---|---|
| IC50 at 72 h (µM) | ||||
| Tamoxifen | 42.7 | 21.8 | 33.8 | 110.0 |
| T5 | 43.3 | 26.3 | 12.5 | 243.5 |
| T15 | 23.0 | 23.8 | 15.1 | n.d. |
| T6 | 4.9 | 64.1 | 51.8 | 263.4 |
| Target Name | Tamoxifen | T5 | T15 | T6 |
|---|---|---|---|---|
| SMAC/Diablo | no change | downregulation | downregulation | upregulation |
| PON2 | no change | downregulation | no change | upregulation |
| HSP70 | upregulation | no change | downregulation | upregulation |
| HSP60 | upregulation | no change | downregulation | upregulation |
| HSP27 | no change | no change | upregulation | upregulation |
| HO-2/HMOX2 | no change | no change | no change | upregulation |
| HO-1/HMOX1/HSP32 | no change | upregulation | upregulation | downregulation |
| Fas/TNFRSF6/CD95 | downregulation | downregulation | no change | upregulation |
| FADD | no change | downregulation | upregulation | downregulation |
| TRAIL R2/DR5 | downregulation | downregulation | upregulation | upregulation |
| TRAIL R1/DR4 | downregulation | downregulation | upregulation | upregulation |
| Cytochrome C | upregulation | downregulation | downregulation | upregulation |
| Molecule | Reference | Tumor Type/Model | Result |
|---|---|---|---|
| Fc-OH-TAM (ferrocenyl-4-hydroxytamoxifen; “ferrocifen”) | [79] | Breast cancer cell lines (MCF-7, T-47D, ZR-75-1; MDA-MB-231, SKBR-3, Hs578T) | Antiproliferative in ER+ and ER− cells; S-phase recruitment; ROS; ER-independent effects. |
| Hydroxyferrocifens | [80] | Breast cancer cell lines (MCF-7, MDA-MB-231) | Active on hormone-dependent & -independent lines; SERM-like antiproliferative effects. |
| Hydroxyferrocifens (cyclodextrin complexes) | [81] | Breast cancer cell lines | Improved formulation/solubility; strong in-vitro antiproliferative activity. |
| Fc-OH-TAM LNCs (“stealth” lipid nanocapsules) | [82] | TNBC xenograft (MDA-MB-231-luc, mouse) | Two 20 mg/kg i.p. doses delayed tumor growth; S-phase arrest preserved in LNCs. |
| Fc-diOH (ferrociphenol) | [83] | Intracranial 9L rat gliosarcoma | longer survival |
| Fc-diOH-LNCs with NFL peptide (targeting) | [84] | Glioma/GBM cells (U87MG) | NFL-peptide coating increased glioma-cell uptake vs. conventional LNCs. |
| P722 ferrocifen in LNCs | [85] | B16F10 melanoma (mouse) | Slower tumor growth, improved survival; intrinsic apoptosis and CD8+ T-cell activation. |
| P53 & P722 ferrocifens (stealth LNCs) ± chemo | [86] | Ovarian adenocarcinoma PDX (High/Low OXPHOS) | P722-LNCs + carboplatin/paclitaxel reduced tumor burden; P722 highlighted as lead. |
| Ferrociphenol/ferrocifen “next-gen” designs | [87] | Multiple cancer cell lines (in vitro) | New ferrociphenols with diverse reactive metabolites; sub-µM antiproliferative activity. |
| Hydroxyferrocifens → TrxR targeting (mechanism) | [88] | Mechanistic/enzymatic + cancer cell assays | Ferrocenyl quinone-methides potently inhibit TrxR; supports redox-based cytotoxicity. |
| Molecule | Reference | Tumor Type/Model | Result |
|---|---|---|---|
| 2,3-Diarylindenes (hydroxylated) | [89] | ER binding (tool compounds) | ER binding measured; orientation considerations for indene ER ligands. |
| 1,3- & 2,3-Diarylindenes (ERα/ERβ) | [90] | MCF-7 | High-affinity ER binding; subtype-selective agonism/antagonism; inhibited E2-stimulated growth. |
| 2,3-Disubstituted indenes | [91] | MCF-7 | Weak steroid-receptor binding; modest/weak antiproliferative effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalabay, M.; Szász, Z.; Lajkó, E.; Bagu, B.; Pállinger, É.; Duró, C.; Jernei, T.; Csámpai, A.; Takács, A.; Kőhidai, L. Comparative Study of Ferrocene- and Indene-Based Tamoxifen Derivatives of Different Molecular Flexibility on High-Mortality Cancer Cell Lines. Pharmaceuticals 2025, 18, 1417. https://doi.org/10.3390/ph18091417
Kalabay M, Szász Z, Lajkó E, Bagu B, Pállinger É, Duró C, Jernei T, Csámpai A, Takács A, Kőhidai L. Comparative Study of Ferrocene- and Indene-Based Tamoxifen Derivatives of Different Molecular Flexibility on High-Mortality Cancer Cell Lines. Pharmaceuticals. 2025; 18(9):1417. https://doi.org/10.3390/ph18091417
Chicago/Turabian StyleKalabay, Márton, Zsófia Szász, Eszter Lajkó, Bálint Bagu, Éva Pállinger, Cintia Duró, Tamás Jernei, Antal Csámpai, Angéla Takács, and László Kőhidai. 2025. "Comparative Study of Ferrocene- and Indene-Based Tamoxifen Derivatives of Different Molecular Flexibility on High-Mortality Cancer Cell Lines" Pharmaceuticals 18, no. 9: 1417. https://doi.org/10.3390/ph18091417
APA StyleKalabay, M., Szász, Z., Lajkó, E., Bagu, B., Pállinger, É., Duró, C., Jernei, T., Csámpai, A., Takács, A., & Kőhidai, L. (2025). Comparative Study of Ferrocene- and Indene-Based Tamoxifen Derivatives of Different Molecular Flexibility on High-Mortality Cancer Cell Lines. Pharmaceuticals, 18(9), 1417. https://doi.org/10.3390/ph18091417










