Interventions for Neglected Diseases Caused by Kinetoplastid Parasites: A One Health Approach to Drug Discovery, Development, and Deployment
Abstract
1. Introduction
2. Kinetoplastid Parasites and the One Health Paradigm
3. Overview of Existing and Emerging Pharmaceutical Interventions
3.1. Leishmaniasis
3.1.1. Current Therapeutics for Leishmaniasis
3.1.2. Emerging Antileishmanial Therapies and Innovations Beyond Traditional Chemotherapy
3.2. Chagas Disease
3.2.1. Current Therapies for Chagas Disease
3.2.2. Pipeline for Anti-Chagas Disease Candidates and Translational Challenges
3.2.3. Perspectives on the One Health Limitations of Current Chagas Disease Therapies
3.3. Human African Trypanosomiasis (HAT)
3.3.1. Historical Therapeutics: Arsenicals and Eflornithine
3.3.2. Recent Advances: Acoziborole (SCYX-7158)
3.3.3. The Veterinary Blind Spot: African Animal Trypanosomiasis (AAT)
3.3.4. Current Developments Towards New Veterinary and Human Trypanocides
4. Comparative Analysis of Anti-Trypanosomatid Drugs for Human vs. Veterinary Diseases: A One-Hundred-Year-Old Trend
5. Control Strategies Other than Chemotherapy
5.1. African Trypanosomiasis: Suppressing Transmission Through Tsetse Control
5.2. Vector Control and Chagas Disease
5.3. Integrating Vector Control with Pharmaceutical Intervention in Leishmaniasis Management
6. Perspectives Toward a One-Health-Aligned Anti-Trypanosomatid Drug Development Strategies
6.1. Holistic Target Prioritisation Across Host and Vector Interfaces
6.2. Co-Development for Human and Veterinary Indications
6.3. Inclusion of Ecological Pharmacology in Preclinical Development
6.4. Harmonisation of Human and Veterinary Regulatory Frameworks
6.5. Local Manufacturing, Delivery Innovation, and Access Equity
6.6. Reframing Drug Development as a Transdisciplinary One Health Enterprise
7. Conclusions
- Expansion of drug target discovery programmes to include pan-kinetoplastid pathways that are amenable to cross-species pharmacology.
- Systematic pharmacokinetic and efficacy studies of new compounds in reservoir hosts.
- Evaluation of the impacts of drugs on vector infectivity and parasite development through transmission-blocking studies.
- Development of field-friendly, heat-stable formulations for both humans and animals.
- Establishment of a joint human–veterinary regulatory review process and post-market pharmacovigilance systems.
- Creation of an integrated access and stewardship programme that safeguards efficacy while ensuring availability and affordability across health sectors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NTDs | Neglected tropical diseases |
| HAT | Human African trypanosomiasis |
| AAT | Animal African trypanosomiasis |
| DNDi | Drugs for neglected diseases initiative |
| kDNA | Kinetoplast deoxyribonucleic acid |
| VL | Visceral leishmaniasis |
| CL | Cutaneous leishmaniasis |
| MCL | Mucocutaneous leishmaniasis |
| PKDL | Post-kala azar dermal leishmaniasis |
| MMP | Mitochondrial membrane potential |
| HIV | Human immunodeficiency virus |
| WHO | World Health Organisation |
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Ortiz-Martínez, Y.; Kouamé, M.G.; Bongomin, F.; Lakoh, S.; Henao-Martínez, A.F. Human African Trypanosomiasis (Sleeping Sickness)—Epidemiology, Clinical Manifestations, Diagnosis, Treatment, and Prevention. Curr. Trop. Med. Rep. 2023, 10, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Desquesnes, M.; Dia, M.L. Trypanosoma Vivax: Mechanical Transmission in Cattle by One of the Most Common African Tabanids, Atylotus Agrestis. Exp. Parasitol. 2003, 103, 35–43. [Google Scholar] [CrossRef]
- Desquesnes, M.; Dargantes, A.; Lai, D.H.; Lun, Z.R.; Holzmuller, P.; Jittapalapong, S. Trypanosoma evansi and Surra: A Review and Perspectives on Transmission, Epidemiology and Control, Impact, and Zoonotic Aspects. Biomed. Res. Int. 2013, 2013, 321237. [Google Scholar] [CrossRef]
- Ungogo, M.A.; de Koning, H.P. Drug Resistance in Animal Trypanosomiases: Epidemiology, Mechanisms and Control Strategies. Int. J. Parasitol. Drugs Drug Resist. 2024, 25, 100533. [Google Scholar] [CrossRef]
- Bern, C.; Messenger, L.A.; Whitman, J.D.; Maguire, J.H. Chagas Disease in the United States: A Public Health Approach. Clin. Microbiol. Rev. 2019, 33, e00023-19. [Google Scholar] [CrossRef] [PubMed]
- Schmunis, G.A.; Yadon, Z.E. Chagas Disease: A Latin American Health Problem Becoming a World Health Problem. Acta Trop. 2010, 115, 14–21. [Google Scholar] [CrossRef]
- Antinori, S.; Galimberti, L.; Bianco, R.; Grande, R.; Galli, M.; Corbellino, M. Chagas Disease in Europe: A Review for the Internist in the Globalized World. Eur. J. Intern. Med. 2017, 43, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Montaner-Angoiti, E.; Llobat, L. Is Leishmaniasis the New Emerging Zoonosis in the World? Vet. Res. Commun. 2023, 47, 1777–1799. [Google Scholar] [CrossRef]
- Maia, C.; Conceição, C.; Pereira, A.; Rocha, R.; Ortuño, M.; Muñozid, C.; Jumakanova, Z.; Pérez-Cutillas, P.; Özbel, Y.; Töz, S.; et al. The Estimated Distribution of Autochthonous Leishmaniasis by Leishmania infantum in Europe in 2005–2020. PLoS Negl. Trop. Dis. 2023, 17, e0011497. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef]
- Ventura-Garcia, L.; Roura, M.; Pell, C.; Posada, E.; Gascón, J.; Aldasoro, E.; Muñoz, J.; Pool, R. Socio-Cultural Aspects of Chagas Disease: A Systematic Review of Qualitative Research. PLoS Negl. Trop. Dis. 2013, 7, e2410. [Google Scholar] [CrossRef]
- Okwor, I.; Uzonna, J. Social and Economic Burden of Human Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489. [Google Scholar] [CrossRef]
- Grifferty, G.; Shirley, H.; McGloin, J.; Kahn, J.; Orriols, A.; Wamai, R. Vulnerabilities to and the Socioeconomic and Psychosocial Impacts of the Leishmaniases: A Review. Res. Rep. Trop. Med. 2021, 12, 135–151. [Google Scholar] [CrossRef]
- World Health Organization. Chagas Disease (Also Known as American Trypanosomiasis). Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 4 August 2025).
- de Koning, H.P. The Drugs of Sleeping Sickness: Their Mechanisms of Action and Resistance, and a Brief History. Trop. Med. Infect. Dis. 2020, 5, 14. [Google Scholar] [CrossRef]
- Alcântara, L.M.; Ferreira, T.C.S.; Gadelha, F.R.; Miguel, D.C. Challenges in Drug Discovery Targeting TriTryp Diseases with an Emphasis on Leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 430–439. [Google Scholar] [CrossRef]
- Tauheed, A.M.; Danazumi, A.U.; Adepoju, O.A.; Kobo, P.I.; Adamu, A.; Balogun, E.O. Kinetoplastid Diseases: Insights into the Mechanisms of Drug Action and Resistance for Novel Drug Discovery. Asp. Mol. Med. 2025, 5, 100071. [Google Scholar] [CrossRef]
- Sunyoto, T.; Potet, J.; Boelaert, M. Why Miltefosine—A Life-Saving Drug for Leishmaniasis—Is Unavailable to People Who Need It the Most. BMJ Glob. Health 2018, 3, e000709. [Google Scholar] [CrossRef]
- Das, A.M.; Chitnis, N.; Burri, C.; Paris, D.H.; Patel, S.; Spencer, S.E.F.; Miaka, E.M.; Castaño, M.S. Modelling the Impact of Fexinidazole Use on Human African Trypanosomiasis (HAT) Transmission in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2021, 15, e0009992. [Google Scholar] [CrossRef]
- Miaka, E.M.; Pasi, C.P.; Hasker, E. Fexinidazole for Human African Trypanosomiasis: The Challenge of Accessibility. Lancet Glob. Health 2025, 13, e789–e790. [Google Scholar] [CrossRef]
- Gómez-Bravo, A.; Cirignoli, S.; Wehrendt, D.; Schijman, A.; León, C.M.; Flores-Chaves, M.; Nieto, J.; Kieran, T.J.; Abril, M.; Guhl, F. Zoonotic Cycle of American Trypanosomiasis in an Endemic Region of the Argentine Chaco, Factors That Influenced a Paradigm Shift. Insects 2024, 15, 471. [Google Scholar] [CrossRef]
- Hodo, C.L.; Hamer, S.A. Toward an Ecological Framework for Assessing Reservoirs of Vector-Borne Pathogens: Wildlife Reservoirs of Trypanosoma cruzi across the Southern United States. ILAR J. 2017, 58, 379–392. [Google Scholar] [CrossRef]
- Segura, G.B.R.; Ochoa, W.H.S.; da Matta, V.L.R.; Martínez, M.; Tercero, C.R.; Gonzalez, R.R.; Pacheco, C.M.S.; Flores, G.V.A.; Silveira, F.T.; Henriquez, M.M.R.; et al. Can Domestic Dogs Be Considered a Good Reservoir of Leishmania (L.) infantum chagasi in an Endemic Area of Nonulcerated Cutaneous Leishmaniasis in Southern Honduras? Rev. Inst. Med. Trop. Sao Paulo 2023, 65, e24. [Google Scholar] [CrossRef]
- Oranjin, S.V.; Kami, H.G.; Tohidi, F.; Mohammadi, Z. Ecological Habitat of Small Mammals as Reservoirs of Leishmania spp. in the Endemic Foci of Cutaneous Leishmaniasis, the North Iran. J. Zoonotic Dis. 2025, 9, 717–729. [Google Scholar] [CrossRef]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; De Koning, H.P.; Barrett, M.P. The Animal Trypanosomiases and Their Chemotherapy: A Review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef]
- Garlapati, R.; Iniguez, E.; Serafim, T.D.; Mishra, P.K.; Rooj, B.; Sinha, B.; Valenzuela, J.G.; Srikantiah, S.; Bern, C.; Kamhawi, S. Towards a Sustainable Vector-Control Strategy in the Post Kala-Azar Elimination Era. Front. Cell. Infect. Microbiol. 2021, 11, 641632. [Google Scholar] [CrossRef]
- Bertram, M.G.; Costi, M.P.; Thoré, E.S.; Sabo-Attwood, T.; Brooks, B.W. One Health. Curr. Biol. 2024, 34, R517–R519. [Google Scholar] [CrossRef]
- Hong, A.; Zampieri, R.A.; Shaw, J.J.; Floeter-Winter, L.M.; Laranjeira-Silva, M.F. One Health Approach to Leishmaniases: Understanding the Disease Dynamics through Diagnostic Tools. Pathogens 2020, 9, 809. [Google Scholar] [CrossRef]
- Khan, Y.; Lin, I.-C.; Khan, S.; Kanwal, M.; Wajid, A.; Khan, A.; Noor, F.; Almajwal, A.M.; Chen, C.-C.; Qadeer, A. Knowledge, Attitudes, and Practices toward Leishmaniasis and One Health: A Cross-Sectional Study among Medical and Veterinary Professionals. Front. Vet. Sci. 2025, 11, 1515370. [Google Scholar] [CrossRef]
- Meisner, J.; Kato, A.; Lemerani, M.M.; Miaka, E.M.; Ismail, A.T.; Wakefield, J.; Rowhani-Rahbar, A.; Pigott, D.; Mayer, J.D.; Lorton, C.; et al. Does a One Health Approach to Human African Trypanosomiasis Control Hasten Elimination? A Stochastic Compartmental Modeling Approach. Acta Trop. 2023, 240, 106804. [Google Scholar] [CrossRef]
- Dean, S.; Gould, M.K.; Dewar, C.E.; Schnaufer, A.C. Single Point Mutations in ATP Synthase Compensate for Mitochondrial Genome Loss in Trypanosomes. Proc. Natl. Acad. Sci. USA 2013, 110, 14741–14746. [Google Scholar] [CrossRef]
- Stuart, K.; Brun, R.; Croft, S.; Fairlamb, A.; Gürtler, R.E.; McKerrow, J.; Reed, S.; Tarleton, R. Kinetoplastids: Related Protozoan Pathogens, Different Diseases. J. Clin. Investig. 2008, 118, 1301–1310. [Google Scholar] [CrossRef]
- Nouvellet, P.; Dumonteil, E.; Gourbière, S. The Improbable Transmission of Trypanosoma cruzi to Human: The Missing Link in the Dynamics and Control of Chagas Disease. PLoS Negl. Trop. Dis. 2013, 7, e2505. [Google Scholar] [CrossRef]
- Babuadze, G.; Alvar, J.; Argaw, D.; de Koning, H.P.; Iosava, M.; Kekelidze, M.; Tsertsvadze, N.; Tsereteli, D.; Chakhunashvili, G.; Mamatsashvili, T.; et al. Epidemiology of Visceral Leishmaniasis in Georgia. PLoS Negl. Trop. Dis. 2014, 8, e2725. [Google Scholar] [CrossRef]
- Bardosh, K.L.; Ryan, S.; Ebi, K.; Welburn, S.; Singer, B. Addressing Vulnerability, Building Resilience: Community-Based Adaptation to Vector-Borne Diseases in the Context of Global Change. Infect. Dis. Poverty 2017, 6, 166. [Google Scholar] [CrossRef]
- Ferreira, B.A.; Martins, T.F.C.; Coser, E.M.; Oliveira, V.d.L.; Yamashiro-Kanashiro, E.H.; Rocha, M.C.; Pinto, M.M.; Cotrim, P.C.; Coelho, A.C. Isolation, Typing, and Drug Susceptibility of Leishmania (Leishmania) infantum Isolates from Dogs of the Municipality of Embu Das Artes, an Endemic Region for Canine Leishmaniasis in Brazil. Parasitol. Res. 2022, 121, 2683–2695. [Google Scholar] [CrossRef]
- Jansen, A.M.; Xavier, S.C.D.C.; Roque, A.L.R. Trypanosoma cruzi Transmission in the Wild and Its Most Important Reservoir Hosts in Brazil. Parasit. Vectors 2018, 11, 502. [Google Scholar] [CrossRef]
- Sandon, L.; Weinberg, D.; Espinosa, M.O.; Abril, M.C.; Chuit, R.; Porcasi, X.; Periago, M.V. Association between Landscape Transformation and the Chagas Disease Vector Dynamics in a Rural Area with Continuous Surveillance and Control. Parasit. Vectors 2025, 18, 203. [Google Scholar] [CrossRef]
- Simo, G.; Asonganyi, T.; Nkinin, S.W.; Njiokou, F.; Herder, S. High Prevalence of Trypanosoma brucei gambiense Group 1 in Pigs from the Fontem Sleeping Sickness Focus in Cameroon. Vet. Parasitol. 2006, 139, 57–66. [Google Scholar] [CrossRef]
- Kasozi, K.I.; Zirintunda, G.; Ssempijja, F.; Buyinza, B.; Alzahrani, K.J.; Matama, K.; Nakimbugwe, H.N.; Alkazmi, L.; Onanyang, D.; Bogere, P.; et al. Epidemiology of Trypanosomiasis in Wildlife—Implications for Humans at the Wildlife Interface in Africa. Front. Vet. Sci. 2021, 8, 621699. [Google Scholar] [CrossRef]
- Stone, C.M.; Chitnis, N. Implications of Heterogeneous Biting Exposure and Animal Hosts on Trypanosomiasis brucei gambiense Transmission and Control. PLoS Comput. Biol. 2015, 11, e1004514. [Google Scholar] [CrossRef]
- Jamonneau, V.; Ravel, S.; Koffi, M.; Kaba, D.; Zeze, D.G.; Ndri, L.; Sane, B.; Coulibaly, B.; Cuny, G.; Solano, P. Mixed Infections of Trypanosomes in Tsetse and Pigs and Their Epidemiological Significance in a Sleeping Sickness Focus of Côte d’Ivoire. Parasitology 2004, 129, 693–702. [Google Scholar] [CrossRef]
- Maia, C.; Cardoso, L. Spread of Leishmania infantum in Europe with Dog Travelling. Vet. Parasitol. 2015, 213, 2–11. [Google Scholar] [CrossRef]
- Carvalho, B.M.; Maia, C.; Courtenay, O.; Llabrés-Brustenga, A.; Lotto Batista, M.; Moirano, G.; Van Daalen, K.R.; Semenza, J.C.; Lowe, R. A Climatic Suitability Indicator to Support Leishmania infantum Surveillance in Europe: A Modelling Study. Lancet Reg. Health–Eur. 2024, 43, 100971. [Google Scholar] [CrossRef]
- Iván, N.; Higuita, A.; Beatty, N.L.; Forsyth, C.; Henao-Martínez, A.F.; Manne-Goehler, J.; Vidal, A. Chagas Disease in the United States: A Call for Increased Investment and Collaborative Research. Lancet Reg. Health–Am. 2024, 34, 100768. [Google Scholar]
- Patrick, D.A.; Bakunov, S.A.; Bakunova, S.M.; Kumar, E.V.K.S.; Lombardy, R.J.; Jones, S.K.; Bridges, A.S.; Zhirnov, O.; Hall, J.E.; Wenzler, T.; et al. Synthesis and in Vitro Antiprotozoal Activities of Dicationic 3,5-Diphenylisoxazoles. J. Med. Chem. 2007, 50, 2468–2485. [Google Scholar] [CrossRef]
- Betu Kumeso, V.K.; Kalonji, W.M.; Rembry, S.; Valverde Mordt, O.; Ngolo Tete, D.; Prêtre, A.; Delhomme, S.; Ilunga Wa Kyhi, M.; Camara, M.; Catusse, J.; et al. Efficacy and Safety of Acoziborole in Patients with Human African Trypanosomiasis Caused by Trypanosoma brucei gambiense: A Multicentre, Open-Label, Single-Arm, Phase 2/3 Trial. Lancet Infect. Dis. 2023, 23, 463–470. [Google Scholar] [CrossRef]
- Ilbeigi, K.; Mabille, D.; Matheeussen, A.; Hendrickx, R.; Claes, M.; Van Reet, N.; Anthonissen, R.; Hulpia, F.; Lin, C.; Maes, L.; et al. Discovery and Development of an Advanced Lead for the Treatment of African Trypanosomiasis. ACS Infect. Dis. 2025, 11, 131–143. [Google Scholar] [CrossRef]
- Pandey, P.; Garg, A.; Garg, S.; Singh, V.; Rai, G.; Mishra, N. Role of Controlled Release in Veterinary Formulations. In Novel Carrier Systems for Targeted and Controlled Drug Delivery; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2024; pp. 231–250. ISBN 9789819749706. [Google Scholar]
- de Souza, R.B.; Guimarães, J.R. Effects of Avermectins on the Environment Based on Its Toxicity to Plants and Soil Invertebrates—A Review. Water Air Soil Pollut. 2022, 233, 259. [Google Scholar] [CrossRef]
- Nicolás de Francisco, O.; Ewbank, A.C.; de la Torre, A.; Sacristán, I.; Afonso Jordana, I.; Planella, A.; Grau, O.; Garcia Ferré, D.; Olmo-Vidal, J.M.; García-Fernández, A.J.; et al. Environmental Contamination by Veterinary Medicinal Products and Their Implications in the Conservation of the Endangered Pyrenean Capercaillie (Tetrao Urogallus Aquitanicus). Ecotoxicol. Environ. Saf. 2024, 288, 117299. [Google Scholar] [CrossRef]
- Van den Kerkhof, M.; Sterckx, Y.G.J.; Leprohon, P.; Maes, L.; Caljon, G. Experimental Strategies to Explore Drug Action and Resistance in Kinetoplastid Parasites. Microorganisms 2020, 8, 950. [Google Scholar] [CrossRef] [PubMed]
- Ready, P.D. Leishmaniasis Emergence and Climate Change. OIE Rev. Sci. Tech. 2008, 27, 399–412. [Google Scholar] [CrossRef]
- Matsumoto, P.S.S.; Hiramoto, R.M.; Pereira, V.B.R.; Camprigher, V.M.; Taniguchi, H.H.; De Raeffray Barbosa, J.E.; De Barros Cortez, L.R.P.; Da Silva Fonseca, E.; Guimarães, R.B.; Tolezano, J.E. Impact of the Dog Population and Household Environment for the Maintenance of Natural Foci of Leishmania infantum Transmission to Human and Animal Hosts in Endemic Areas for Visceral Leishmaniasis in Sao Paulo State, Brazil. PLoS ONE 2021, 16, e0256534. [Google Scholar] [CrossRef]
- Costa-Da-silva, A.C.; Nascimento, D.d.O.; Ferreira, J.R.M.; Guimarães-Pinto, K.; Freire-De-lima, L.; Morrot, A.; Decote-Ricardo, D.; Filardy, A.A.; Freire-De-lima, C.G. Immune Responses in Leishmaniases: An Overview. Trop. Med. Infect. Dis. 2022, 7, 54. [Google Scholar]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, R.; Liu, Y.; Yu, M.; He, Z.; Xiao, J.; Li, K.; Liu, G.; Ning, Q.; Li, Y. Progress in Antileishmanial Drugs: Mechanisms, Challenges, and Prospects. PLoS Negl. Trop. Dis. 2025, 19, e0012735. [Google Scholar] [CrossRef]
- Imamura, H.; Downing, T.; van den Broeck, F.; Sanders, M.J.; Rijal, S.; Sundar, S.; Mannaert, A.; Vanaerschot, M.; Berg, M.; de Muylder, G.; et al. Evolutionary Genomics of Epidemic Visceral Leishmaniasis in the Indian Subcontinent. eLife 2016, 5, e12613. [Google Scholar] [CrossRef]
- Potvin, J.E.; Leprohon, P.; Queffeulou, M.; Sundar, S.; Ouellette, M. Mutations in an Aquaglyceroporin as a Proven Marker of Antimony Clinical Resistance in the Parasite Leishmania donovani. Clin. Infect. Dis. 2021, 72, e526–e532. [Google Scholar] [CrossRef] [PubMed]
- Haldar, A.K.; Sen, P.; Roy, S. Use of Antimony in the Treatment of Leishmaniasis: Current Status and Future Directions. Mol. Biol. Int. 2011, 2011, 571242. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; More, D.K.; Singh, M.K.; Singh, V.P.; Sharma, S.; Makharia, A.; Kumar, P.C.K.; Murray, H.W. Failure of Pentavalent Antimony in Visceral Leishmaniasis in India: Report from the Center of the Indian Epidemic. Clin. Infect. Dis. 2000, 31, 1104–1107. [Google Scholar] [CrossRef]
- Singh, O.P.; Singh, B.; Chakravarty, J.; Sundar, S. Current Challenges in Treatment Options for Visceral Leishmaniasis in India: A Public Health Perspective. Infect. Dis. Poverty 2016, 5, 19. [Google Scholar] [CrossRef]
- Schoenbach, E.B.; Greenspan, E.M. The Pharmacology, Mode of Action and Therapeutic Potentialities of Stilbamidine, Pentamidine, Propamidine and Other Aromatic Diamidines—A Review. Medicine 1948, 27, 327–377. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Drug Resistance in Leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 111–126. [Google Scholar] [CrossRef]
- Briones Nieva, C.A.; Cid, A.G.; Romero, A.I.; García-Bustos, M.F.; Villegas, M.; Bermúdez, J.M. An Appraisal of the Scientific Current Situation and New Perspectives in the Treatment of Cutaneous Leishmaniasis. Acta Trop. 2021, 221, 105988. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.M.; Cruz-Saldarriaga, M.; Llanos-Cuentas, A.; Luz-Cjuno, M.; Echevarria, J.; Miranda-Verastegui, C.; Colina, O.; Berman, J.D. Comparison of Meglumine Antimoniate and Pentamidine for Peruvian Cutaneous Leishmaniasis. Am. J. Trop. Med. Hyg. 2005, 72, 133–137. [Google Scholar] [CrossRef]
- Soto-Mancipe, J.; Grogl, M.; Berman, J.D. Evaluation of Pentamidine for the Treatment of Cutaneous Leishmaniasis in Colombia. Clin. Infect. Dis. 1993, 16, 417–425. [Google Scholar] [CrossRef]
- Sheikh, S.Y.; Ansari, W.A.; Hassan, F.; Faruqui, T.; Khan, M.F.; Akhter, Y.; Khan, A.R.; Siddiqui, M.A.; Al-Khedhairy, A.A.; Nasibullah, M. Drug Repositioning to Discover Novel Ornithine Decarboxylase Inhibitors against Visceral Leishmaniasis. J. Mol. Recognit. 2023, 36, e3021. [Google Scholar] [CrossRef]
- Basselin, M.; Lawrence, F.; Robert-Gero, M. Pentamidine Uptake in Leishmania Donovani and Leishmania Amazonensis Promastigotes and Axenic Amastigotes. Biochem. J. 1996, 315, 631. [Google Scholar] [CrossRef]
- Tiwari, N.; Kumar, A.; Singh, A.K.; Bajpai, S.; Agrahari, A.K.; Kishore, D.; Tiwari, V.K.; Singh, R.K. Leishmaniasis Control: Limitations of Current Drugs and Prospects of Natural Products. In Discovery and Development of Therapeutics from Natural Products Against Neglected Tropical Diseases; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Vercesi, A.E.; Docampo, R. Ca2+ Transport by Digitonin-Permeabilized Leishmania Donovani. Effects of Ca2+, Pentamidine and WR-6026 on Mitochondrial Membrane Potential in Situ. Biochem. J. 1992, 284, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Brazil, R.P. Effect of Pentamidine Isethionate on the Ultrastructure and Morphology of Leishmania Mexicana Amazonensis in Vitro. Ann. Trop. Med. Parasitol. 1982, 76, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Basselin, M.; Denise, H.; Coombs, G.H.; Barrett, M.P. Resistance to Pentamidine in Leishmania mexicana Involves Exclusion of the Drug from the Mitochondrion. Antimicrob. Agents Chemother. 2002, 46, 3731–3738. [Google Scholar] [CrossRef]
- De Koning, H.P. Uptake of Pentamidine in Trypanosoma Brucei Brucei Is Mediated by Three Distinct Transporters: Implications for Cross-Resistance with Arsenicals. Mol. Pharmacol. 2001, 59, 586–592. [Google Scholar] [CrossRef]
- Munday, J.C.; Eze, A.A.; Baker, N.; Glover, L.; Clucas, C.; Andrés, D.A.; Natto, M.J.; Teka, I.A.; Mcdonald, J.; Lee, R.S.; et al. Trypanosoma brucei Aquaglyceroporin 2 Is a High-Affinity Transporter for Pentamidine and Melaminophenyl Arsenic Drugs and the Main Genetic Determinant of Resistance to These Drugs. J. Antimicrob. Chemother. 2014, 69, 651–663. [Google Scholar] [CrossRef]
- Davidson, R.N.; den Boer, M.; Ritmeijer, K. Paromomycin. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.M.; Shepherd, M. A Mini-Review: Environmental and Metabolic Factors Affecting Aminoglycoside Efficacy. World J. Microbiol. Biotechnol. 2023, 39, 7. [Google Scholar] [CrossRef]
- Shalev-Benami, M.; Zhang, Y.; Rozenberg, H.; Nobe, Y.; Taoka, M.; Matzov, D.; Zimmerman, E.; Bashan, A.; Isobe, T.; Jaffe, C.L.; et al. Atomic Resolution Snapshot of Leishmania Ribosome Inhibition by the Aminoglycoside Paromomycin. Nat. Commun. 2017, 8, 1589. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, M.; De Kouchkovsky, Y.; Brown, S.; Petit, P.X.; Robert-Gero, M. In Vivo Interference of Paromomycin with Mitochondrial Activity of Leishmania. Exp. Cell Res. 1997, 232, 339–348. [Google Scholar] [CrossRef]
- Kimutai, R.; Musa, A.M.; Njoroge, S.; Omollo, R.; Alves, F.; Hailu, A.; Khalil, E.A.G.; Diro, E.; Soipei, P.; Musa, B.; et al. Safety and Effectiveness of Sodium Stibogluconate and Paromomycin Combination for the Treatment of Visceral Leishmaniasis in Eastern Africa: Results from a Pharmacovigilance Programme. Clin. Drug Investig. 2017, 37, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, A.; Agrawal, N.; Chakravarty, J. Effectiveness of Single-Dose Liposomal Amphotericin b in Visceral Leishmaniasis in Bihar. Am. J. Trop. Med. Hyg. 2019, 101, 795. [Google Scholar] [CrossRef]
- Heidari-Kharaji, M.; Rodrigues, P.; Petersen, C. Solid Lipid Nanoparticles Encapsulated with Paromomycin: An Effective Oral Formulation Against Leishmania major in Mouse Model. Parasit. Immunol. 2025, 47, e70002. [Google Scholar] [CrossRef]
- Kamiński, D.M. Recent Progress in the Study of the Interactions of Amphotericin B with Cholesterol and Ergosterol in Lipid Environments. Eur. Biophys. J. 2014, 43, 453–467. [Google Scholar] [CrossRef]
- Readio, J.D.; Bittman, R. Equilibrium Binding of Amphotericin B and Its Methyl Ester and Borate Complex to Sterols. BBA—Biomembr. 1982, 685, 219–224. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Gagos, M.; Kernen, P. Polyene Antibiotic Amphotericin B in Monomolecular Layers: Spectrophotometric and Scanning Force Microscopic Analysis. FEBS Lett. 2002, 524, 92–96. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, V.; Tiwari, R.K.; Ravidas, V.; Pandey, K.; Kumar, A. Amphotericin B: A Drug of Choice for Visceral Leishmaniasis. Acta Trop. 2022, 235, 106661. [Google Scholar] [CrossRef]
- Mondal, D.; Alvar, J.; Hasnain, M.G.; Hossain, M.S.; Ghosh, D.; Huda, M.M.; Nabi, S.G.; Sundar, S.; Matlashewski, G.; Arana, B. Efficacy and Safety of Single-Dose Liposomal Amphotericin B for Visceral Leishmaniasis in a Rural Public Hospital in Bangladesh: A Feasibility Study. Lancet Glob. Health 2014, 2, e51–e57. [Google Scholar] [CrossRef] [PubMed]
- Wijnant, G.J.; Dumetz, F.; Dirkx, L.; Bulté, D.; Cuypers, B.; Van Bocxlaer, K.; Hendrickx, S. Tackling Drug Resistance and Other Causes of Treatment Failure in Leishmaniasis. Front. Trop. Dis. 2022, 3, 837460. [Google Scholar] [CrossRef]
- Frézard, F.; Aguiar, M.M.G.; Ferreira, L.A.M.; Ramos, G.S.; Santos, T.T.; Borges, G.S.M.; Vallejos, V.M.R.; De Morais, H.L.O. Liposomal Amphotericin B for Treatment of Leishmaniasis: From the Identification of Critical Physicochemical Attributes to the Design of Effective Topical and Oral Formulations. Pharmaceutics 2023, 15, 99. [Google Scholar] [CrossRef]
- Croft, S.L.; Neal, R.A.; Pendergast, W.; Chan, J.H. The Activity of Alkyl Phosphorylcholines and Related Derivatives against Leishmania donovani. Biochem. Pharmacol. 1987, 36, 2633–2636. [Google Scholar] [CrossRef]
- Croft, S.L.; Engel, J. Miltefosine—Discovery of the Antileishmanial Activity of Phospholipid Derivatives. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Jha, T.K.; Thakur, C.P.; Bhattacharya, S.K.; Rai, M. Oral Miltefosine for the Treatment of Indian Visceral Leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, S26–S33. [Google Scholar] [CrossRef]
- Rakotomanga, M.; Loiseau, P.M.; Saint-Pierre-Chazalet, M. Hexadecylphosphocholine Interaction with Lipid Monolayers. Biochim. Biophys. Acta Biomembr. 2004, 1661, 212–218. [Google Scholar] [CrossRef]
- Vincent, I.M.; Weidt, S.; Rivas, L.; Burgess, K.; Smith, T.K.; Ouellette, M. Untargeted Metabolomic Analysis of Miltefosine Action in Leishmania infantum Reveals Changes to the Internal Lipid Metabolism. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 20–27. [Google Scholar] [CrossRef]
- Armitage, E.G.; Alqaisi, A.Q.I.; Godzien, J.; Peña, I.; Mbekeani, A.J.; Alonso-Herranz, V.; López-Gonzálvez, Á.; Martín, J.; Gabarro, R.; Denny, P.W.; et al. Complex Interplay between Sphingolipid and Sterol Metabolism Revealed by Perturbations to the Leishmania Metabolome Caused by Miltefosine. Antimicrob. Agents Chemother. 2018, 62, e02095-17. [Google Scholar] [CrossRef]
- Benaim, G.; Paniz-Mondolfi, A. Unmasking the Mechanism behind Miltefosine: Revealing the Disruption of Intracellular Ca2+ Homeostasis as a Rational Therapeutic Target in Leishmaniasis and Chagas Disease. Biomolecules 2024, 14, 406. [Google Scholar] [CrossRef] [PubMed]
- Wadhone, P.; Maiti, M.; Agarwal, R.; Kamat, V.; Martin, S.; Saha, B. Miltefosine Promotes IFN-γ-Dominated Anti-Leishmanial Immune Response. J. Immunol. 2009, 182, 7146–7154. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.C.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A Review of Its Pharmacology and Therapeutic Efficacy in the Treatment of Leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef]
- Barratt, G.; Saint-Pierre-Chazalet, M.; Loiseau, P. Cellular Transport and Lipid Interactions of Miltefosine. Curr. Drug Metab. 2009, 10, 247–255. [Google Scholar] [CrossRef]
- Pérez-Victoria, F.J.; Sánchez-Cañete, M.P.; Castanys, S.; Gamarro, F. Phospholipid Translocation and Miltefosine Potency Require Both L. donovani Miltefosine Transporter and the New Protein LdRos3 in Leishmania Parasites. J. Biol. Chem. 2006, 281, 23766–23775. [Google Scholar] [CrossRef]
- Coelho, A.C.; Boisvert, S.; Mukherjee, A.; Leprohon, P.; Corbeil, J.; Ouellette, M. Multiple Mutations in Heterogeneous Miltefosine-Resistant Leishmania Major Population as Determined by Whole Genome Sequencing. PLoS Negl. Trop. Dis. 2012, 6, e15120. [Google Scholar] [CrossRef]
- Prasad, R.; Kumar, R.; Jaiswal, B.P.; Singh, U.K. Miltefosine: An Oral Drug for Visceral Leishmaniasis. Indian J. Pediatr. 2004, 71, 143–144. [Google Scholar] [CrossRef]
- Ramesh, V.; Singh, R.; Avishek, K.; Verma, A.; Deep, D.K.; Verma, S.; Salotra, P. Decline in Clinical Efficacy of Oral Miltefosine in Treatment of Post Kala-Azar Dermal Leishmaniasis (PKDL) in India. PLoS Negl. Trop. Dis. 2015, 9, e0004093. [Google Scholar] [CrossRef]
- Pijpers, J.; Den Boer, M.L.; Essink, D.R.; Ritmeijer, K. The Safety and Efficacy of Miltefosine in the Long-Term Treatment of Post-Kala-Azar Dermal Leishmaniasis in South Asia—A Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007173. [Google Scholar] [CrossRef]
- Astman, N.; Arbel, C.; Katz, O.; Barzilai, A.; Solomon, M.; Schwartz, E. Tolerability and Safety of Miltefosine for the Treatment of Cutaneous Leishmaniasis. Trop. Med. Infect. Dis. 2024, 9, 218. [Google Scholar] [CrossRef]
- Castelo Branco, P.V.; Soares, R.E.P.; de Jesus, L.C.L.; Moreira, V.R.; Alves, H.J.; de Castro Belfort, M.R.; Silva, V.L.M.; Ferreira Pereira, S.R. The Antileishmanial Drug Miltefosine (Impavido®) Causes Oxidation of DNA Bases, Apoptosis, and Necrosis in Mammalian Cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 806, 34–39. [Google Scholar] [CrossRef]
- Dos Santos Nogueira, F.; Avino, V.C.; Galvis-Ovallos, F.; Pereira-Chioccola, V.L.; Moreira, M.A.B.; Romariz, A.P.P.L.; Molla, L.M.; Menz, I. Use of Miltefosine to Treat Canine Visceral Leishmaniasis Caused by Leishmania Infantum in Brazil. Parasit. Vectors 2019, 12, 79. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Michalick, M.S.M.; Da Silva, M.E.; Dos Santos, C.C.P.; Frézard, F.J.G.; Da Silva, S.M. Canine Leishmaniasis: An Overview of the Current Status and Strategies for Control. Biomed. Res. Int. 2018, 2018, 3296893. [Google Scholar] [CrossRef]
- Manna, L.; Corso, R.; Galiero, G.; Cerrone, A.; Muzj, P.; Gravino, A.E. Long-Term Follow-up of Dogs with Leishmaniosis Treated with Meglumine Antimoniate plus Allopurinol versus Miltefosine plus Allopurinol. Parasit. Vectors 2015, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, S.; Patterson, S.; Stojanovski, L.; Simeons, F.R.C.; Norval, S.; Kime, R.; Read, K.D.; Fairlamb, A.H. The Anti-Trypanosome Drug Fexinidazole Shows Potential for Treating Visceral Leishmaniasis. Sci. Transl. Med. 2012, 4, 119re1. [Google Scholar] [CrossRef] [PubMed]
- Reguera, R.M.; Pérez-Pertejo, Y.; Gutiérrez-Corbo, C.; Domínguez-Asenjo, B.; Ordóñez, C.; García-Estrada, C.; Martínez-Valladares, M.; Balaña-Fouce, R. Current and Promising Novel Drug Candidates against Visceral Leishmaniasis. Pure Appl. Chem. 2019, 91, 1385–1404. [Google Scholar] [CrossRef]
- do Damasio, D.S.N.; Antunes, P.A.; Lages, E.B.; de Morais-Teixeira, E.; Vital, K.D.; Cardoso, V.N.; Fernandes, S.O.A.; Aguiar, M.G.; Ferreira, L.A.M. A New Oral Self-Emulsifying Drug Delivery System Improves the Antileishmania Efficacy of Fexinidazole in Vivo. Int. J. Pharm. 2023, 631, 122505. [Google Scholar] [CrossRef]
- Wyllie, S.; Brand, S.; Thomas, M.; De Rycker, M.; Chung, C.W.; Pena, I.; Bingham, R.P.; Bueren-Calabuig, J.A.; Cantizani, J.; Cebrian, D.; et al. Preclinical Candidate for the Treatment of Visceral Leishmaniasis That Acts through Proteasome Inhibition. Proc. Natl. Acad. Sci. USA 2019, 116, 9318–9323. [Google Scholar] [CrossRef]
- Nagle, A.; Biggart, A.; Be, C.; Srinivas, H.; Hein, A.; Caridha, D.; Sciotti, R.J.; Pybus, B.; Kreishman-Deitrick, M.; Bursulaya, B.; et al. Discovery and Characterization of Clinical Candidate LXE408 as a Kinetoplastid-Selective Proteasome Inhibitor for the Treatment of Leishmaniases. J. Med. Chem. 2020, 63, 10773–10781. [Google Scholar] [CrossRef]
- DNDi. Visceral Leishmaniasis: LXE408 Novartis for VL. Available online: https://dndi.org/research-development/portfolio/lxe408-novartis-visceral-leishmaniasis/?utm_source=chatgpt.com (accessed on 3 September 2025).
- Mowbray, C.E.; Braillard, S.; Glossop, P.A.; Whitlock, G.A.; Jacobs, R.T.; Speake, J.; Pandi, B.; Nare, B.; Maes, L.; Yardley, V.; et al. DNDI-6148: A Novel Benzoxaborole Preclinical Candidate for the Treatment of Visceral Leishmaniasis. J. Med. Chem. 2021, 64, 16159–16176. [Google Scholar] [CrossRef]
- DNDi. New Hope for Novel Drugs for Leishmaniasis: Update of DNDi’s Leishmaniasis R&D Pipeline. Available online: https://dndi.org/wp-content/uploads/2017/05/DNDi_LeishmaniasisPipeline_2017.pdf?utm_source=chatgpt.com (accessed on 3 September 2025).
- Wijnant, G.-J.; Croft, S.L.; de la Flor, R.; Alavijeh, M.; Yardley, V.; Braillard, S.; Mowbray, C.; Van Bocxlaer, K. Pharmacokinetics and Pharmacodynamics of the Nitroimidazole DNDI-0690 in Mouse Models of Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2019, 63, e00829-19. [Google Scholar] [CrossRef] [PubMed]
- García-Estrada, C.; Pérez-Pertejo, Y.; Domínguez-Asenjo, B.; Holanda, V.N.; Murugesan, S.; Martínez-Valladares, M.; Balaña-Fouce, R.; Reguera, R.M. Further Investigations of Nitroheterocyclic Compounds as Potential Antikinetoplastid Drug Candidates. Biomolecules 2023, 13, 637. [Google Scholar] [CrossRef]
- Braillard, S.; Keenan, M.; Breese, K.J.; Heppell, J.; Abbott, M.; Islam, R.; Shackleford, D.M.; Katneni, K.; Crighton, E.; Chen, G.; et al. DNDI-6174 Is a Preclinical Candidate for Visceral Leishmaniasis That Targets the Cytochrome Bc1. Sci. Transl. Med. 2023, 15, eadh9902. [Google Scholar] [CrossRef]
- Elamin, M.; Al-Olayan, E.; Abdel-Gaber, R.; Yehia, R.S. Anti-Proliferative and Apoptosis Induction Activities of Curcumin on Leishmania major. Rev. Argent. Microbiol. 2021, 53, 240–247. [Google Scholar] [CrossRef]
- Changtam, C.; de Koning, H.P.; Ibrahim, H.; Sajid, M.S.; Gould, M.K.; Suksamrarn, A. Curcuminoid Analogs with Potent Activity against Trypanosoma and Leishmania Species. Eur. J. Med. Chem. 2010, 45, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.; Ebiloma, G.U.; Williams, R.; Alfayez, I.A.; Natto, M.J.; Alenezi, S.; Siheri, W.; AlQarni, M.; Igoli, J.O.; Fearnley, J.; et al. Activity of Compounds from Temperate Propolis against Trypanosoma brucei and Leishmania mexicana. Molecules 2021, 26, 3912. [Google Scholar] [CrossRef] [PubMed]
- Boniface, P.K.; Ferreira, E.I. Flavonoids as Efficient Scaffolds: Recent Trends for Malaria, Leishmaniasis, Chagas Disease, and Dengue. Phytother. Res. 2019, 33, 2473–2517. [Google Scholar] [CrossRef]
- Sen, G.; Mukhopadhyay, S.; Ray, M.; Biswas, T. Quercetin Interferes with Iron Metabolism in Leishmania donovani and Targets Ribonucleotide Reductase to Exert Leishmanicidal Activity. J. Antimicrob. Chemother. 2008, 61, 1066–1075. [Google Scholar] [CrossRef]
- Mohajeri, M.; Saghaei, L.; Ghanadian, M.; Saberi, S.; Pestechian, N.; Ostadhusseini, E. Synthesis and In Vitro Leishmanicidal Activities of Six Quercetin Derivatives. Adv. Biomed. Res. 2018, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Dubey, A.K.; Verma, P.R.P.; Singh, S.K.; Singh, S.K. Therapeutic Potential of Quercetin-Loaded Nanoemulsion against Experimental Visceral Leishmaniasis: In Vitro/Ex Vivo Studies and Mechanistic Insights. Mol. Pharm. 2022, 19, 3367–3384. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Bhattacharyya, F.K.; Ghosh, D.K. Leishmania Donovani: Amastigote Inhibition and Mode of Actior of Berberine. Exp. Parasitol. 1985, 60, 404–413. [Google Scholar] [CrossRef]
- Calvo, A.; Moreno, E.; Larrea, E.; Sanmartín, C.; Irache, J.M.; Espuelas, S. Berberine-Loaded Liposomes for the Treatment of Leishmania infantum-Infected BALB/c Mice. Pharmaceutics 2020, 12, 858. [Google Scholar] [CrossRef]
- Chowdhury, A.R.; Mandal, S.; Goswami, A.; Ghosh, M.; Mandal, L.; Chakraborty, D.; Ganguly, A.; Tripathi, G.; Mukhopadhyay, S.; Bandyopadhyay, S.; et al. Dihydrobetulinic Acid Induces Apoptosis in Leishmania donovani by Targeting DNA Topoisomerase I and II: Implications in Antileishmanial Therapy. Mol. Med. 2003, 9, 26–36. [Google Scholar] [CrossRef]
- Alakurtti, S.; Bergström, P.; Sacerdoti-Sierra, N.; Jaffe, C.L.; Yli-Kauhaluoma, J. Anti-Leishmanial Activity of Betulin Derivatives. J. Antibiot. 2010, 63, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Youssefi, M.R.; Moghaddas, E.; Tabari, M.A.; Moghadamnia, A.A.; Hosseini, S.M.; Farash, B.R.H.; Ebrahimi, M.A.; Mousavi, N.N.; Fata, A.; Maggi, F.; et al. In Vitro and In Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania infantum. Molecules 2019, 24, 2072. [Google Scholar] [CrossRef] [PubMed]
- Arruda, D.C.; Miguel, D.C.; Yokoyama-Yasunaka, J.K.U.; Katzin, A.M.; Uliana, S.R.B. Inhibitory Activity of Limonene against Leishmania Parasites in Vitro and in Vivo. Biomed. Pharmacother. 2009, 63, 643–649. [Google Scholar] [CrossRef]
- Sosa, A.M.; Moya Álvarez, A.; Bracamonte, E.; Korenaga, M.; Marco, J.D.; Barroso, P.A. Efficacy of Topical Treatment with (−)-Epigallocatechin Gallate, A Green Tea Catechin, in Mice with Cutaneous Leishmaniasis. Molecules 2020, 25, 1741. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Veronica, J.; Gundampati, R.K.; Sundar, S.; Maurya, R. Exploring the Inhibitory Activity of Withaferin-A against Pteridine Reductase-1 of L. Donovani. J. Enzym. Inhib. Med. Chem. 2016, 31, 1029–1037. [Google Scholar] [CrossRef][Green Version]
- Asilian, A.; Sharif, A.; Faghihi, G.; Enshaeieh, S.; Shariati, F.; Siadat, A.H. Evaluation of CO2 Laser Efficacy in the Treatment of Cutaneous Leishmaniasis. Int. J. Dermatol. 2004, 43, 736–738. [Google Scholar] [CrossRef]
- Osman, A.M.; Almuslet, N.A. Evaluation of CO2 Laser Efficacy in the Treatment of Cutaneous Leishmaniasis in a Group of 10 Sudanese Patients. Photonics Lasers Med. 2015, 4, 259–263. [Google Scholar] [CrossRef]
- Valencia, B.M.; Miller, D.; Witzig, R.S.; Boggild, A.K.; Llanos-Cuentas, A. Novel Low-Cost Thermotherapy for Cutaneous Leishmaniasis in Peru. PLoS Negl. Trop. Dis. 2013, 7, e2196. [Google Scholar] [CrossRef] [PubMed]
- Siadat, A.; Iraji, F.; Zolfaghari, A.; Shariat, S.; Jazi, S. Heat Therapy for Cutaneous Leishmaniasis: A Literature Review. J. Res. Med. Sci. 2021, 26, 15. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Yadagiri, G.; Gedda, M.R.; Singh, A.; Singh, O.P.; Verma, A.; Sundar, S.; Mudavath, S.L. Modified Solid Lipid Nanoparticles Encapsulated with Amphotericin B and Paromomycin: An Effective Oral Combination against Experimental Murine Visceral Leishmaniasis. Sci. Rep. 2020, 10, 12243. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, J.; Singh, V.K.; Agrawal, N.; Kumar, R. Current and Emerging Therapies for the Treatment of Leishmaniasis. Expert. Opin. Orphan Drugs 2024, 12, 19–32. [Google Scholar] [CrossRef]
- van Henten, S.; Bialfew, F.; Hassen, S.; Tilahun, F.; van Griensven, J.; Abdela, S.G. Treatment of Cutaneous Leishmaniasis with Sodium Stibogluconate and Allopurinol in a Routine Setting in Ethiopia: Clinical and Patient-Reported Outcomes and Operational Challenges. Trop. Med. Infect. Dis. 2023, 8, 414. [Google Scholar] [CrossRef]
- Musa, A.M.; Mbui, J.; Mohammed, R.; Olobo, J.; Ritmeijer, K.; Alcoba, G.; Muthoni Ouattara, G.; Egondi, T.; Nakanwagi, P.; Omollo, T.; et al. Paromomycin and Miltefosine Combination as an Alternative to Treat Patients with Visceral Leishmaniasis in Eastern Africa: A Randomized, Controlled, Multicountry Trial. Clin. Infect. Dis. 2023, 76, e1177–e1185. [Google Scholar] [CrossRef]
- Rijal, S.; Ostyn, B.; Uranw, S.; Rai, K.; Bhattarai, N.R.; Dorlo, T.P.C.; Beijnen, J.H.; Vanaerschot, M.; Decuypere, S.; Dhakal, S.S.; et al. Increasing Failure of Miltefosine in the Treatment of Kala-Azar in Nepal and the Potential Role of Parasite Drug Resistance, Reinfection, or Noncompliance. Clin. Infect. Dis. 2013, 56, 1530–1538. [Google Scholar] [CrossRef]
- Goyal, V.; Mahajan, R.; Pandey, K.; Singh, S.N.; Singh, R.S.; Strub-Wourgaft, N.; Alves, F.; Rabi Das, V.N.; Topno, R.K.; Sharma, B.; et al. Field Safety and Effectiveness of New Visceral Leishmaniasis Treatment Regimens within Public Health Facilities in Bihar, India. PLoS Negl. Trop. Dis. 2018, 12, e0006830. [Google Scholar] [CrossRef]
- van Griensven, J.; Dorlo, T.P.; Diro, E.; Costa, C.; Burza, S. The Status of Combination Therapy for Visceral Leishmaniasis: An Updated Review. Lancet Infect. Dis. 2024, 24, e36–e46. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, J.A.; Perez, A.M.; Norman, F.F.; Monge-Maillo, B.; López-Vélez, R. Old and New Challenges in Chagas Disease. Lancet Infect. Dis. 2015, 15, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.; Agudelo Higuita, N.I.; Hamer, S.A.; Ibarra-Cerdeña, C.N.; Valdez-Tah, A.; Stigler Granados, P.; Hamer, G.L.; Vingiello, M.; Beatty, N.L. Climate Change and Trypanosoma cruzi Transmission in North and Central America. Lancet Microbe 2024, 5, 100946. [Google Scholar] [CrossRef]
- Lewis, M.D.; Francisco, A.F.; Jayawardhana, S.; Langston, H.; Taylor, M.C.; Kelly, J.M. Imaging the Development of Chronic Chagas Disease after Oral Transmission. Sci. Rep. 2018, 8, 11292. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.V.; Repetto, S.A. American Trypanosomiasis (Chagas Disease) in Solid Organ Transplantation. Transpl. Infect. Dis. 2020, 22, e13429. [Google Scholar] [CrossRef]
- Howard, E.J.; Xiong, X.; Carlier, Y.; Sosa-Estani, S.; Buekens, P. Frequency of the Congenital Transmission of Trypanosoma cruzi: A Systematic Review and Meta-Analysis. BJOG 2014, 121, 22–33. [Google Scholar] [CrossRef]
- da Gama, A.N.S.; Soeiro, M.d.N.C. Trypanosoma cruzi Transmission Through Blood Samples and Derivatives: Main Routes, Control Strategies, and Recent Advancements in Blood Banks. Pathogens 2025, 14, 133. [Google Scholar] [CrossRef]
- Rodríguez-Monguí, E.; Cantillo-Barraza, O.; Prieto-Alvarado, F.E.; Cucunubá, Z.M. Heterogeneity of Trypanosoma cruzi Infection Rates in Vectors and Animal Reservoirs in Colombia: A Systematic Review and Meta-Analysis. Parasit. Vectors 2019, 12, 308. [Google Scholar] [CrossRef]
- Caldas, I.S.; da Matta Guedes, P.M.; dos Santos, F.M.; de Figueiredo Diniz, L.; Martins, T.A.F.; da Silva do Nascimento, A.F.; Azevedo, M.A.; de Lima, W.G.; Neto, R.M.N.; Torres, R.M.; et al. Myocardial Scars Correlate with Eletrocardiographic Changes in Chronic Trypanosoma cruzi Infection for Dogs Treated with Benznidazole. Trop. Med. Int. Health 2013, 18, 75–84. [Google Scholar] [CrossRef]
- Bryan, L.K.; Hamer, S.A.; Shaw, S.; Curtis-Robles, R.; Auckland, L.D.; Hodo, C.L.; Chaffin, K.; Rech, R.R. Chagas Disease in a Texan Horse with Neurologic Deficits. Vet. Parasitol. 2016, 216, 13–17. [Google Scholar] [CrossRef]
- Desquesnes, M.; Gonzatti, M.; Sazmand, A.; Thévenon, S.; Bossard, G.; Boulangé, A.; Gimonneau, G.; Truc, P.; Herder, S.; Ravel, S.; et al. A Review on the Diagnosis of Animal Trypanosomoses. Parasit. Vectors 2022, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A Mechanism for Cross-Resistance to Nifurtimox and Benznidazole in Trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Díaz de Toranzo, E.G.; Castro, J.A.; Franke de Cazzulo, B.M.; Cazzulo, J.J. Interaction of Benznidazole Reactive Metabolites with Nuclear and Kinetoplastic DNA, Proteins and Lipids from Trypanosoma cruzi. Experientia 1988, 44, 880–881. [Google Scholar] [CrossRef]
- Belmino, A.C.d.C.; Sousa, E.K.S.; da Silva Filho, J.D.; Rocha, E.A.; Nunes, F.M.M.; Sampaio, T.L.; Evangelista, L.F.; Duque, B.R.; Araújo, I.C.d.S.; Jacó, J.I.d.O.; et al. Causality and Severity of Adverse Reactions and Biochemical Changes to Benznidazole Treatment in Patients with Chronic Chagas Disease. Arq. Bras. Cardiol. 2024, 121, e20230787. [Google Scholar] [CrossRef]
- Baker, N.; Alsford, S.; Horn, D. Genome-Wide RNAi Screens in African Trypanosomes Identify the Nifurtimox Activator NTR and the Eflornithine Transporter AAT6. Mol. Biochem. Parasitol. 2011, 176, 55–57. [Google Scholar] [CrossRef]
- Gallardo-Garrido, C.; Cho, Y.; Cortés-Rios, J.; Vasquez, D.; Pessoa-Mahana, C.D.; Araya-Maturana, R.; Pessoa-Mahana, H.; Faundez, M. Nitrofuran Drugs beyond Redox Cycling: Evidence of Nitroreduction-Independent Cytotoxicity Mechanism. Toxicol. Appl. Pharmacol. 2020, 401, 115104. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Jaramillo, A.M.; Fernández, G.J.; Palacio, L.; Triana-Chávez, O. Gene Expression Study Using Real-Time PCR Identifies an NTR Gene as a Major Marker of Resistance to Benznidazole in Trypanosoma cruzi. Parasit. Vectors 2011, 4, 169. [Google Scholar] [CrossRef]
- Brisse, S.; Barnabé, C.; Tibayrenc, M. Identification of Six Trypanosoma Cruzi Phylogenetic Lineages by Random Amplified Polymorphic DNA and Multilocus Enzyme Electrophoresis. Int. J. Parasitol. 2000, 30, 35–44. [Google Scholar] [CrossRef]
- Vela, A.; Coral-Almeida, M.; Sereno, D.; Costales, J.A.; Barnabé, C.; Brenière, S.F. In Vitro Susceptibility of Trypanosoma cruzi Discrete Typing Units (Dtus) to Benznidazole: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009269. [Google Scholar] [CrossRef]
- Jackson, Y.; Alirol, E.; Getaz, L.; Wolff, H.; Combescure, C.; Chappuis, F. Tolerance and Safety of Nifurtimox in Patients with Chronic Chagas Disease. Clin. Infect. Dis. 2010, 51, e69–e75. [Google Scholar] [CrossRef]
- Levi, G.C.; Lobo, I.M.F.; Kallás, E.G.; Amato Neto, V. Etiological Drug Treatment of Human Infection by Trypanosoma cruzi. Rev. Inst. Med. Trop. Sao Paulo 1996, 38, 35–38. [Google Scholar] [CrossRef]
- Ramos, L.G.; de Souza, K.R.; Júnior, P.A.S.; Câmara, C.C.; Castelo-Branco, F.S.; Boechat, N.; Carvalho, S.A. Tackling the Challenges of Human Chagas Disease: A Comprehensive Review of Treatment Strategies in the Chronic Phase and Emerging Therapeutic Approaches. Acta Trop. 2024, 256, 107264. [Google Scholar] [CrossRef]
- Villar, J.C.; Perez, J.G.; Cortes, O.L.; Riarte, A.; Pepper, M.; Marin-Neto, J.A.; Guyatt, G.H. Trypanocidal Drugs for Chronic Asymptomatic Trypanosoma cruzi Infection. Cochrane Database Syst. Rev. 2014, 2014, CD003463. [Google Scholar] [PubMed]
- Ribeiro, I.; Sevcsik, A.-M.; Alves, F.; Diap, G.; Don, R.; Harhay, M.O.; Chang, S.; Pecoul, B. New, Improved Treatments for Chagas Disease: From the R&D Pipeline to the Patients. PLoS Negl. Trop. Dis. 2009, 3, e484. [Google Scholar] [CrossRef]
- Bahia, M.T.; de Andrade, I.M.; Martins, T.A.F.; Nascimento, Á.F.d.S.d.; Diniz, L.d.F.; Caldas, I.S.; Talvani, A.; Trunz, B.B.; Torreele, E.; Ribeiro, I. Fexinidazole: A Potential New Drug Candidate for Chagas Disease. PLoS Negl. Trop. Dis. 2012, 6, e1870. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, M.J.; Forsyth, C.; Losada, I.; Esteban, E.T.; García-Rodríguez, M.; Villegas, M.L.; Molina, I.; Crespillo-Andújar, C.; Gállego, M.; Ballart, C.; et al. Efficacy and Safety of Fexinidazole for Treatment of Chronic Indeterminate Chagas Disease (FEXI-12): A Multicentre, Randomised, Double-Blind, Phase 2 Trial. Lancet Infect. Dis. 2024, 24, 395–403. [Google Scholar] [CrossRef]
- Urbina, J.A. Ergosterol Biosynthesis and Drug Development for Chagas Disease. Mem. Inst. Oswaldo Cruz 2009, 104, 311–318. [Google Scholar] [CrossRef]
- De Ornelas Toledo, M.J.; Bahia, M.T.; Carneiro, C.M.; Martins-Filho, O.A.; Tibayrenc, M.; Barnabé, C.; Tafuri, W.L.; De Lana, M. Chemotherapy with Benznidazole and Itraconazole for Mice Infected with Different Trypanosoma cruzi Clonal Genotypes. Antimicrob. Agents Chemother. 2003, 47, 223–230. [Google Scholar] [CrossRef]
- Chatelain, E. Chagas Disease Drug Discovery: Toward a New Era. J. Biomol. Screen. 2015, 20, 22–35. [Google Scholar] [CrossRef]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; del Carmen Bangher, M.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascon, J.; et al. Benznidazole and Posaconazole in Eliminating Parasites in Asymptomatic T. cruzi Carriers: The STOP-CHAGAS Trial. J. Am. Coll. Cardiol. 2017, 69, 939–947. [Google Scholar] [CrossRef]
- Pinazo, M.J.; Malchiodi, E.; Ioset, J.R.; Bivona, A.; Gollob, K.J.; Dutra, W.O. Challenges and Advancements in the Development of Vaccines and Therapies against Chagas Disease. Lancet Microbe 2024, 5, 100972. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.J.; Rico, E.; Lukac, I.; Zuccotto, F.; Elg, S.; Gilbert, I.H.; Freund, Y.; Alley, M.R.K.; Field, M.C.; Wyllie, S.; et al. Clinical and Veterinary Trypanocidal Benzoxaboroles Target CPSF3. Proc. Natl. Acad. Sci. USA 2018, 115, 9616–9621. [Google Scholar] [CrossRef]
- Padilla, A.M.; Wang, W.; Akama, T.; Carter, D.S.; Easom, E.; Freund, Y.; Halladay, J.S.; Liu, Y.; Hamer, S.A.; Hodo, C.L.; et al. Discovery of an Orally Active Benzoxaborole Prodrug Effective in the Treatment of Chagas Disease in Non-Human Primates. Nat. Microbiol. 2022, 7, 1536–1546. [Google Scholar] [CrossRef]
- Helleberg, B.B.; Gudmundsson, K.S.S.; Kurtzhals, J.A.L.; Helleberg, M. Second stage human African Trypanosomiasis with Trypanosoma brucei rhodesiense treated with fexinidazole. Lancet Infect. Dis. 2003, 23, e505. [Google Scholar] [CrossRef]
- Drugs for Neglected Diseases initiative (DNDi). DNDI-6148 Chagas Disease. Available online: https://dndi.org/research-development/portfolio/dndi-6148-chagas/ (accessed on 7 August 2025).
- DNDi Chagas Disease: UW Series. Available online: https://dndi.org/research-development/portfolio/uw-series/ (accessed on 3 September 2025).
- González, S.; Wall, R.J.; Thomas, J.; Braillard, S.; Brunori, G.; Camino Díaz, I.; Cantizani, J.; Carvalho, S.; Castañeda Casado, P.; Chatelain, E.; et al. Short-Course Combination Treatment for Experimental Chronic Chagas Disease. Sci. Transl. Med. 2023, 15, eadg8105. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.-T.; Periferakis, A.; Periferakis, K.; Caruntu, A.; Anca Badarau, I.; Savulescu-Fiedler, I.; Scheau, C.; Caruntu, C. Antimicrobial Properties of Capsaicin: Available Data and Future Research Perspectives. Nutrients 2023, 15, 4097. [Google Scholar] [CrossRef] [PubMed]
- Valera-Vera, E.A.; Reigada, C.; Sayé, M.; Sayé, S.; Digirolamo, F.A.; Galceran, F.; Miranda, M.R.; Pereira, C.A. Effect of Capsaicin on the Protozoan Parasite Trypanosoma cruzi. FEMS Microbiol. Lett. 2020, 367, fnaa194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, M.; Zhang, J.; Peng, W.; Kesse Firempong, C.; Deng, W.; Wang, Q.; Wang, S.; Shi, F.; Yu, J.; et al. Improved Oral Bioavailability of Capsaicin via Liposomal Nanoformulation: Preparation, in Vitro Drug Release and Pharmacokinetics in Rats. Arch. Pharm. Res. 2015, 38, 512–521. [Google Scholar] [CrossRef]
- Vanrell, M.C.; Martinez, S.J.; Muñoz, L.I.; Salassa, B.N.; Tudela, J.G.; Romano, P.S.; Touz, M.C.; Pereira De Sa, N.; Tudela, G.J.; Romano, P.S. Induction of Autophagy by Ursolic Acid Promotes the Elimination of Trypanosoma cruzi Amastigotes from Macrophages and Cardiac Cells. Cell. Infect. Microbiol. 2022, 12, 919096. [Google Scholar] [CrossRef]
- da Silva Ferreira, D.; Esperandim, V.R.; Toldo, M.P.A.; Kuehn, C.C.; do Prado Júnior, J.C.; Cunha, W.R.; Silva, M.L.A.E.; Albuquerque, S. de In Vivo Activity of Ursolic and Oleanolic Acids during the Acute Phase of Trypanosoma cruzi Infection. Exp. Parasitol. 2013, 134, 455–459. [Google Scholar] [CrossRef]
- Valera Vera, E.A.; Sayé, M.; Reigada, C.; Damasceno, F.S.; Silber, A.M.; Miranda, M.R.; Pereira, C.A. Resveratrol Inhibits Trypanosoma cruzi Arginine Kinase and Exerts a Trypanocidal Activity. Int. J. Biol. Macromol. 2016, 87, 498–503. [Google Scholar] [CrossRef]
- Vilar-Pereira, G.; Carneiro, V.C.; Mata-Santos, H.; R Vicentino, A.R.; Ramos, I.P.; L Giarola, N.L.; Feijó, D.F.; Meyer-Fernandes, J.R.; Paula-Neto, H.A.; Medei, E.; et al. Resveratrol Reverses Functional Chagas Heart Disease in Mice. Int. J. Biol. Macromol. 2016, 12, e1005947. [Google Scholar] [CrossRef]
- Barbosa, J.M.C.; Nicoletti, C.D.; Da Silva, P.B.; Melo, T.G.; Bora, D.; Futuro, O.; Ferreira, V.F.; Salomãoid, K. Characterization and Trypanocidal Activity of a β-Lapachone-Containing Drug Carrier. PLoS ONE 2021, 16, e0246811. [Google Scholar] [CrossRef]
- dos Anjos, D.O.; Sobral Alves, E.S.; Gonçalves, V.T.; Fontes, S.S.; Nogueira, M.L.; Suarez-Fontes, A.M.; Neves da Costa, J.B.; Rios-Santos, F.; Vannier-Santos, M.A. Effects of a Novel β–Lapachone Derivative on Trypanosoma cruzi: Parasite Death Involving Apoptosis, Autophagy and Necrosis. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 207–219. [Google Scholar] [CrossRef]
- Barr, S.C. Canine Chagas’ Disease (American Trypanosomiasis) in North America. Vet. Clin. N. Am.—Small Anim. Pract. 2009, 39, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L.; Palmer, R.H.; Hodges, C.C.; Hall, D.G. Neurologic Manifestations of Trypanosomiasis in a Dog. J. Am. Vet. Med. Assoc. 1991, 198, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Michimuko-Nagahara, Y.; Nakagama, Y.; Rodriguez, M.S.; Kaku, N.; Nitahara, Y.; Candray, K.; Tshibangu-Kabamba, E.; Hamano, S.; Hirayama, K.; Kaneko, A.; et al. Natural Reservoir of Trypanosoma cruzi Found in Triatomines Targeting Humans: Results from Nation-Wide Vector Surveillance in El Salvador. JMA J. 2025, 8, 432–443. [Google Scholar] [CrossRef]
- Busselman, R.E.; Meyers, A.C.; Zecca, I.B.; Auckland, L.D.; Castro, A.H.; Dowd, R.E.; Curtis-Robles, R.; Hodo, C.L.; Saunders, A.B.; Hamer, S.A. High Incidence of Trypanosoma cruzi Infections in Dogs Directly Detected through Longitudinal Tracking at 10 Multi-Dog Kennels, Texas, Usa. PLoS Negl. Trop. Dis. 2021, 15, e0009935. [Google Scholar] [CrossRef]
- Dario, M.A.; Furtado, C.; Lisboa, C.V.; de Oliveira, F.; Santos, F.M.; D’Andrea, P.S.; Roque, A.L.R.; Xavier, S.C.d.C.; Jansen, A.M. Trypanosomatid Richness Among Rats, Opossums, and Dogs in the Caatinga Biome, Northeast Brazil, a Former Endemic Area of Chagas Disease. Front. Cell Infect. Microbiol. 2022, 12, 851903. [Google Scholar] [CrossRef] [PubMed]
- Durães-Oliveira, J.; Palma-Marques, J.; Moreno, C.; Rodrigues, A.; Monteiro, M.; Alexandre-Pires, G.; da Fonseca, I.P.; Santos-Gomes, G. Chagas Disease: A Silent Threat for Dogs and Humans. Int. J. Mol. Sci. 2024, 25, 3840. [Google Scholar] [CrossRef]
- Freitas, N.E.M.; Habib, F.L.; Santos, E.F.; Silva, Â.A.O.; Fontes, N.D.; Leony, L.M.; Sampaio, D.D.; de Almeida, M.C.; Dantas-Torres, F.; Santos, F.L.N. Technological Advances in the Serological Diagnosis of Chagas Disease in Dogs and Cats: A Systematic Review. Parasit. Vectors 2022, 15, 343. [Google Scholar] [CrossRef]
- Despommier, D.; Ellis, B.R.; Wilcox, B.A. The Role of Ecotones in Emerging Infectious Diseases. Ecohealth 2006, 3, 281–289. [Google Scholar] [CrossRef]
- Laiño, M.A.; Cardinal, M.V.; Enriquez, G.F.; Alvedro, A.; Gaspe, M.S.; Gürtler, R.E. An Oral Dose of Fluralaner Administered to Dogs Kills Pyrethroid-Resistant and Susceptible Chagas Disease Vectors for at Least Four Months. Vet. Parasitol. 2019, 268, 98–104. [Google Scholar] [CrossRef]
- Laiño, M.A.; Cardinal, M.V.; Gaspe, M.S.; Enriquez, G.F.; Alvedro, A.; Macchiaverna, N.P.; Gürtler, R.E. Control of Pyrethroid-Resistant Populations of Triatoma infestans, the Main Vector of Trypanosoma cruzi, by Treating Dogs with Fluralaner in the Argentine Chaco. Med. Vet. Entomol. 2022, 36, 149–158. [Google Scholar] [CrossRef]
- World Health Organization. Trypanosomiasis, Human African (Sleeping Sickness). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 4 August 2025).
- Meisner, J.; Kato, A.; Lemerani, M.M.; Miaka, E.M.; Taban, A.I.; Wakefield, J.; Rowhani-Rahbar, A.; Pigott, D.M.; Mayer, J.D.; Rabinowitz, P.M. The Effect of Livestock Density on Trypanosoma brucei gambiense and T. b. rhodesiense: A Causal Inference-Based Approach. PLoS Negl. Trop. Dis. 2022, 16, e0010155. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.; Milord, F.; Khonde, A.N.; Niyonsenga, T.; Loko, L.; Mpia, B.; De Wals, P. Risk Factors for Encephalopathy and Mortality during Melarsoprol Treatment of Trypanosoma brucei gambiense Sleeping Sickness. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.; de Koning, H.P.; Mäser, P.; Horn, D. Drug Resistance in African Trypanosomiasis: The Melarsoprol and Pentamidine Story. Trends Parasitol. 2013, 29, 110–118. [Google Scholar] [CrossRef]
- Bray, P.G.; Barrett, M.P.; Ward, S.A.; De Koning, H.P. Pentamidine Uptake and Resistance in Pathogenic Protozoa: Past, Present and Future. Trends Parasitol. 2003, 19, 232–239. [Google Scholar] [CrossRef]
- Zoltner, M.; Horn, D.; de Koning, H.P.; Field, M.C. Exploiting the Achilles’ Heel of Membrane Trafficking in Trypanosomes. Curr. Opin. Microbiol. 2016, 34, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pertejo, Y.; García-Estrada, C.; Martínez-Valladares, M.; Murugesan, S.; Reguera, R.M.; Balaña-Fouce, R. Polyamine Metabolism for Drug Intervention in Trypanosomatids. Pathogens 2024, 13, 79. [Google Scholar] [CrossRef]
- Alirol, E.; Schrumpf, D.; Amici Heradi, J.; Riedel, A.; De Patoul, C.; Quere, M.; Chappuis, F. Nifurtimox-Eflornithine Combination Therapy for Second-Stage Gambiense Human African Trypanosomiasis: Médecins Sans Frontières Experience in the Democratic Republic of the Congo. Clin. Infect. Dis. 2013, 56, 195–203. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Alshammari, M.K.; Alqahtani, A.M.; Alanazi, T.A.; Kamal, M.; Jawaid, T.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. Discovery, Development, Inventions and Patent Review of Fexinidazole: The First All-Oral Therapy for Human African Trypanosomiasis. Pharmaceuticals 2022, 15, 128. [Google Scholar] [CrossRef]
- Lindner, A.K.; Lejon, V.; Barrett, M.P.; Blumberg, L.; Bukachi, S.A.; Chancey, R.J.; Edielu, A.; Matemba, L.; Mesha, T.; Mwanakasale, V.; et al. New WHO Guidelines for Treating Rhodesiense Human African Trypanosomiasis: Expanded Indications for Fexinidazole and Pentamidine. Lancet Infect. Dis. 2025, 25, e77–e85. [Google Scholar] [CrossRef] [PubMed]
- Waithaka, A.; Clayton, C. Clinically Relevant Benzoxaboroles Inhibit MRNA Processing in Trypanosoma brucei. BMC Res. Notes 2022, 15, 371. [Google Scholar] [CrossRef]
- Barrett, M.P. Transforming the Chemotherapy of Human African Trypanosomiasis. Clin. Microbiol. Rev. 2025, 38, e0015323. [Google Scholar] [CrossRef]
- Steverding, D. The Development of Drugs for Treatment of Sleeping Sickness: A Historical Review. Parasit. Vectors 2010, 3, 15. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Programme Against African Trypanosomosis (PAAT). Available online: https://www.fao.org/paat/the-programme/the-disease/en/ (accessed on 6 August 2025).
- Akazue, P.I.; Ebiloma, G.U.; Ajibola, O.; Isaac, C.; Onyekwelu, K.; Ezeh, C.O.; Eze, A.A. Sustainable Elimination (Zero Cases) of Sleeping Sickness: How Far Are We from Achieving This Goal? Pathogens 2019, 8, 135. [Google Scholar] [CrossRef]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Rationale for Continued Investment in Tackling Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2021.
- Akama, T.; Zhang, Y.K.; Freund, Y.R.; Berry, P.; Lee, J.; Easom, E.E.; Jacobs, R.T.; Plattner, J.J.; Witty, M.J.; Peter, R.; et al. Identification of a 4-Fluorobenzyl L-Valinate Amide Benzoxaborole (AN11736) as a Potential Development Candidate for the Treatment of Animal African Trypanosomiasis (AAT). Bioorg Med. Chem. Lett. 2018, 28, 6–10. [Google Scholar] [CrossRef]
- Giordani, F.; Paape, D.; Vincent, I.M.; Pountain, A.W.; Fernández-Cortés, F.; Rico, E.; Zhang, N.; Morrison, L.J.; Freund, Y.; Witty, M.J.; et al. Veterinary Trypanocidal Benzoxaboroles Are Peptidase-Activated Prodrugs. PLoS Pathog. 2020, 16, e1008932. [Google Scholar] [CrossRef]
- Sonoiki, E.; Ng, C.L.; Lee, M.C.S.; Guo, D.; Zhang, Y.K.; Zhou, Y.; Alley, M.R.K.; Ahyong, V.; Sanz, L.M.; Lafuente-Monasterio, M.J.; et al. A Potent Antimalarial Benzoxaborole Targets a Plasmodium falciparum Cleavage and Polyadenylation Specificity Factor Homologue. Nat. Commun. 2017, 8, 14574. [Google Scholar] [CrossRef]
- Palencia, A.; Bougdour, A.; Brenier-Pinchart, M.; Touquet, B.; Bertini, R.; Sensi, C.; Gay, G.; Vollaire, J.; Josserand, V.; Easom, E.; et al. Targeting Toxoplasma Gondii CPSF 3 as a New Approach to Control Toxoplasmosis. EMBO Mol. Med. 2017, 9, 385–394. [Google Scholar] [CrossRef]
- Rottenberg, M.E.; Masocha, W.; Ferella, M.; Petitto-Assis, F.; Goto, H.; Kristensson, K.; McCaffrey, R.; Wigzell, H. Treatment of African Trypanosomiasis with Cordycepin and Adenosine Deaminase Inhibitors in a Mouse Model. J. Infect. Dis. 2005, 192, 1658–1665. [Google Scholar] [CrossRef]
- Vodnala, S.K.; Lundbäck, T.; Yeheskieli, E.; Sjöberg, B.; Gustavsson, A.L.; Svensson, R.; Olivera, G.C.; Eze, A.A.; De Koning, H.P.; Hammarström, L.G.J.; et al. Structure-Activity Relationships of Synthetic Cordycepin Analogues as Experimental Therapeutics for African Trypanosomiasis. J. Med. Chem. 2013, 56, 9861–9873. [Google Scholar] [CrossRef] [PubMed]
- Hulpia, F.; Mabille, D.; Campagnaro, G.D.; Schumann, G.; Maes, L.; Roditi, I.; Hofer, A.; de Koning, H.P.; Caljon, G.; Van Calenbergh, S. Combining Tubercidin and Cordycepin Scaffolds Results in Highly Active Candidates to Treat Late-Stage Sleeping Sickness. Nat. Commun. 2019, 10, 5564. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.S.; Fairlamb, A.H. Arsenical-Resistant Trypanosomes Lack an Unusual Adenosine Transporter. Nature 1993, 361, 173–176. [Google Scholar] [CrossRef]
- De Koning, H.P.; MacLeod, A.; Barrett, M.P.; Cover, B.; Jarvis, S.M. Further Evidence for a Link between Melarsoprol Resistance and P2 Transporter Function in African Trypanosomes. Mol. Biochem. Parasitol. 2000, 106, 181–185. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P.; Anderson, L.F.; Stewart, M.; Burchmore, R.J.S.; Wallace, L.J.M.; Barrett, M.P. The Trypanocide Diminazene Aceturate Is Accumulated Predominantly through the TbAT1 Purine Transporter: Additional Insights on Diamidine Resistance in African Trypanosomes. Antimicrob. Agents Chemother. 2004, 48, 1515–1519. [Google Scholar] [CrossRef]
- Munday, J.C.; Rojas López, K.E.; Eze, A.A.; Delespaux, V.; Van Den Abbeele, J.; Rowan, T.; Barrett, M.P.; Morrison, L.J.; de Koning, H.P. Functional Expression of TcoAT1 Reveals It to Be a P1-Type Nucleoside Transporter with No Capacity for Diminazene Uptake. Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 69–76. [Google Scholar] [CrossRef]
- Ungogo, M.A.; Aldfer, M.M.; Natto, M.J.; Zhuang, H.; Chisholm, R.; Walsh, K.; McGee, M.C.; Ilbeigi, K.; Asseri, J.I.; Burchmore, R.J.S.; et al. Cloning and Characterization of Trypanosoma congolense and T. vivax Nucleoside Transporters Reveal the Potential of P1-Type Carriers for the Discovery of Broad-Spectrum Nucleoside-Based Therapeutics against Animal African Trypanosomiasis. Int. J. Mol. Sci. 2023, 24, 3144. [Google Scholar] [CrossRef]
- Francisco, K.R.; Monti, L.; Yang, W.; Park, H.; Liu, L.J.; Watkins, K.; Amarasinghe, D.K.; Nalli, M.; Roberto Polaquini, C.; Regasini, L.O.; et al. Structure-Activity Relationship of Dibenzylideneacetone Analogs against the Neglected Disease Pathogen, Trypanosoma brucei. Bioorg. Med. Chem. Lett. 2023, 81, 129123. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of Curcumin, a Component of Golden Spice, and Its Miraculous Biological Activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Alkhaldi, A.A.M.; Creek, D.J.; Ibrahim, H.; Kim, D.-H.; Quashie, N.B.; Burgess, K.E.; Changtam, C.; Barrett, M.P.; Suksamrarn, A.; de Koning, H.P. Potent Trypanocidal Curcumin Analogs Bearing a Monoenone Linker Motif Act on Trypanosoma brucei by Forming an Adduct with Trypanothione. Mol. Pharmacol. 2015, 87, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Sirak, B.; Bizuneh, G.K.; Imming, P.; Asres, K. In Vitro and in Vivo Antitrypanosomal Activity of the Fresh Leaves of Ranunculus Multifidus Forsk and Its Major Compound Anemonin against Trypanosoma congolense Field Isolate. BMC Vet. Res. 2024, 20, 32. [Google Scholar] [CrossRef]
- Ebiloma, G.U.; Igoli, J.O.; Katsoulis, E.; Donachie, A.-M.; Eze, A.; Gray, A.I.; de Koning, H.P. Bioassay-Guided Isolation of Active Principles from Nigerian Medicinal Plants Identifies New Trypanocides with Low Toxicity and No Cross-Resistance to Diamidines and Arsenicals. J. Ethnopharmacol. 2017, 202, 256–264. [Google Scholar] [CrossRef]
- Ebiloma, G.U.; Katsoulis, E.; Igoli, J.O.; Gray, A.I.; De Koning, H.P. Multi-Target Mode of Action of a Clerodane-Type Diterpenoid from Polyalthia longifolia Targeting African Trypanosomes. Sci. Rep. 2018, 8, 4613. [Google Scholar] [CrossRef]
- Nwodo, N.; Ibezim, A.; Ntie-Kang, F.; Adikwu, M.; Mbah, C. Anti-Trypanosomal Activity of Nigerian Plants and Their Constituents. Molecules 2015, 20, 7750–7771. [Google Scholar] [CrossRef]
- Ungogo, M.A.; Ebiloma, G.U.; Ichoron, N.; Igoli, J.O.; de Koning, H.P.; Balogun, E.O. A Review of the Antimalarial, Antitrypanosomal, and Antileishmanial Activities of Natural Compounds Isolated from Nigerian Flora. Front. Chem. 2020, 8, 617448. [Google Scholar] [CrossRef]
- Nekoei, S.; Khamesipour, F.; Habtemariam, S.; de Souza, W.; Mohammadi Pour, P.; Hosseini, S.R. The Anti- Trypanosoma Activities of Medicinal Plants: A Systematic Review of the Literature. Vet. Med. Sci. 2022, 8, 2738–2772. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.D.; Spivak, M. Increased Resin. Collection after Parasite Challenge: A Case of Self-Medication in Honey Bees? PLoS ONE 2012, 7, e34601. [Google Scholar] [CrossRef] [PubMed]
- Mura, A.; Pusceddu, M.; Theodorou, P.; Angioni, A.; Floris, I.; Paxton, R.J.; Satta, A. Propolis Consumption Reduces Nosema Ceranae Infection of European Honey Bees (Apis Mellifera). Insects 2020, 11, 124. [Google Scholar] [CrossRef]
- Siheri, W.; Ebiloma, G.U.; Igoli, J.O.; Gray, A.I.; Biddau, M.; Akrachalanont, P.; Alenezi, S.; Alwashih, M.A.; Edrada-Ebel, R.; Muller, S.; et al. Isolation of a Novel Flavanonol and an Alkylresorcinol with Highly Potent Anti-Trypanosomal Activity from Libyan Propolis. Molecules 2019, 24, 1041. [Google Scholar] [CrossRef]
- Alenezi, S.S.; Alenezi, N.D.; Ebiloma, G.U.; Natto, M.J.; Ungogo, M.A.; Igoli, J.O.; Ferro, V.A.; Gray, A.I.; Fearnley, J.; de Koning, H.P.; et al. The Antiprotozoal Activity of Papua New Guinea Propolis and Its Triterpenes. Molecules 2022, 27, 1622. [Google Scholar] [CrossRef]
- Alotaibi, A.; Ebiloma, G.U.; Williams, R.; Alenezi, S.; Donachie, A.-M.; Guillaume, S.; Igoli, J.O.; Fearnley, J.; De Koning, H.P.; Watson, D.G. European Propolis Is Highly Active against Trypanosomatids Including Crithidia fasciculata. Sci. Rep. 2019, 9, 11364. [Google Scholar] [CrossRef]
- Omar, R.; Igoli, J.O.; Zhang, T.; Gray, A.I.; Ebiloma, G.U.; Clements, C.J.; Fearnley, J.; Edrada Ebel, R.; Paget, T.; de Koning, H.P.; et al. The Chemical Characterization of Nigerian Propolis samples and Their Activity Against Trypanosoma brucei. Sci. Rep. 2017, 7, 923. [Google Scholar] [CrossRef]
- Ebiloma, G.U.; Ichoron, N.; Siheri, W.; Watson, D.G.; Igoli, J.O.; De Koning, H.P. The Strong Anti-Kinetoplastid Properties of Bee Propolis: Composition and Identification of the Active Agents and Their Biochemical Targets. Molecules 2020, 25, 5155. [Google Scholar] [CrossRef]
- Alghamdi, A.H.; Munday, J.C.; Campagnaro, G.D.; Gurvic, D.; Svensson, F.; Okpara, C.E.; Kumar, A.; Quintana, J.; Martin Abril, M.E.; Milić, P.; et al. Positively Selected Modifications in the Pore of TbAQP2 Allow Pentamidine to Enter Trypanosoma brucei. eLife 2020, 9, e56416. [Google Scholar] [CrossRef]
- Piccica, M.; Lagi, F.; Bartoloni, A.; Zammarchi, L. Efficacy and Safety of Pentamidine Isethionate for Tegumentary and Visceral Human Leishmaniasis: A Systematic Review. J. Travel. Med. 2021, 28, taab065. [Google Scholar] [CrossRef] [PubMed]
- Wiedemar, N.; Graf, F.E.; Zwyer, M.; Ndomba, E.; Kunz Renggli, C.; Cal, M.; Schmidt, R.S.; Wenzler, T.; Mäser, P. Beyond Immune Escape: A Variant Surface Glycoprotein Causes Suramin Resistance in Trypanosoma brucei. Mol. Microbiol. 2018, 107, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Zoltner, M.; Campagnaro, G.D.; Taleva, G.; Burrell, A.; Cerone, M.; Leung, K.F.; Achcar, F.; Horn, D.; Vaughan, S.; Gadelha, C.; et al. Suramin Exposure Alters Cellular Metabolism and Mitochondrial Energy Production in African Trypanosomes. J. Biol. Chem. 2020, 295, 8331–8347. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Richer, M.; Bilenge, C.M.M.; Josenando, T.; Chappuis, F.; Manthelot, C.R.; Nangouma, A.; Doua, F.; Asumu, P.N.; Simarro, P.P.; et al. Effectiveness of a 10-Day Melarsoprol Schedule for the Treatment of Late-Stage Human African Trypanosomiasis: Confirmation from a Multinational Study (IMPAMEL II). J. Infect. Dis. 2005, 191, 1922–1931. [Google Scholar] [CrossRef][Green Version]
- Matovu, E.; Stewart, M.L.; Geiser, F.; Brun, R.; Mäser, P.; Wallace, L.J.M.; Burchmore, R.J.; Enyaru, J.C.K.; Barrett, M.P.; Kaminsky, R.; et al. Mechanisms of Arsenical and Diamidine Uptake and Resistance in Trypanosoma brucei. Eukaryot. Cell 2003, 2, 1003–1008. [Google Scholar] [CrossRef]
- Burri, C.; Brun, R. Eflornithine for the Treatment of Human African Trypanosomiasis. Parasitol. Res. 2003, 90, S49–S52. [Google Scholar] [CrossRef]
- World Health Organization. Control of the Leishmaniases: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2010.
- Das, V.N.; Ranjan, A.; Sinha, A.N.; Verma, N.; Lal, C.S.; Gupta, A.K.; A Siddiqui, N.; Kar, S.K. A Randomized Clinical Trial of Low Dosage Combination of Pentamidine and Allopurinol in the Treatment of Antimony Unresponsive Cases of Visceral Leishmaniasis. J. Assoc. Physicians India 2001, 49, 609–613. [Google Scholar]
- Rassi, A.; Luquetti, A.O.; Rassi, A.; Rassi, G.G.; Rassi, S.G.; Da Silva, I.G.; Rassi, A.G. Specific Treatment for Trypanosoma cruzi: Lack of Efficacy of Allopurinol in the Human Chronic Phase of Chagas Disease. Am. J. Trop. Med. Hyg. 2007, 76, 58–61. [Google Scholar] [CrossRef]
- Mazzeti, A.L.; Diniz, L.d.F.; Gonçalves, K.R.; WonDollinger, R.S.; Assíria, T.; Ribeiro, I.; Bahia, M.T. Synergic Effect of Allopurinol in Combination with Nitroheterocyclic Compounds against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2019, 63, e02264-18. [Google Scholar] [CrossRef]
- Pennisi, M.G.; Persichetti, M.F. Feline Leishmaniosis: Is the Cat a Small Dog? Vet. Parasitol. 2018, 251, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Dorn, P.L.; Monroy, M.C.; Stevens, L. Sustainable, Integrated Control of Native Vectors: The Case of Chagas Disease in Central America. Front. Trop. Dis. 2022, 3, 971000. [Google Scholar] [CrossRef]
- Geerts, S.; Holmes, P.H.; Diall, O.; Eisler, M.C. African Bovine Trypanosomiasis: The Problem of Drug Resistance. Parasitol. Today 2001, 17, 25–28. [Google Scholar] [CrossRef]
- Bouyer, J.; Stachurski, F.; Gouro, A.S.; Lancelot, R. Control of Bovine Trypanosomosis by Restricted Application of Insecticides to Cattle Using Footbaths. Vet. Parasitol. 2009, 161, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, U.; Hendrichs, J. Integrating the Sterile Insect Technique as a Key Component of Area-Wide Tsetse and Trypanosomiasis Intervention; PAAT Technical and Scientific Series; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2001; Volume 3. [Google Scholar]
- Lord, J.S.; Lea, R.S.; Allan, F.K.; Byamungu, M.; Hall, D.R.; Lingley, J.; Mramba, F.; Paxton, E.; Vale, G.A.; Hargrove, J.W.; et al. Assessing the Effect of Insecticide-Treated Cattle on Tsetse Abundance and Trypanosome Transmission at the Wildlife-Livestock Interface in Serengeti, Tanzania. PLoS Negl. Trop. Dis. 2020, 14, e0008288. [Google Scholar] [CrossRef]
- Baylis, M.; Stevenson, P. Trypanosomiasis and Tsetse Control with Insecticidal Pour-Ons—Fact and Fiction? Parasitol. Today 1998, 14, 77–82. [Google Scholar] [CrossRef]
- Gimonneau, G.; Alioum, Y.; Abdoulmoumini, M.; Zoli, A.; Cene, B.; Adakal, H.; Bouyer, J. Insecticide and Repellent Mixture Pour-On Protects Cattle against Animal Trypanosomosis. PLoS Negl. Trop. Dis. 2016, 10, e0005248. [Google Scholar] [CrossRef]
- STACHURSKI, F.; LANCELOT, R. Footbath Acaricide Treatment to Control Cattle Infestation by the Tick Amblyomma variegatum. Med. Vet. Entomol. 2006, 20, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.C.P. Southern Cone Initiative for the Elimination of Domestic Populations of Triatoma Infestans and the Interruption of Transfusional Chagas Disease. Historical Aspects, Present Situation, and Perspectives. Mem. Inst. Oswaldo Cruz 2007, 102, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, X.N. Preventing the Transmission of American Trypanosomiasis and Its Spread into Non-Endemic Countries. Infect. Dis. Poverty 2015, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Timm, B.L.; da Gama, A.N.S.; Batista, M.M.; Batista, D.d.G.J.; Boykin, D.W.; De Koning, H.P.; Correia Soeiro, M.d.N. Arylimidamides Have Potential for Chemoprophylaxis Against Blood-Transmitted Chagas Disease. Pathogens 2023, 12, 701. [Google Scholar] [CrossRef]
- Lima, J.F. Subregional Initiatives for Chagas Disease. A Path of Technical Cooperation, Opened by the Countries, as an Approach to a Neglected Disease. Mem. Inst. Oswaldo Cruz 2022, 117, e210130chgsa. [Google Scholar] [CrossRef] [PubMed]
- Schofield, C.J.; Dias, J.C.P. The Southern Cone Initiative against Chagas Disease. Adv. Parasitol. 1999, 42, 1–27. [Google Scholar] [CrossRef]
- de Arias, A.R.; Monroy, C.; Guhl, F.; Sosa-Estani, S.; Santos, W.S.; Abad-Franch, F. Chagas Disease Control-Surveillance in the Americas: The Multinational Initiatives and the Practical Impossibility of Interrupting Vector-Borne Trypanosoma cruzi Transmission. Mem. Inst. Oswaldo Cruz 2021, 116, e210130. [Google Scholar] [CrossRef]
- Reid, E.; Deb, R.M.; Ali, A.; Singh, R.P.; Mishra, P.K.; Shepherd, J.; Singh, A.M.; Bharti, A.; Singh, C.; Sharma, S.; et al. Molecular Surveillance of Insecticide Resistance in Phlebotomus Argentipes Targeted by Indoor Residual Spraying for Visceral Leishmaniasis Elimination in India. PLoS Negl. Trop. Dis. 2023, 17, e0011734. [Google Scholar] [CrossRef] [PubMed]
- Jha, T.K. Epidemiology of Drug-Resistant Kala-Azar in India and Neighboring Countries. In Kala Azar in South Asia: Current Status and Sustainable Challenges, 2nd ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Denton-Schneider, J.M.E. Disease, Disparities, and Development: Evidence from Chagas Disease Control in Brazil; NBER Working Paper; National Bureau of Economic Research, Inc.: Cambridge, MA, USA, 2025. [Google Scholar]
- Alvar, J.; den Boer, M.; Dagne, D.A. Towards the Elimination of Visceral Leishmaniasis as a Public Health Problem in East Africa: Reflections on an Enhanced Control Strategy and a Call for Action. Lancet Glob. Health 2021, 9, e1763–e1769. [Google Scholar] [CrossRef]
- Makau-Barasa, L.K.; Ochol, D.; Yotebieng, K.A.; Adera, C.B.; de Souza, D.K. Moving from Control to Elimination of Visceral Leishmaniasis in East Africa. Front. Trop. Dis. 2022, 3, 965609. [Google Scholar] [CrossRef]
- World Health Organization. Developing a Strategic Plan for the Elimination of Visceral Leishmaniasis in Eastern Africa: Report of a Stakeholder Meeting, Nairobi, Kenya, 24–27 January 2023; World Health Organization: Geneva, Switzerland, 2024.
- Strelkova, M.V.; Ponirovsky, E.N.; Morozov, E.N.; Zhirenkina, E.N.; Razakov, S.A.; Kovalenko, D.A.; Schnur, L.F.; Schönian, G. A Narrative Review of Visceral Leishmaniasis in Armenia, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, the Crimean Peninsula and Southern Russia. Parasit. Vectors 2015, 8, 330. [Google Scholar] [CrossRef]
- Sergiev, V.; Kondrashin, A.; Litvinov, S.; Morozova, L.; Turbabina, N.; Stepanova, E.; Maksimova, M.; Shevchenko, S.; Morozov, E. Epidemiology and Control of Leishmaniasis in the Former USSR: A Review Article. Iran. J. Parasitol. 2018, 13, 342. [Google Scholar]
- Isaev, L.M. Eradication of Leishmaniasis in the USSR during the 7-Year-Plan. Med. Parazitol. 1959, 28, 323–327. [Google Scholar]
- Babuadze, G.; Farlow, J.; De Koning, H.P.; Carrillo, E.; Chakhunashvili, G.; Murskvaladze, M.; Kekelidze, M.; Karseladze, I.; Kokaia, N.; Kalandadze, I.; et al. Seroepidemiology and Molecular Diversity of Leishmania donovani Complex in Georgia. Parasit. Vectors 2016, 9, 279. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; de Boer, M. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Özbel, Y.; Töz, S.; Muñoz, C.; Ortuño, M.; Jumakanova, Z.; Pérez-Cutillas, P.; Maia, C.; Conceição, C.; Baneth, G.; Pereira, A.; et al. The Current Epidemiology of Leishmaniasis in Turkey, Azerbaijan and Georgia and Implications for Disease Emergence in European Countries. Zoonoses Public Health 2022, 69, 395–407. [Google Scholar] [CrossRef]
- Tarral, A.; Hovsepian, L.; Duvauchelle, T.; Donazzolo, Y.; Latreille, M.; Felices, M.; Gualano, V.; Delhomme, S.; Valverde Mordt, O.; Blesson, S.; et al. Determination of the Optimal Single Dose Treatment for Acoziborole, a Novel Drug for the Treatment of Human African Trypanosomiasis: First-in-Human Study. Clin. Pharmacokinet. 2023, 62, 481–491. [Google Scholar] [CrossRef]
- Ashiwaju, B.I.; Uzougbo, C.G.; Orikpete, O.F. Environmental Impact of Pharmaceuticals: A Comprehensive Review. Matrix Sci. Pharma 2023, 7, 85–94. [Google Scholar] [CrossRef]
- Lima, C.M.; Uliassi, E.; Thoré, E.S.J.; Bertram, M.G.; Cardoso, L.; Cordeiro da Silva, A.; Costi, M.P.; de Koning, H.P. Environmental Impacts of Drugs against Parasitic Vector-Borne Diseases and the Need to Integrate Sustainability into Their Development and Use. Open Res. Eur. 2024, 4, 207. [Google Scholar] [CrossRef] [PubMed]
- Ilbeigi, K.; Barata, C.; Barbosa, J.; Bertram, M.G.; Caljon, G.; Costi, M.P.; Kroll, A.; Margiotta-Casaluci, L.; Thoré, E.S.J.; Bundschuh, M. Assessing Environmental Risks during the Drug Development Process for Parasitic Vector-Borne Diseases: A Critical Reflection. ACS Infect. Dis. 2024, 10, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The One Health Approach and Key Recommendations of the Quadripartite. 2023. Available online: https://www.who.int/publications/m/item/the-one-health-approach-and-key-recommendations-of-the-quadripartite (accessed on 5 August 2025).
- Williamson, G.L.; Bachelder, E.M.; Ainslie, K.M. Clinical and Preclinical Methods of Heat-Stabilization of Human Vaccines. Mol. Pharm. 2024, 21, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. One Health Joint Plan of Action (2022–2026): Working Together for the Health of Humans, Animals, Plants and the Environment; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240059139.
- Zhou, X.; Zheng, J. Building a Transdisciplinary Science of One Health with a Global Vision. Glob. Health J. 2024, 8, 99–102. [Google Scholar] [CrossRef]
- Hegewisch-Taylor, J.; Dreser, A.; Aragón-Gama, A.C.; Moreno-Reynosa, M.A.; Ramos Garcia, C.; Ruckert, A.; Labonté, R. Analyzing One Health Governance and Implementation Challenges in Mexico. Glob. Public Health 2024, 19, 2377259. [Google Scholar] [CrossRef]

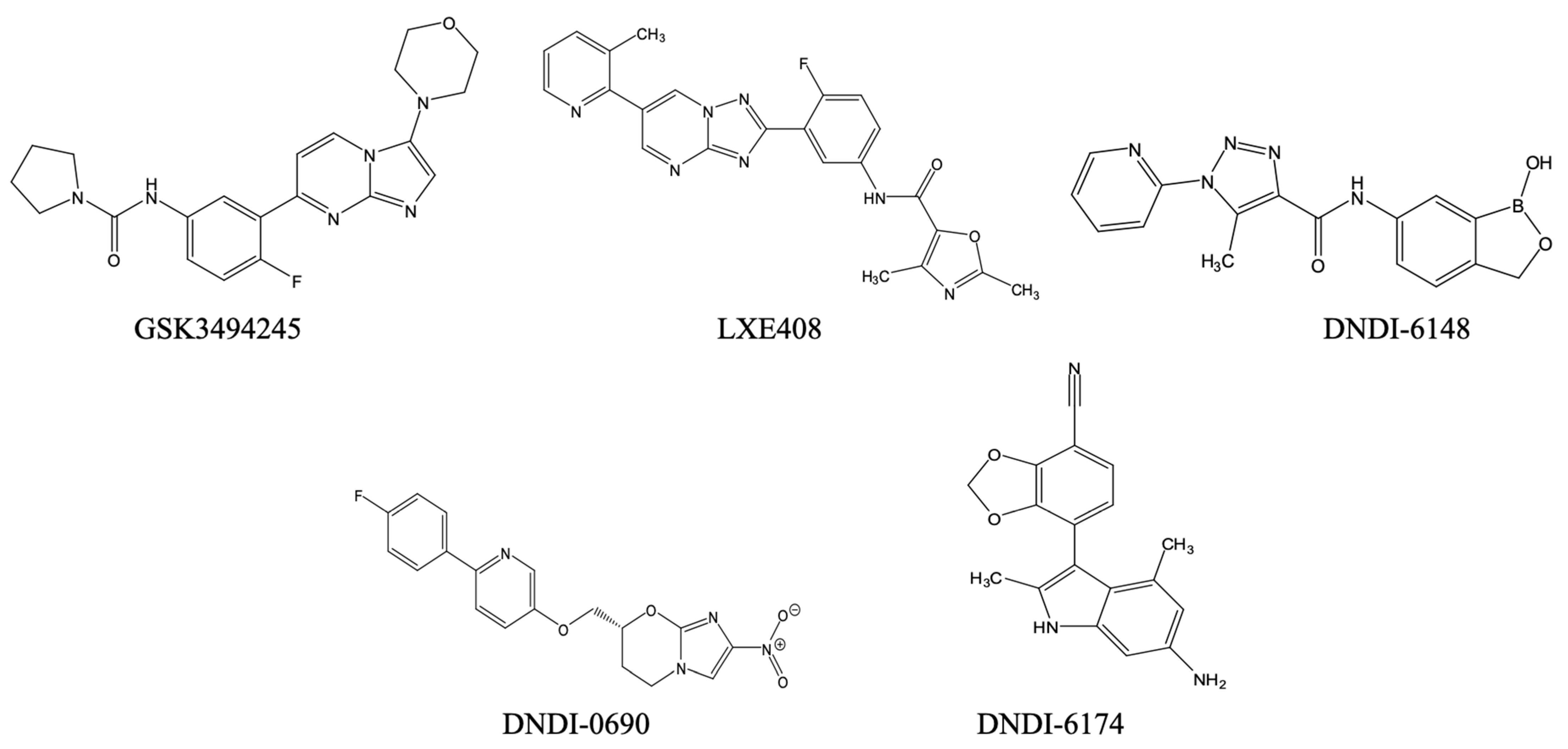
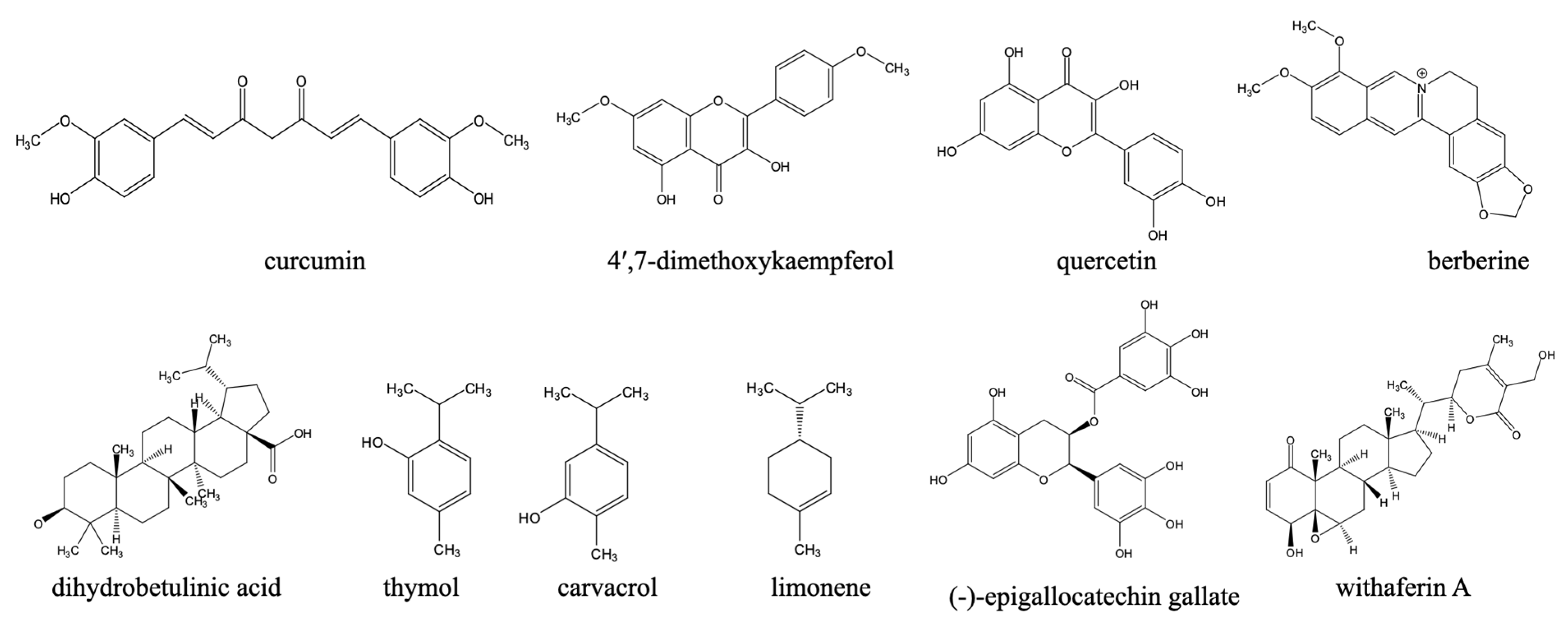
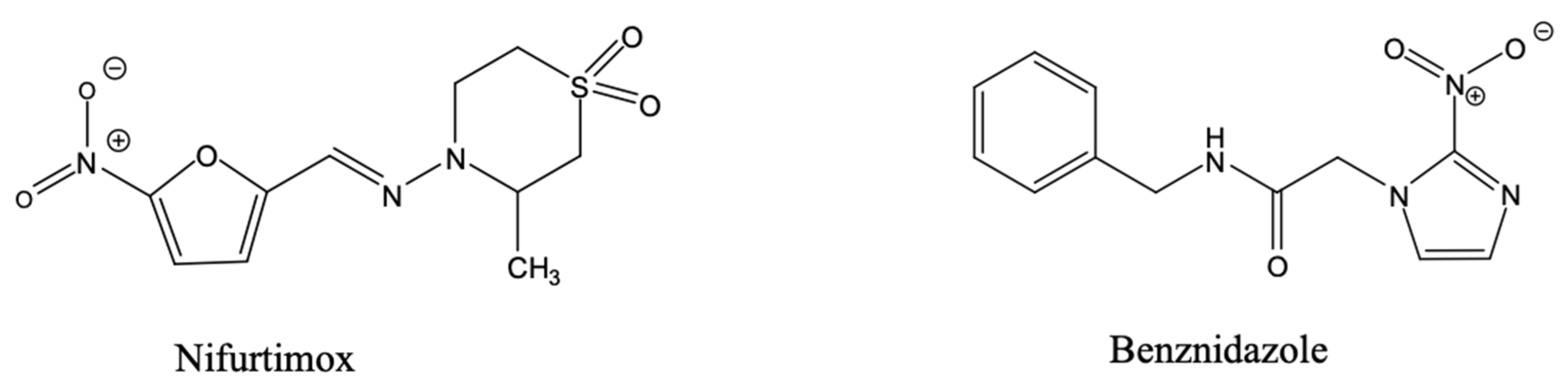
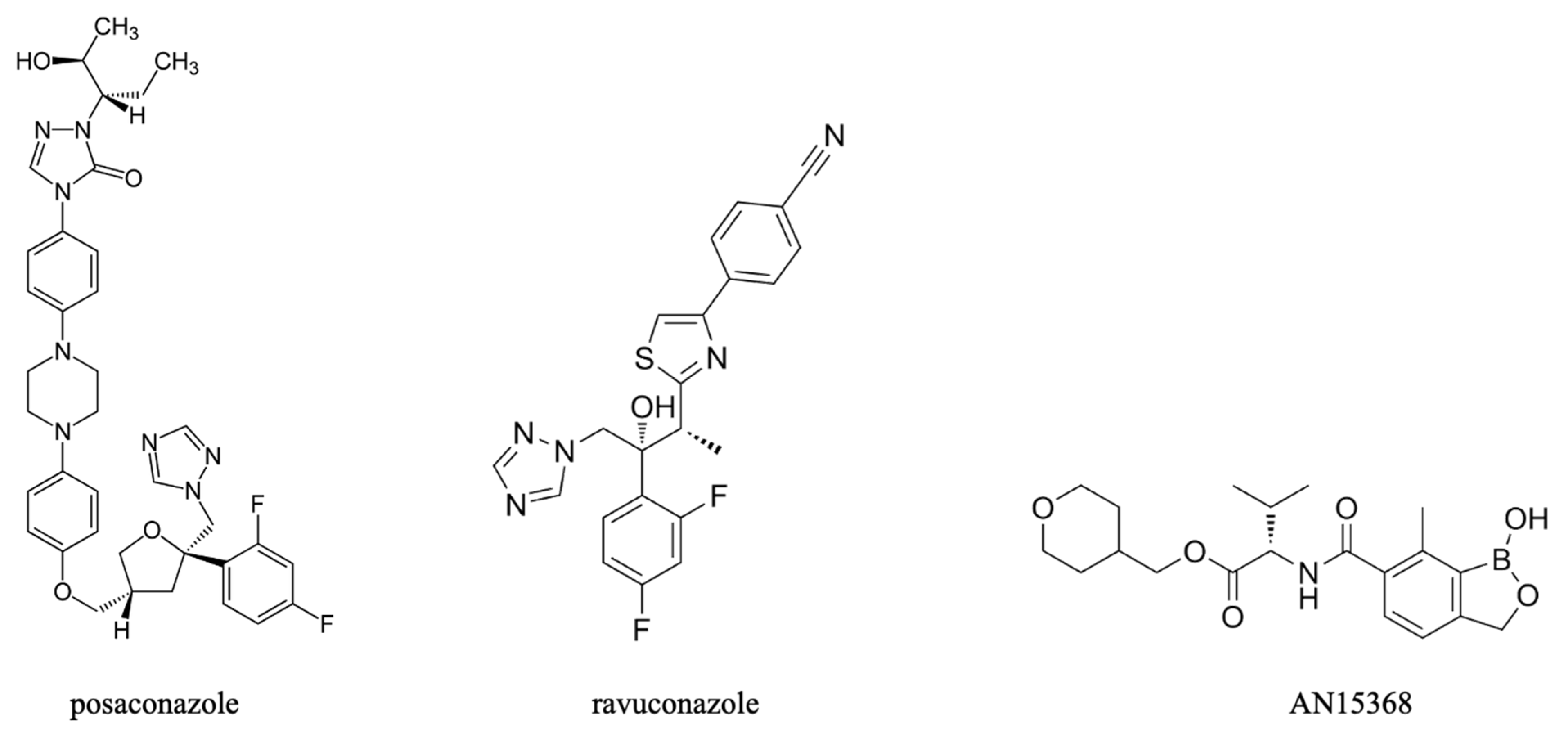
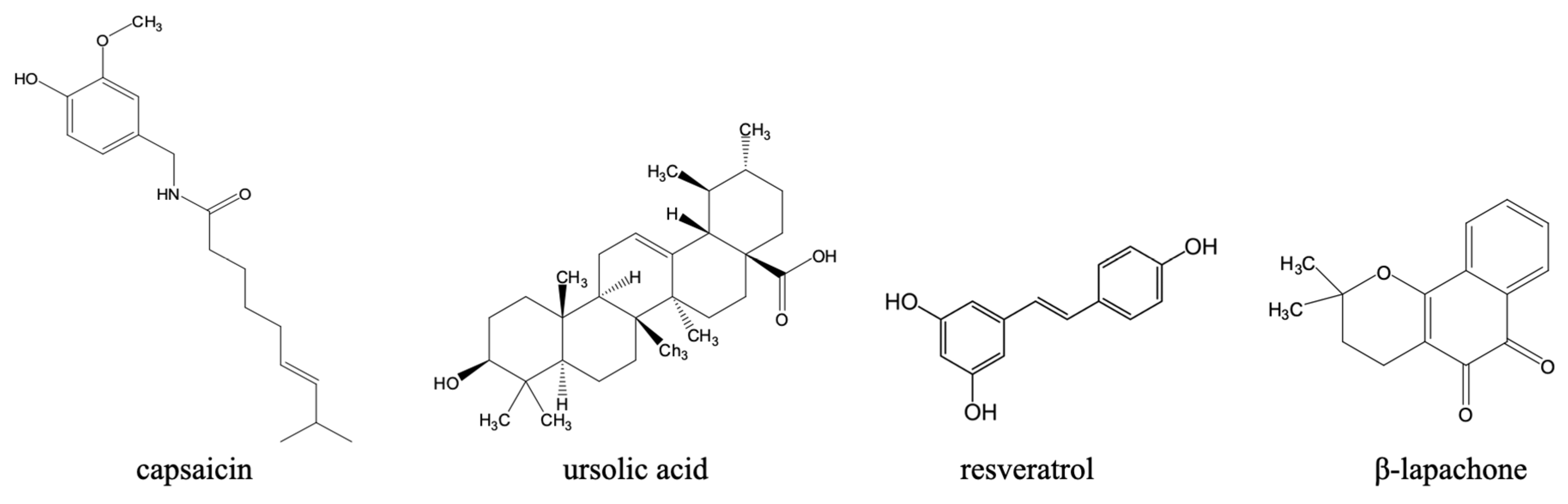
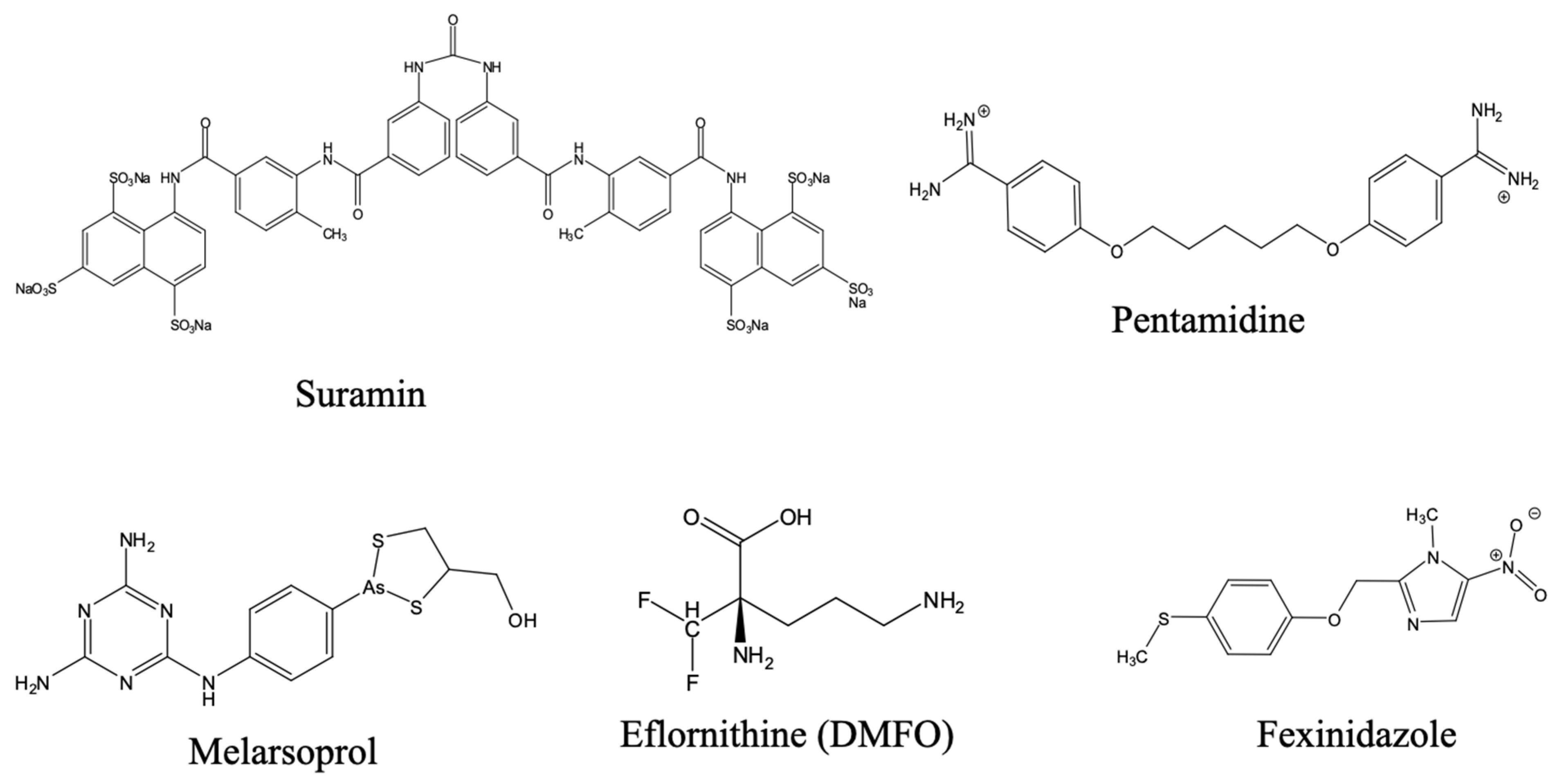
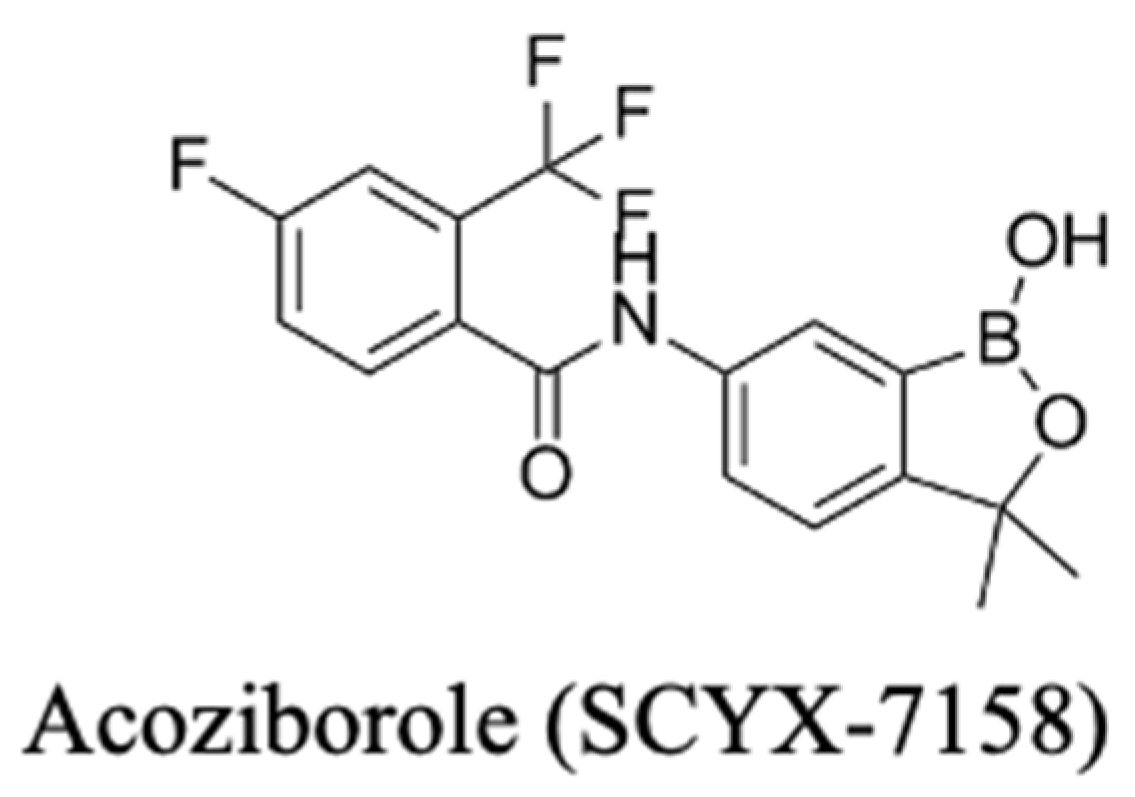
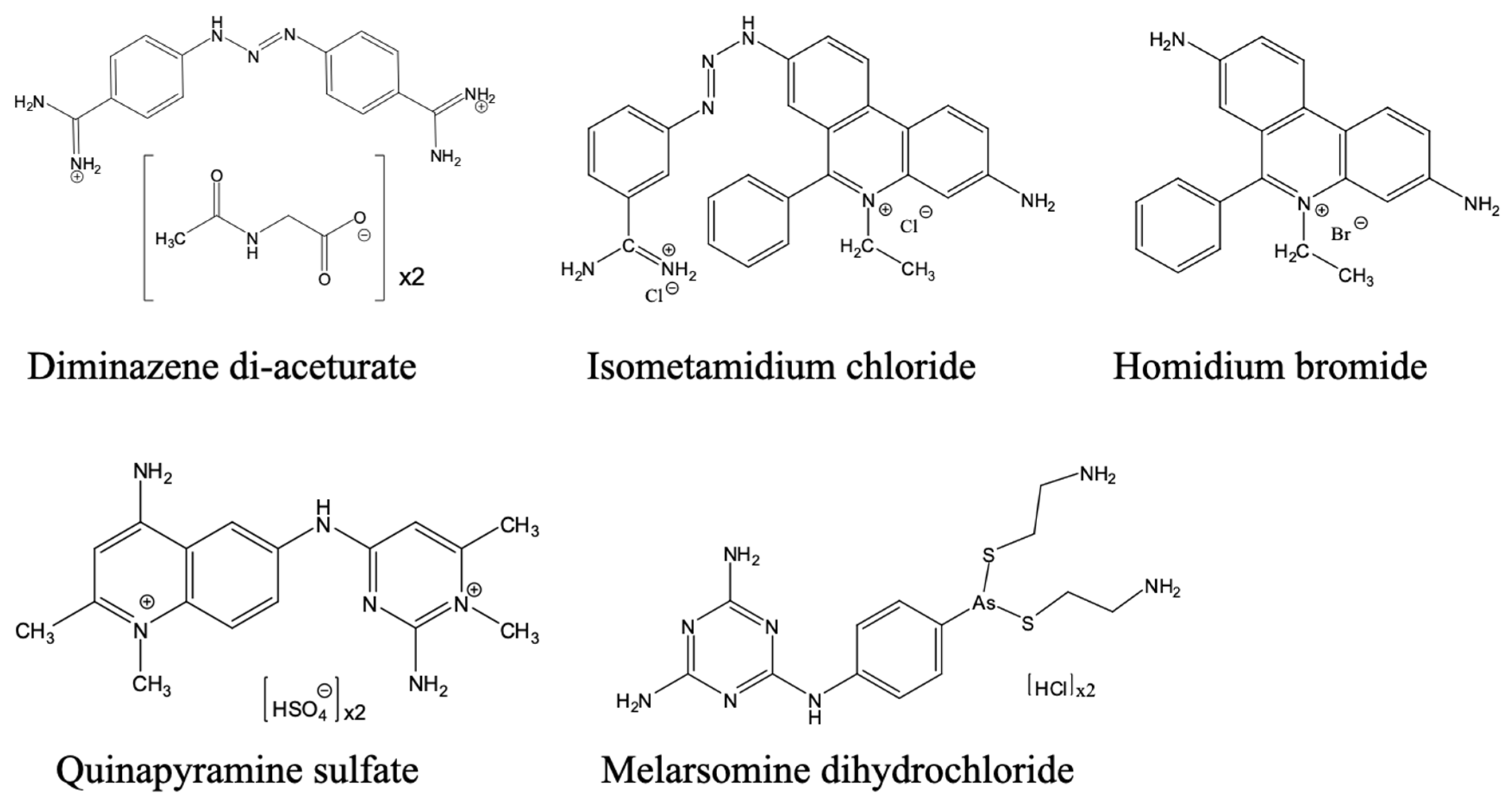
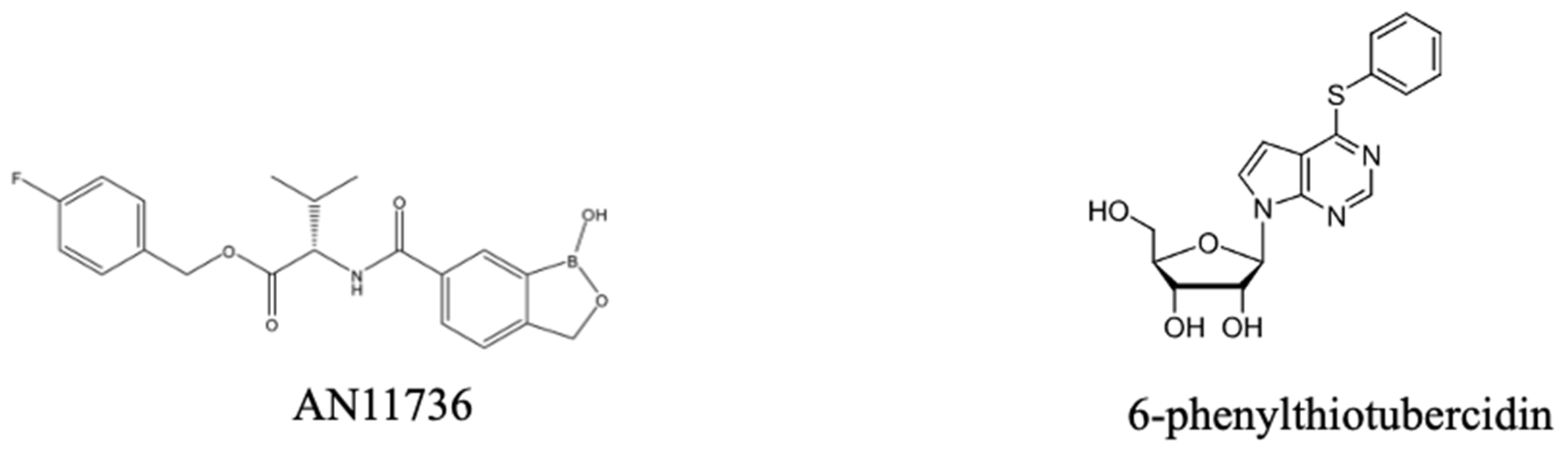

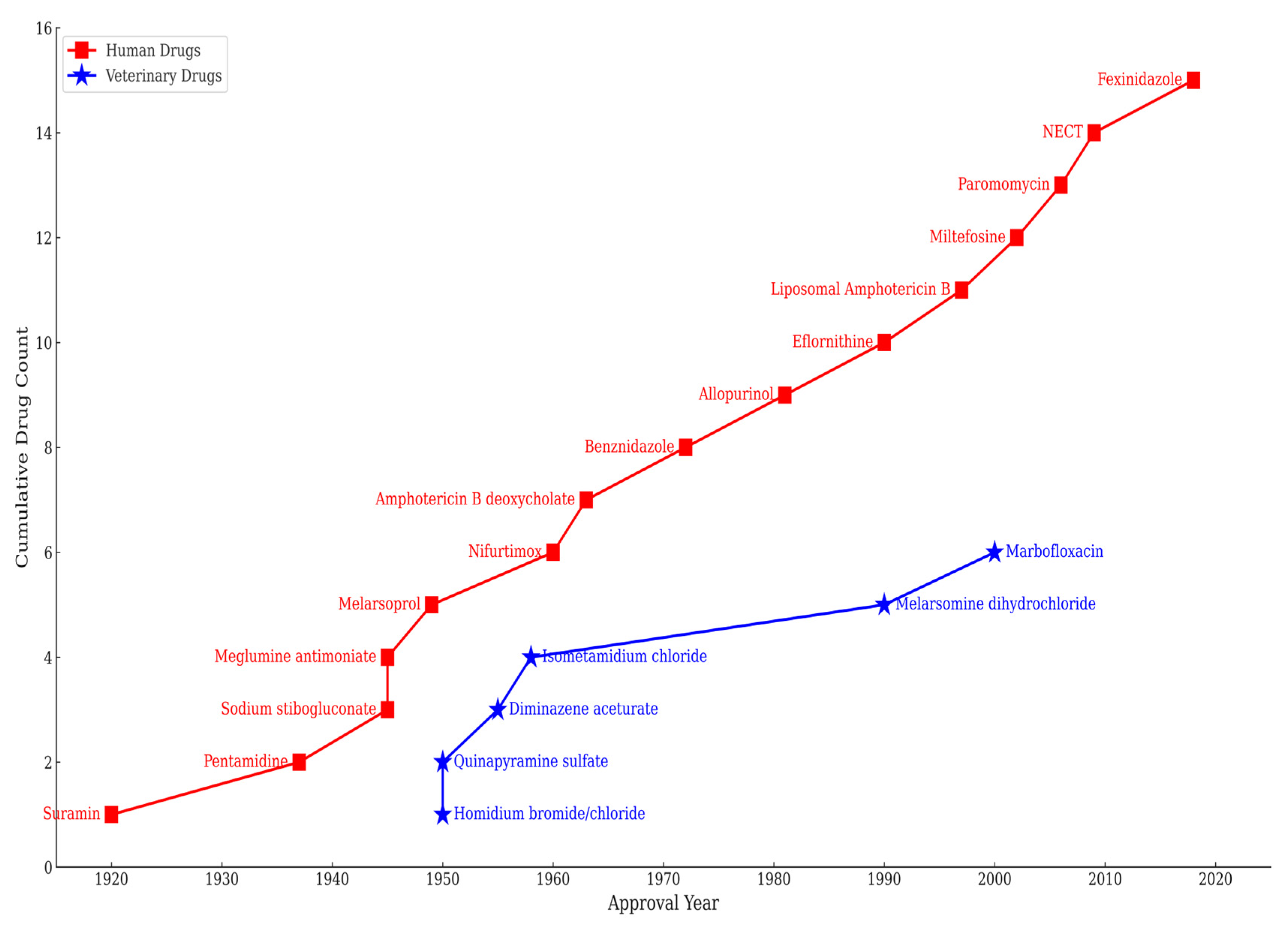
| Drug Name | Year of First Use | Target Parasite/ Disease Form | Mechanism of Action | Mechanism of Resistance | Side Effects/Major Limitations | Reference |
|---|---|---|---|---|---|---|
| Pentamidine | 1937 | T. brucei—effective against first stage T. b. gambiense infection. Though relatively less effective, it is also used in leishmaniasis, particularly the cutaneous form. | Not fully understood, but the drug is reported to bind to parasite DNA, inhibiting type II topoisomerase, and disrupting mitochondrial DNA. | Via reduced drug uptake due to alterations in transmembrane pentamidine transport proteins, specifically the TbAT1 adenosine transporter and the aquaglyceroporin TbAQP2. | Serious toxicity, including hypotension, hypoglycaemia, drug resistance. It is only used for treating stage 1 HAT as it does not permeate the BBB. | [58,65,207,247,248] |
| Suramin | 1920 | T. brucei—effective against first stage T. b. rhodesiense | Unclear but reported to be via induced collapse of cellular ATP which ultimately leads to trypanosome cell death. Also, it inhibits glycolysis enzymes, and non-specifically binds to L-α- glycerophosphate oxidase. | Unclear but linked to changes in variant surface glycoprotein, which confers resistance by binding suramin and potentially altering its uptake pathway. Mutations in a DNA helicase of T. brucei (RuvBL1) are reported to reduce suramin’s effectiveness. | Severe toxicity issues—nephrotoxicity, allergic reactions. It is highly polar and does not cross the BBB, meaning it can only be used for treating stage 1 HAT. It has a short half-life. | [208,249,250] |
| Melarsoprol | 1949 | T. brucei—first-line treatment for second stage T. rhodesiense HAT | Disruption of redox balance via inhibition of trypanothione reductase. | Mutations in transporters AQP2 and TbAT1. | Highly toxic with a narrow therapeutic window, drug counterfeiting, and prolonged treatment regimens. Reactive encephalopathy frequently occurs. Can cross the BBB, has a long half-life of 35 h and has been widely used for treating the late stages of HAT, although the accompanying encephalopathy and widespread drug resistance limit its use. | [18,206,251,252] |
| Eflornithine | 1990 | Meningoencephalitic (Second) stage T. b. gambiense. | Ornithine analogue that inhibits ornithine decarboxylase. | Deletion or mutation of the TbAAT6 gene in trypanosomes. | Has a short half-life; hence, a high dose (400 mg/kg/day) is needed. The i.v. regimen is also complex and cumbersome to apply. Note: Eflornithine is trypanostatic and not trypanocidal; so, it might not be effective in immunosuppressed patients. | [209,253] |
| NECT Nifurtimox/Eflornithine Combination Therapy | 2009 | T. brucei—Stage 2 T. b. gambiense infection | Combination of DNA damage and polyamine synthesis inhibition | Reduced activity of the enzyme T. brucei type I nitroreductase, crucial for activating nifurtimox. Also, reduced uptake of eflornithine, or mutations in the drug target. | It is not effective against T. b. rhodesiense. Requires inpatient care, trained personnel, and sterile supplies. | [210] |
| Fexinidazole | 2018 | T. brucei—First and non-severe second stage T. b. gambiense and T. b. rhodesiense | Activation via nitroreductases causes free radical damage selective damage of the parasites’ DNA and proteins. | Similar to Nifurtimox. Loss of activity of a type I nitroreductase (NTR) enzyme via gene deletion or decreased transcription. | Decreased cure rates and unacceptable side effects in patients with severe stage 2 HAT. At the time of writing, safety and efficacy studies have not been established in children < 6 years or weighing <20 kg. | [180,181,207] |
| Benznidazole | 1972 | T. cruzi | Nitroreductase enzyme mediated DNA damage and oxidative stress from free radicals. | Mutations in the nitroreductase (TcNTR-1) gene, changes in chromosome copy number, and activation of other genes involved in drug resistance. | Several unwanted side effects have been reported, including abdominal pain, rash or skin lesions, weight loss, nausea, vomiting, diarrhoea, dizziness, and tremor. Potentially teratogenic—cannot be used by pregnant women or women who may become pregnant. | [143,208,209] |
| Nifurtimox | 1960 | T. cruzi | See benznidazole. | See benznidazole. | Several, including decreased appetite, diarrhoea, dizziness, fever, headache, nausea, stomach pain, vomiting, and weight loss. Highly toxic, existence of drug counterfeiting, and prolonged treatment regimens. Potentially teratogenic—cannot be used by pregnant women or women who may become pregnant. | [18,210,211] |
| Miltefosine | 2002 | Leishmania species | Interferes with phospholipid metabolism, disrupts Ca2+ homeostasis, and inhibits mitochondrial cytochrome C oxidase. | Aminophospholipid transporter gene mutation linked with decreased drug uptake, or increased drug efflux via overexpressed ABC transporters. Also, suppression of oxidative stress-induced programmed cell death. | Adverse effects include renal toxicity and gastrointestinal disturbances, but these symptoms are reversible. Teratogenic. Susceptibility varies by Leishmania species and geographic regions. | [58,101,212,213] |
| Amphotericin B deoxycholate | 1963 | Leishmania species | Binds to (leishmania-specific) ergosterol, and forms membrane pores. | Alterations in the parasite’s sterol composition and membrane properties. | Reports of serious adverse reactions, including fever with rigor and chills, renal dysfunction, severe hypokalaemia, occasional serious myocarditis and death. Its use requires close monitoring and prolonged hospitalisation. | [84,88,89,214,215] |
| Liposomal Amphotericin B | 1997 | Leishmania species | See Amphotericin B deoxycholate. | See Amphotericin B deoxycholate. | In the lipid formulations (AmBisome), deoxycholate is replaced with other lipids that mask Amphotericin B. AmBisome is considered relatively non-toxic and highly effective form of treatment for VL. The need for cold-chain storage is a challenge in rural areas. | [88,89] |
| Sodium stibogluconate | 1945 | Leishmania species | Reduces to Sb(III) and disrupts redox balance. | Changes in gene expression for antimony transport protein LdAQP1 and glutathione metabolism leading to decreased drug uptake, increased drug efflux, and changes in parasite metabolism. | Toxic, severe side effects, including anorexia, nausea/vomiting, abdominal pain, headache, raised liver enzymes, coughing and substernal pain, fever, sweating, flushing, bleeding from nose or gum, jaundice, and rash. | [61,62,65,216] |
| Meglumine antimoniate | 1945 | Leishmania species | The drug reduces to Sb(III) and disrupts redox balance by disrupting glutathione (GSH) (trypanothione). | Reduced drug uptake, increased drug efflux, and alterations in antioxidant defence mechanisms resulting in diminished biological reduction of Sbv to Sb (III). | Associated severe side effects and toxicity concerns, including local pain at the site of intramuscular injection, cardiotoxicity, hepatotoxicity, pancreatitis, and nephrotoxicity. Also, there are reports of emergence of resistance and therapeutic failures in certain regions. | [61,216,217] |
| Paromomycin | 2006 | Leishmania species especially L. donovani | Inhibits leishmania protein synthesis by binding to the ribosomal subunit and via depolarisation of mitochondrial membrane potential (MMP). | Decreased drug accumulation due to alterations in the parasite’s cell membrane composition. | Common side effect includes ototoxicity, and problems with liver function. | [58,78,80,218] |
| Drug Name/Chemical Formula | Approval Year | Host Species | Target Parasite | Mechanism of Action | Mechanism of Resistance | Side effects & Other Treatment Issues | Reference |
|---|---|---|---|---|---|---|---|
| Diminazene aceturate | 1955 | Cattle, goats, sheep, pigs | Trypanosoma spp. (vivax, evansi, congolense, brucei) | Binds kinetoplast DNA, inhibits replication. | Reduced drug uptake by T. brucei due to mutations in transporter gene TbAT1. In T. congolense, reduced mitochondrial membrane potential (MMP). | Increase in body temperature (in sheep), decrease in body temperature (in cow): respiratory and heart rates increased (in sheep and horses), and decreased respiratory rates (in cows). Widespread resistance in multiple regions. | [5,197,219] |
| Isometamidium chloride | 1958 | Cattle, goats | Trypanosoma spp. (vivax, evansi, congolense) | Accumulates in mitochondria, disrupts kinetoplast. | Impaired drug uptake, reduced MMP and altered drug targets (kDNA). | Resistance is widespread in West Africa. | [26,220,221,222] |
| Homidium bromide/chloride | 1950s | Equids | Widely used in the treatment of AAT caused by Trypanosoma spp. Including T. vivax, T. evansi, T. congolense. Low efficacy in T. b. bucei infection. | It disrupts the kinetoplast DNA (kDNA) network by intercalating parasite DNA, disrupting its structure and replication, leading to the loss of kDNA, and at high doses, inhibits nuclear DNA replication. | Mutations in genes encoding for drug transporters, resulting in reduced drug uptake, or alterations in drug targets. | Widespread drug resistance issues. The drug exists in powder form. It is highly carcinogenic. Administration is via IM; it causes toxic effects in horses. | [223,224] |
| Suramin sodium | 1920s | Horses, camels, dogs | Trypanosoma spp. (brucei, evansi) | Linked to inhibition of energy metabolism by inhibiting enzymes involved in glycolysis, particularly in the glycosomes. Effects on the mitochondrial membrane potential. | Multiple, including loss of function of the suramin receptor (ISG75), endosomal proteins, lysosomal proteases (Cathepsin L), and lysosome-based major facilitator superfamily (MFST). | Rarely studied in treated animals and underreported. | [203,225,226] |
| Quinapyramine sulfate | 1950s | Horses, camels, cattle | Trypanosoma spp. (evansi, vivax, congolense, brucei, equiperdum, simiae) | Interferes with parasite mitochondrial function, inhibits DNA and protein synthesis–potentially by displacing magnesium ions and polyamines from ribosomes. Causes morphological changes in the parasites–increased vacuolation of lysosomes and mitochondrial swelling. | Uncertain. | In equines, it causes salivation, diarrhoea, trembling, sometimes collapse in sensitive animals within minutes of treatment It is not frequently used in livestock, at least in Africa, because quinapyramine-resistant T. congolense displays cross-resistance to diminazene, homidium, and isometamidium. | [5,224,227,228,229] |
| Melarsomine dihydrochloride | 1990s | Camels | T. evansi | Precise mechanism unknown but linked to binding thiol-containing enzymes and trypanothione; alters the parasite redox balance, and causes cellular damage, leading to parasite death. | Mutations in the adenine–adenosine transporter (P2/TbAT1), reduced uptake of the drug due to alterations in other membrane transport proteins, and increased expression of efflux pumps. | Clinical relapses can occur months to 1 or 2 years after treatment, more common with shorter duration of treatment (i.e., less than 4 weeks). | [224,230,231] |
| Meglumine antimoniate (N-methylglucamine antimoniate) | 1996 | Dogs | L. infantum | The drug reduces to Sb(III), and disrupts redox balance by disrupting glutathione (GSH) (trypanothione). | Reduced drug uptake via decreased Aquaglyceroporin-1 Expression. Increased detoxification via increase in the production of thiols, like glutathione, and enzymes involved in thiol metabolism, which can directly reduce Sb(V) to Sb(III), making it less toxic to the parasite. | Can cause pain and inflammation at the site of injection, potentially nephrotoxicosis, and rarely pancreatitis. Emerging resistance in endemic areas. Relapse or recrudescence can occur after 6–12 months of treatment. | [59,60,232] |
| Allopurinol | 1980s | Dogs | L. infantum | Allopurinol is metabolised by the parasite into a toxic compound (4-amino-pyrazole-pyrimidine riboside monophosphate), which is incorporated into RNA, disrupting its structure and function. | Variations in gene copy numbers, including reduction in the S-adenosylmethionine synthetase (METK) gene. | Can cause nephrolithiasis, xanthine crystalluria, and urolithiasis. Recrudescence and relapse can occur after 4–6 months of treatment. Reduced efficacy due to chronic use. | [63,218,233,234] |
| Miltefosine | 2000s | Dogs | L. infantum | Alters membrane lipid metabolism, induces apoptosis-like cell death, disrupts calcium homeostasis and inhibits cytochrome c oxidase. | Mutations in the miltefosine transporter (MT), a complex encoded by the MT and its regulatory subunit ROS3 genes. | Causes vomiting, diarrhoea, and dysorexia. Recrudescence and relapse are frequent—can occur after 4–6 months. Widespread resistance in Latin America and Mediterranean. Unable to fully clear the parasite from infected dogs. | [235,236] |
| Paromomycin (Aminosidine) | 2006 | Dogs | L. infantum | Inhibitor of bacterial protein synthesis through irreversible binding to the 30S ribosomal subunit in mitochondria. | Associated with changes in membrane fluidity and increased expression of ABC transporters, which facilitate drug efflux. | Can cause ototoxicosis and nephrotoxicosis. Recrudescence or relapse can occur after 3–4 months. | [58,80,237] |
| Pentamidine | 1980s | Dogs | L. infantum | Precise mode of action remains to be elucidated but acts on mitochondrial targets including the kinetoplast and affects the mitochondrial membrane potential. | Unclear, but likely due to reduced drug uptake and increased drug efflux. | Severe adverse effects: pain and necrosis at the injection site, diarrhoea, vomiting, systemic hypotension, hypersalivation, and anaphylactic shock | [235,238] |
| Marbofloxacin | 2000s | Dogs | L. infantum | Unknown, but like other quinolones, it may selectively inhibit the enzyme DNA gyrase (or topoisomerase IV). | Mutations in the parasite’s DNA gyrase or topoisomerase II, the enzyme targeted by the drug. | Vomiting, decrease in appetite. | [239,240,241] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebiloma, G.U.; Alhejeli, A.; de Koning, H.P. Interventions for Neglected Diseases Caused by Kinetoplastid Parasites: A One Health Approach to Drug Discovery, Development, and Deployment. Pharmaceuticals 2025, 18, 1415. https://doi.org/10.3390/ph18091415
Ebiloma GU, Alhejeli A, de Koning HP. Interventions for Neglected Diseases Caused by Kinetoplastid Parasites: A One Health Approach to Drug Discovery, Development, and Deployment. Pharmaceuticals. 2025; 18(9):1415. https://doi.org/10.3390/ph18091415
Chicago/Turabian StyleEbiloma, Godwin U., Amani Alhejeli, and Harry P. de Koning. 2025. "Interventions for Neglected Diseases Caused by Kinetoplastid Parasites: A One Health Approach to Drug Discovery, Development, and Deployment" Pharmaceuticals 18, no. 9: 1415. https://doi.org/10.3390/ph18091415
APA StyleEbiloma, G. U., Alhejeli, A., & de Koning, H. P. (2025). Interventions for Neglected Diseases Caused by Kinetoplastid Parasites: A One Health Approach to Drug Discovery, Development, and Deployment. Pharmaceuticals, 18(9), 1415. https://doi.org/10.3390/ph18091415







