Effect of Combination Therapy with Probiotic Bulgarian Goat Yoghurt Enriched with Aronia melanocarpa Fruit Juice in Patients with Type 2 Diabetes Mellitus and Complication of Diabetic Nephropathy: A Pilot Study
Abstract
1. Introduction
2. Results
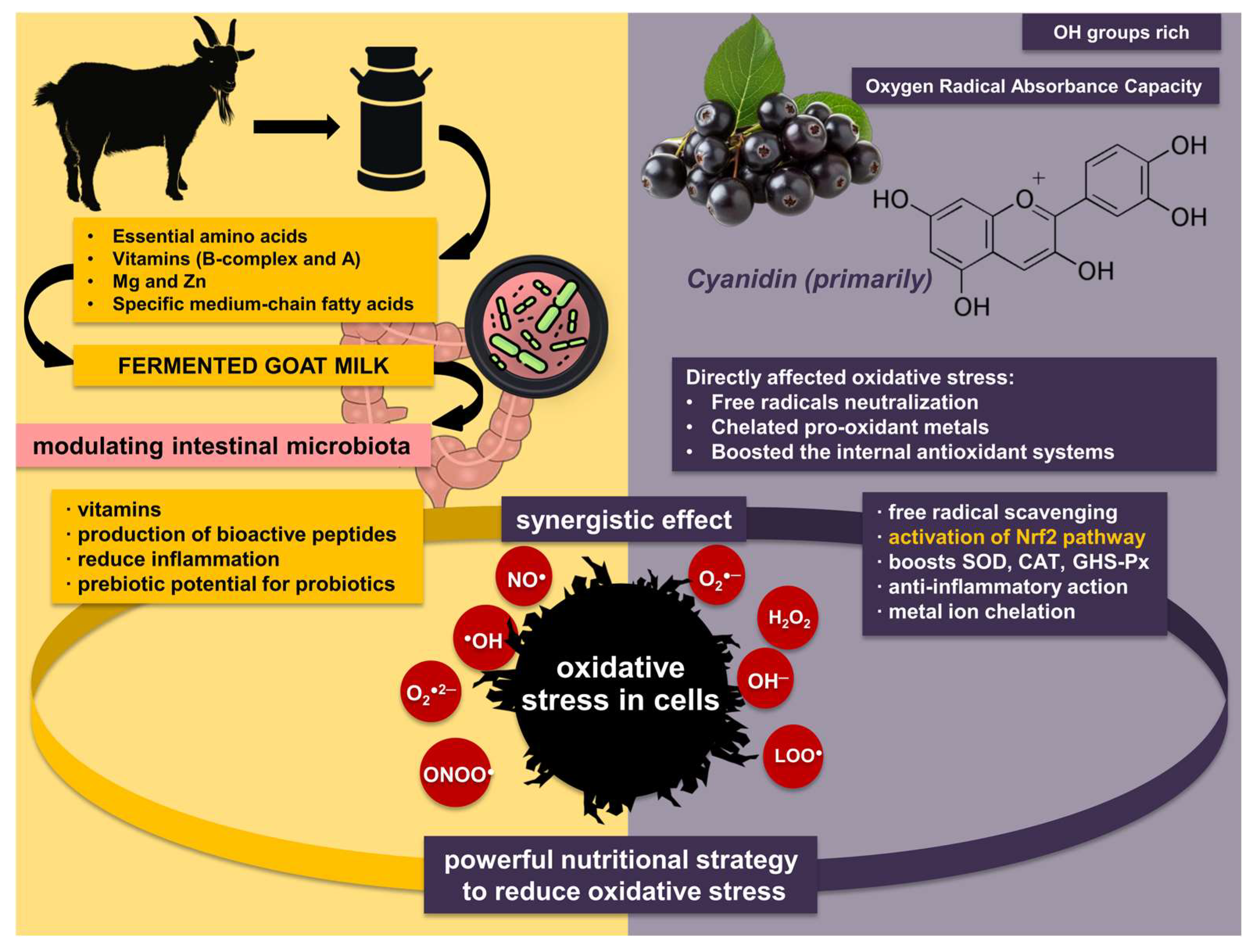
2.1. Goat Yoghurt with Aronia
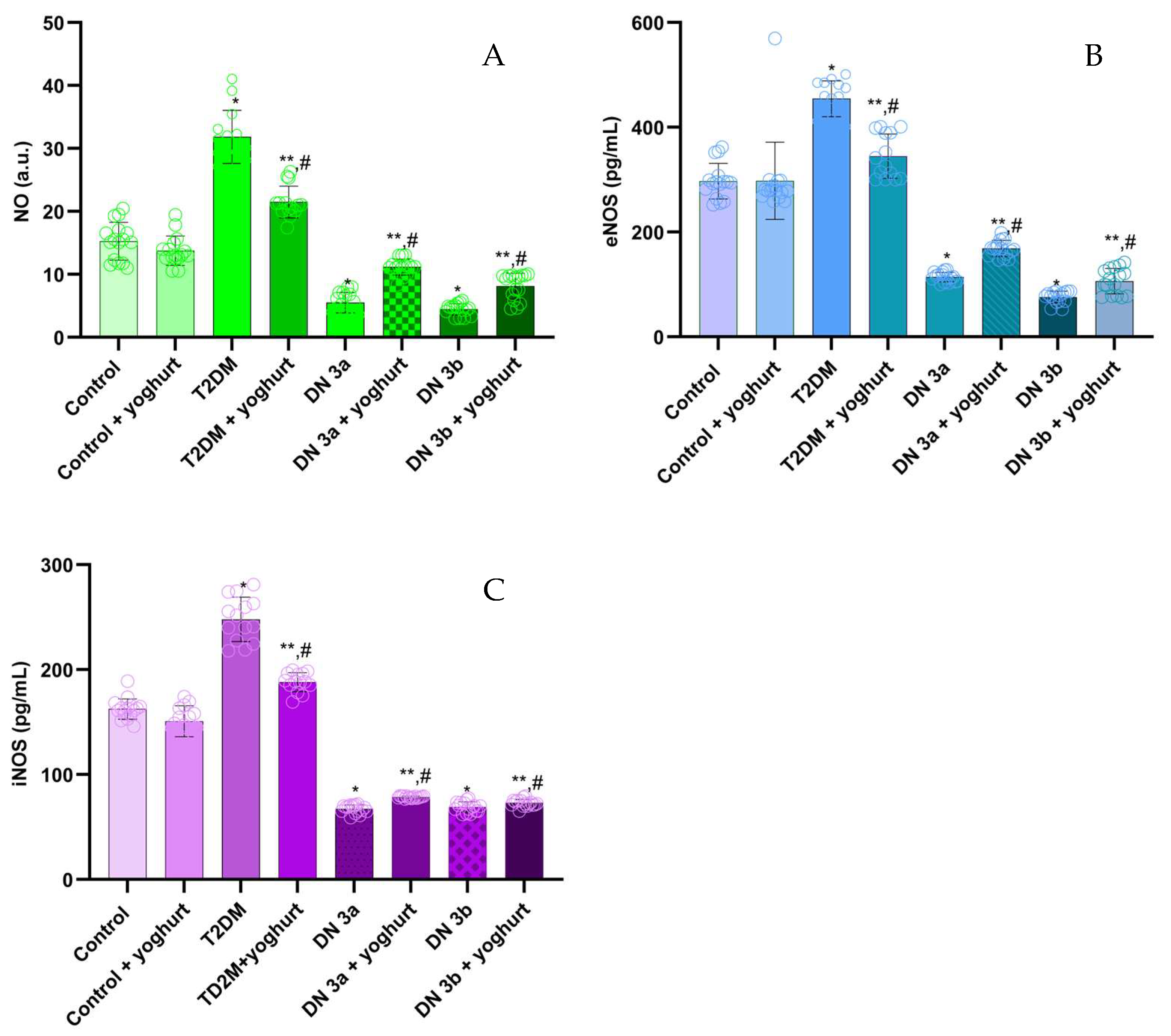
2.2. NO, eNOS and iNOS Assay
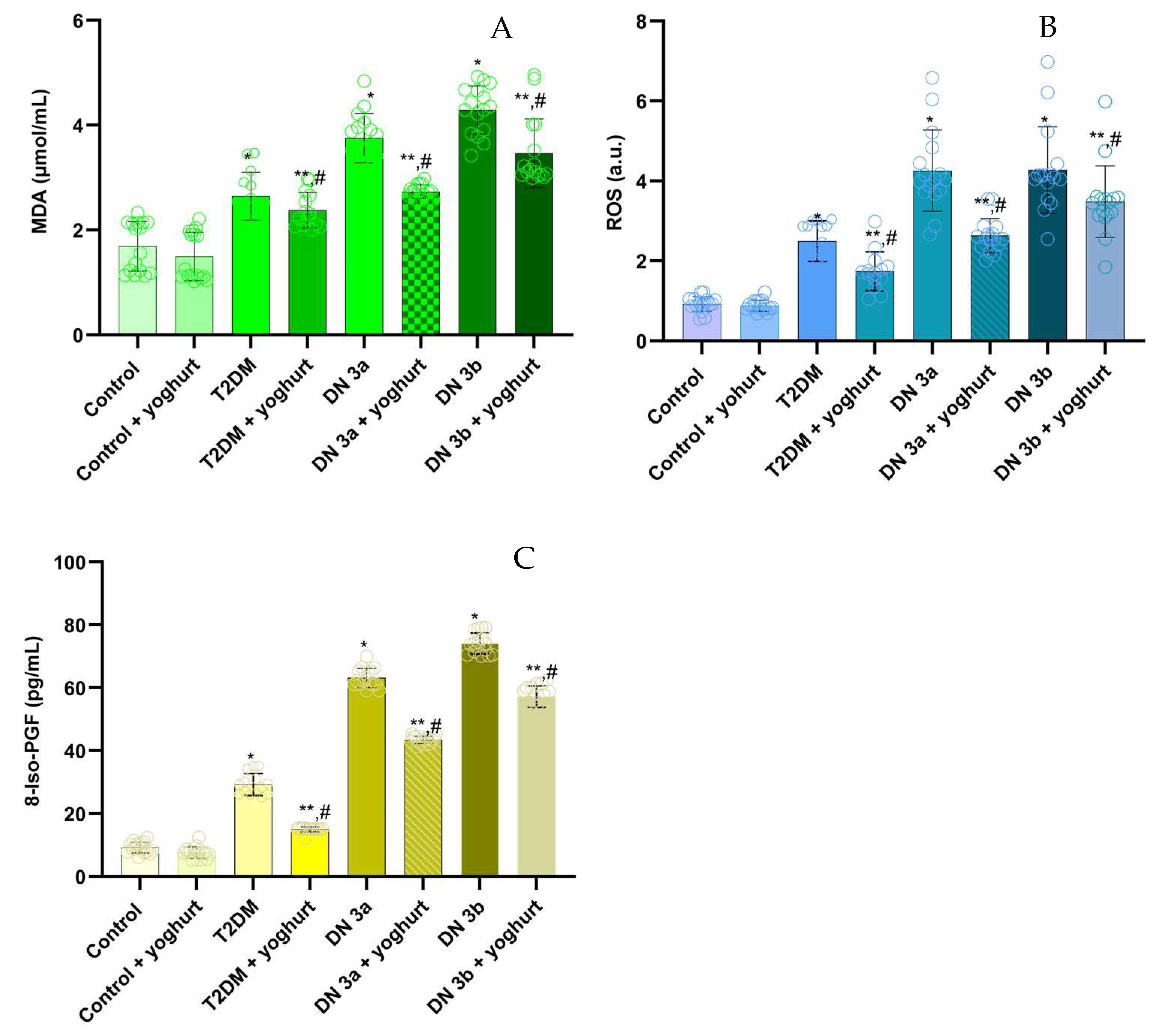
2.3. Measuring the Levels of Malondialdehyde (MDA), ROS, and 8-Iso-Prostaglandins (8-IsoPr)
2.4. Levels of Protein Modification
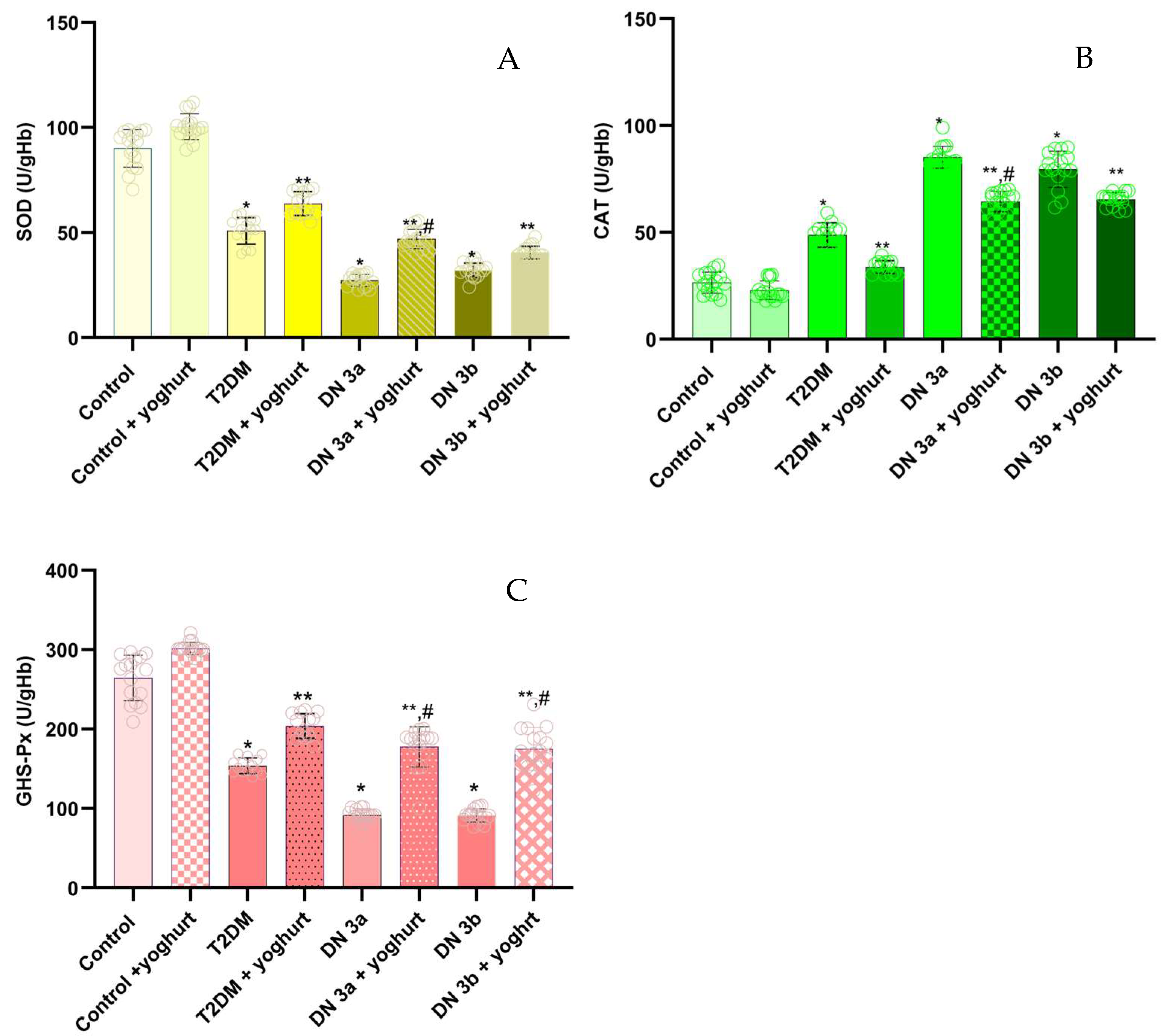
2.5. Antioxidant Enzymes
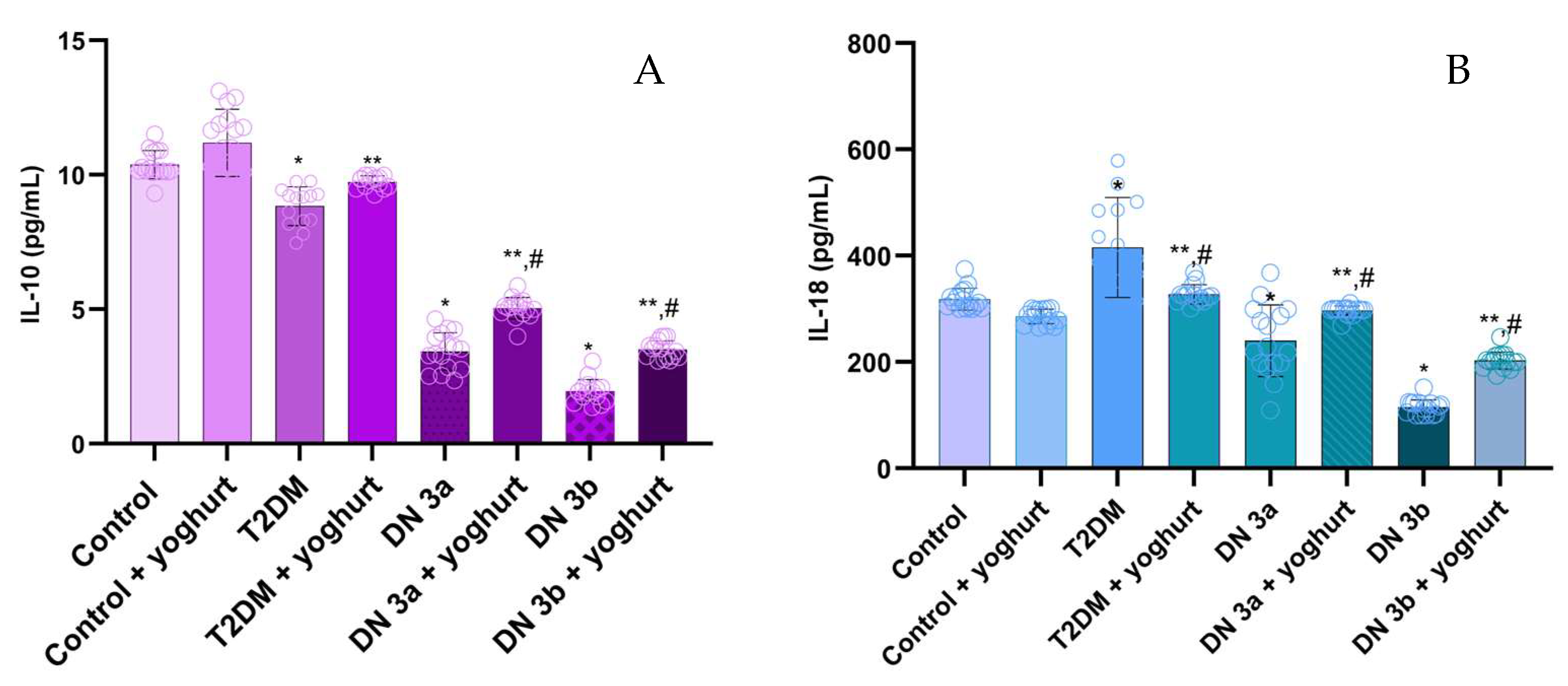
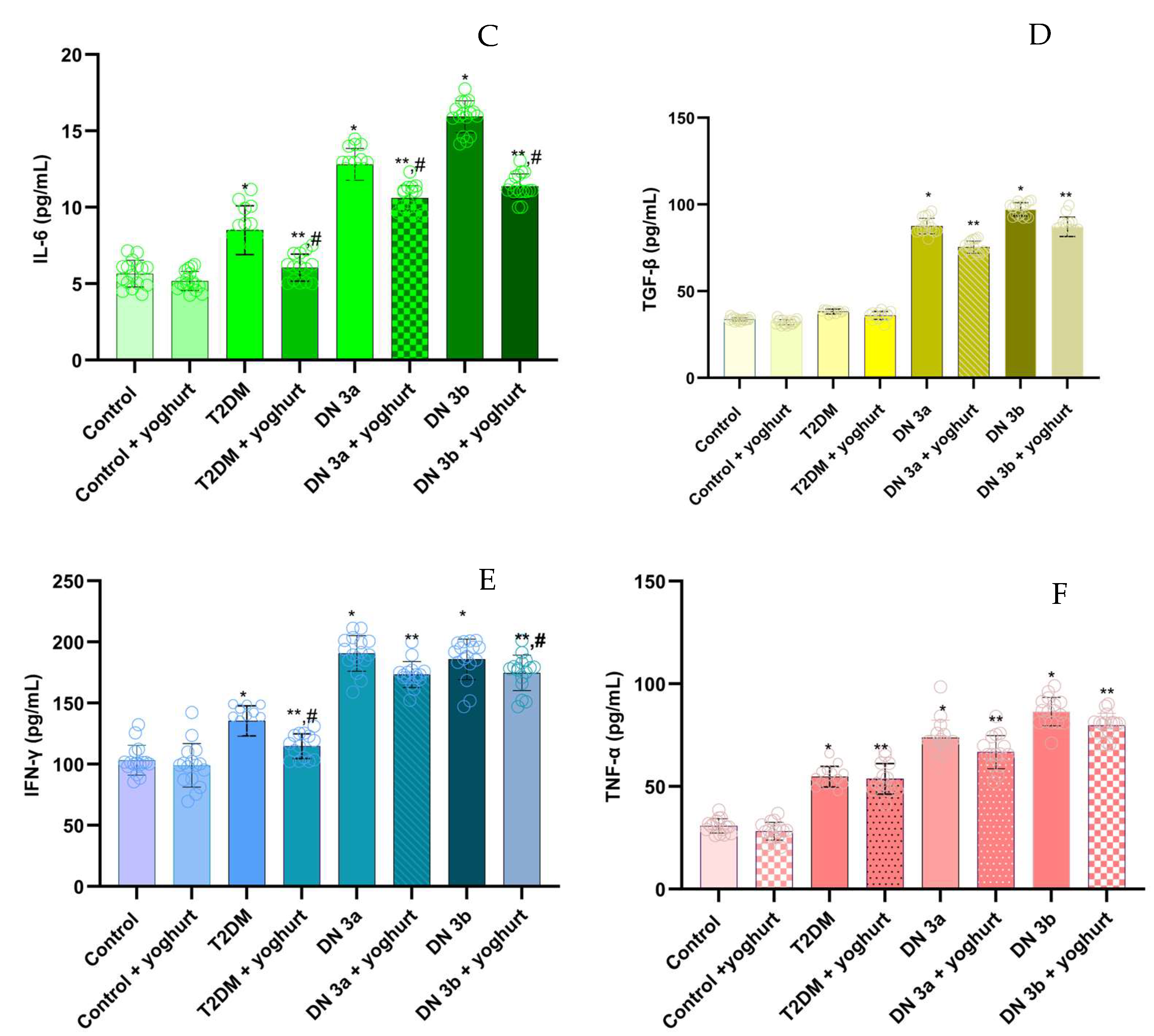
2.6. Pro-Inflammatory Cytokine Levels
3. Discussion
4. Materials and Methods
4.1. Source of Raw Materials
4.2. Yoghurt Preparation Protocol
4.3. Chemicals
4.4. Subjects and Study Design
4.5. Electron Paramagnetic Resonance (EPR) Spectroscopy
4.5.1. Evaluation the ROS Products Levels
4.5.2. Evaluation of NO Levels
4.5.3. Protein Oxidation with 3-Maleimido Proxyl (5-MSL)
4.6. Enzyme-Linked Immunosorbent Assay
4.7. Limitations of the Study
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ALKaisy, Q.H.; Al-Saadi, J.S.; Al-Rikabi, A.J.; Altemimi, A.B.; Hesarinejad, M.A.; Abedelmaksoud, T.G. Exploring the health benefits and functional properties of goat milk proteins. Food Sci. Nutr. 2023, 11, 5641–5656. [Google Scholar] [CrossRef]
- Lazarova, M. Mineral Composition of Yoghurt from Goat Milk Supplemented with Aronia Fruit Juice. J. Mt. Agric. Balk. (JMAB) 2024, 1, 27. [Google Scholar]
- Boukrouh, S.; Noutfia, A.; Moula, N.; Avril, C.; Hornick, J.-L.; Chentouf, M.; Cabaraux, J.-F. Effects of Sulla Flexuosa Hay as Alternative Feed Resource on Goat’s Milk Production and Quality. Animals 2023, 13, 709. [Google Scholar] [CrossRef]
- Sanjulián, L.; Fernández-Rico, S.; González-Rodríguez, N.; Cepeda, A.; Miranda, J.M.; Fente, C.; Lamas, A.; Regal, P. The Role of Dairy in Human Nutrition: Myths and Realities. Nutrients 2025, 17, 646. [Google Scholar] [CrossRef]
- Ratan, Y.; Rajput, A.; Pareek, A.; Pareek, A.; Singh, G. Comprehending the Role of Metabolic and Hemodynamic Factors Alongside Different Signaling Pathways in the Pathogenesis of Diabetic Nephropathy. Int. J. Mol. Sci. 2025, 26, 3330. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2016, 1, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Qasim, N.; Arif, A.; Mahmood, R. Hyperglycemia enhances the generation of ROS and RNS that impair antioxidant power and cause oxidative damage in human erythrocytes. Biochem. Cell Biol. 2022, 101, 64–76. [Google Scholar] [CrossRef]
- Bashan, N.; Kovsan, J.; Kachko, I.; Ovadia, H.; Rudich, A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol. Rev. 2009, 89, 27–71. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Darenskaya, M.; Kolesnikov, S.; Semenova, N.; Kolesnikova, L. Diabetic Nephropathy: Significance of Determining Oxidative Stress and Opportunities for Antioxidant Therapies. Int. J. Mol. Sci. 2023, 24, 12378. [Google Scholar] [CrossRef]
- Lindblom, R.; Higgins, G.; Coughlan, M.; de Haan, J.B. Targeting Mitochondria and Reactive Oxygen Species-Driven Pathogenesis in Diabetic Nephropathy. Rev. Diabet. Stud. 2015, 12, 134–156. [Google Scholar] [CrossRef] [PubMed]
- Boukria, O.; El Hadrami, E.M.; Sameen, A.; Sahar, A.; Khan, S.; Safarov, J.; Sultanova, S.; Leriche, F.; Aït-Kaddour, A. Biochemical, Physicochemical and Sensory Properties of Yoghurts Made from Mixing Milks of Different Mammalian Species. Foods 2020, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Paszczyk, B.; Czarnowska-Kujawska, M.; Klepacka, J.; Tońska, E. Health-Promoting Ingredients in Goat’s Milk and Fermented Goat’s Milk Drinks. Animals 2023, 13, 907. [Google Scholar] [CrossRef]
- Marquez, A.; Russo, M.; Tomei, C.; Castellano, P.; Puglisi, E.; Medina, R.; Gauffin-Cano, P. Effects of Fermented Goat Milk on Adiposity and Gut Microbiota in a Diet-Induced Obesity Murine Model. Fermentation 2024, 10, 155. [Google Scholar] [CrossRef]
- Künili, İ.E.; Akdeniz, V.; Akpınar, A.; Budak, Ş.Ö.; Curiel, J.A.; Guzel, M.; Karagözlü, C.; Berkel Kasikci, M.; Caruana, G.P.M.; Starowicz, M.; et al. Bioactive compounds in fermented foods: A systematic narrative review. Front. Nutr. 2025, 12, 1625816. [Google Scholar] [CrossRef]
- Mirzaei, H.; Sharafati Chaleshtori, R. Role of fermented goat milk as a nutritional product to improve anemia. J. Food Biochem. 2022, 46, e13969. [Google Scholar] [CrossRef]
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.; Díaz-Castro, J.; López-Aliaga, I. New perspectives in fermented dairy products and their health relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Hasheminasabgorji, E.; Jha, J.C. Dyslipidemia, Diabetes and Atherosclerosis: Role of Inflammation and ROS-Redox-Sensitive Factors. Biomedicines 2021, 9, 1602. [Google Scholar] [CrossRef]
- Ganesh, G.V.; Ramkumar, K. MDysregulation of Nrf2 redox pathway in macrophages under diabetic microenvironment. Exp. Gerontol. 2021, 152, 111479. [Google Scholar] [CrossRef]
- Konno, T.; Melo, E.P.; Chambers, J.E.; Avezov, E. Intracellular Sources of ROS/H2O2 in Health and Neurodegeneration: Spotlight on Endoplasmic Reticulum. Cells 2021, 10, 233. [Google Scholar] [CrossRef]
- Goycheva, P.; Petkova-Parlapanska, K.; Georgieva, E.; Karamalakova, Y.; Nikolova, G. Biomarkers of Oxidative Stress in Diabetes Mellitus with Diabetic Nephropathy Complications. Int. J. Mol. Sci. 2023, 24, 13541. [Google Scholar] [CrossRef]
- Alicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Takahashi, T.; Harris, R.C. Role of endothelial nitric oxide synthase in diabetic nephropathy: Lessons from diabetic eNOS knockout mice. J. Diabetes Res. 2014, 2014, 590541. [Google Scholar] [CrossRef] [PubMed]
- Młynarska, E.; Buławska, D.; Czarnik, W.; Hajdys, J.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Novel Insights into Diabetic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 10222. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef] [PubMed]
- Dellamea, B.S.; Leitão, C.B.; Friedman, R.; Canani, L.H. Nitric oxide system and diabetic nephropathy. Diabetol. Metab. Syndr. 2014, 6, 17. [Google Scholar] [CrossRef]
- Ahmad, M.; Wolberg, A.; Kahwaji, C.I. Biochemistry, Electron Transport Chain; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Milutinović, M.; Radovanović, R.V.; Šavikin, K.; Radenković, S.; Arvandi, M.; Pešić, M.; Kostić, M.; Miladinović, B.; Branković, S.; Kitić, D. Chokeberry juice supplementation in type 2 diabetic patients-impact on health status. J. Appl. Biomed. 2019, 17, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, S.B.; Botushanov, N.P.; Karahanian, E.B.; Pavlova, M.B.; Husianitis, H.K.; Troev, D.M. Effects of Aronia melanocarpa juice as part of the dietary regimen in patients with diabetes mellitus. Folia Medica 2002, 44, 20–23. [Google Scholar]
- Yamane, T.; Kozuka, M.; Wada-Yoneta, M.; Sakamoto, T.; Nakagaki, T.; Nakano, Y.; Ohkubo, I. Aronia juice suppresses the elevation of postprandial blood glucose levels in adult healthy Japanese. Clin. Nutr. Exp. 2017, 12, 20–26. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Ríos, L.; Guerrero-Hue, M.; García-Caballero, C.; Vázquez-Carballo, C.; Mas, S.; Sanz, A.B.; Herencia, C.; Mezzano, S.; et al. Pathogenic Pathways and Therapeutic Approaches Targeting Inflammation in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 3798. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Martínez-Orgado, J.; Martínez-Vega, M.; Silva, L.; Romero, A.; de Hoz-Rivera, M.; Villa, M.; Del Pozo, A. Protein Carbonylation as a Biomarker of Oxidative Stress and a Therapeutic Target in Neonatal Brain Damage. Antioxidants 2023, 12, 1839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Tu, C.; Chen, X.; He, R. Advanced Glycation End Products in Disease Development and Potential Interventions. Antioxidants 2025, 14, 492. [Google Scholar] [CrossRef]
- Lima, M.J.R.; Teixeira-Lemos, E.; Oliveira, J.; Teixeira-Lemos, L.P.; Monteiro, A.M.; Costa, J.M. Nutritional and health profile of goat products: Focus on health benefits of goat milk. In Goat Science; IntechOpen: London, UK, 2018; pp. 189–232. [Google Scholar] [CrossRef]
- Shi, H.; Sui, Y.; Wang, X.; Luo, Y.; Ji, L. Hydroxyl radical production and oxidative damage induced by cadmium and naphthalene in liver of Carassius auratus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 115–121. [Google Scholar] [CrossRef]
- Yokoyama, K.; Hashiba, K.; Wakabayashi, H.; Hashimoto, K.; Satoh, K.; Kurihara, T.; Sakagami, H. Inhibition of LPS-stimulated NO production in mouse macrophage-like cells by tropolones. Anticancer Res. 2004, 24, 3917. [Google Scholar]
- Yoshioka, T.; Iwamoto, N.; Ito, K. An application of electron paramagnetic resonance to evaluate nitric oxide and its quenchers. J. Am. Soc. Nephrol. 1996, 7, 961. [Google Scholar] [CrossRef]
- Petkova-Parlapanska, K.; Stefanov, I.; Ananiev, J.; Georgiev, T.; Hadzhibozheva, P.; Petrova-Tacheva, V.; Kaloyanov, N.; Georgieva, E.; Nikolova, G.; Karamalakova, Y. Sambucus nigra-Lyophilized Fruit Extract Attenuated Acute Redox-Homeostatic Imbalance via Mutagenic and Oxidative Stress Modulation in Mice Model on Gentamicin-Induced Nephrotoxicity. Pharmaceuticals 2025, 18, 85. [Google Scholar]

| Variables | Controls n = 42 | T2DM Normal Renal Function (n = 24) Stage 1 | DN Mild to Moderate Loss of Renal Failure (n = 47); Stage 3a | DN Moderate to Severe Renal Failure (n = 51) Stage 3b |
|---|---|---|---|---|
| Age | 41.25 ± 7.78 | 51.27 ± 6.07 | 54.41 ± 5.74 | 56.68 ± 8.91 |
| Sex (M/F) | 14 M/24 F | 10 M/11 F | 10 M/13 F | 14 M/10 F |
| Disease duration | - | 10.7 ± 7.3 | 12.7 ± 8.6 | 14.8 ± 6.1 |
| BMI (kg/m2) mean ± SD | 26.07 ± 3.52 | 32.11 ± 5.44 | 33.56 ± 6.21 | 34.76 ± 6.14 |
| Blood shugar mmol/L, mean ± SD | 4.98 ± 0.32 | 8.05 ± 5.11 | 9.55 ± 4.89 | 9.82 ± 5.41 |
| HbA1C% | 5.69 ± 0.44 | 6.37 ± 0.85 | 9.87 ± 1.27 | 9.99 ± 1.98 |
| GFR mL/min mL/min/1.73 m2 | 110.08 ± 9.32 | 95.66 ± 5.60 | 49.83 ± 2.57 | 36.95 ±4.50 |
| UAE mg/day | 1.20 ± 0.35 | 21.80 ± 1.78 | 213.55 ± 6.85 | 648.73 ± 8.92 |
| FBC, mmol/L | 5.31 ± 0.28 | 6.43 ± 0.89 | 12.72 ± 5.15 | 5.47± 0.6 |
| Cholesterol mmol/L | 4.17 ± 0.3 | 5.01 ± 0.7 | 5.47 ± 0.6 | 5.48 ± 0.7 |
| Triglycerides mmol/L | 1.31 ± 0.15 | 2.13 ± 0.2 | 2.65 ± 0.2 | 3.01 |
| HDL, mmol/L | 0.91 ± 0.019 | 1.19 ± 0.04 | 1.32 ± 0.05 | 1.33 ± 0.01 |
| LDL mmol/L | 2.03 ± 0.11 | 2.72 ± 0.24 | 2.96 ± 0.2 | 3.2 ± 0.5 |
| Variables | Controls n = 42 | T2DM Normal Renal Function (n = 24) Stage 1 | DN Mild to Moderate Loss of Renal Failure (n = 47); Stage 3a | DN Moderate to Severe Renal Failure (n = 51) Stage 3b |
|---|---|---|---|---|
| Age | 41.25 ± 7.78 | 51.27 ± 6.07 | 54.41 ± 5.74 | 56.68 ± 8.91 |
| Sex (M/F) | 14 M/24 F | 10 M/11 F | 10 M/13 F | 14 M/10 F |
| Disease duration | - | 10.7 ± 7.3 | 12.7 ± 8.6 | 14.8 ± 6.1 |
| BMI (kg/m2) mean ± SD | 25.69 ± 3.01 | 31.88 ± 4.04 | 32.98 ± 5.88 | 33.72 ± 5.55 |
| Blood shugar mmol/L, mean ± SD | 4.94 ± 0.24 | 8.05 ± 5.11 | 9.55 ± 4.89 | 9.82 ± 5.41 |
| HbA1C% | 5.50 ± 0.12 | 6.12 ± 0.44 | 9.15 ± 1.04 | 9.01 ± 0.97 |
| GFR mL/min mL/min/1.73 m2 | 110.08 ± 9.32 | 97.74 ± 7.01 | 52.55 ± 4.74 | 38.98 ± 5.32 |
| UAE mg/day | 1.24 ± 0.47 | 18.35 ± 0.98 | 198.12 ± 8.74 | 601.28 ± 7.69 |
| FBC, mmol/L | 5.24 ± 0.31 | 6.07 ± 0.58 | 11.64 ± 4.79 | 5.69 ± 0.7 |
| Cholesterol mmol/L | 4.24 ± 0.4 | 5.11 ± 0.7 | 5.34 ± 0.7 | 5.32 ± 0.6 |
| Triglycerides mmol/L | 1.32 ± 0.17 | 2.12 ± 0.2 | 2.7 ± 0.2 | 2.95± 0.2 |
| HDL, mmol/L | 0.91 ± 0.019 | 1.19 ± 0.04 | 1.32 ± 0.05 | 1.33 ± 0.01 |
| LDL mmol/L | 2.03 ± 0.11 | 2.72 ± 0.24 | 2.96 ± 0.2 | 3.2 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goycheva, P.; Petkova-Parlapanska, K.; Georgieva, E.; Zheleva, N.; Lazarova, M.; Karamalakova, Y.; Nikolova, G. Effect of Combination Therapy with Probiotic Bulgarian Goat Yoghurt Enriched with Aronia melanocarpa Fruit Juice in Patients with Type 2 Diabetes Mellitus and Complication of Diabetic Nephropathy: A Pilot Study. Pharmaceuticals 2025, 18, 1409. https://doi.org/10.3390/ph18091409
Goycheva P, Petkova-Parlapanska K, Georgieva E, Zheleva N, Lazarova M, Karamalakova Y, Nikolova G. Effect of Combination Therapy with Probiotic Bulgarian Goat Yoghurt Enriched with Aronia melanocarpa Fruit Juice in Patients with Type 2 Diabetes Mellitus and Complication of Diabetic Nephropathy: A Pilot Study. Pharmaceuticals. 2025; 18(9):1409. https://doi.org/10.3390/ph18091409
Chicago/Turabian StyleGoycheva, Petya, Kamelia Petkova-Parlapanska, Ekaterina Georgieva, Nikolina Zheleva, Mariya Lazarova, Yanka Karamalakova, and Galina Nikolova. 2025. "Effect of Combination Therapy with Probiotic Bulgarian Goat Yoghurt Enriched with Aronia melanocarpa Fruit Juice in Patients with Type 2 Diabetes Mellitus and Complication of Diabetic Nephropathy: A Pilot Study" Pharmaceuticals 18, no. 9: 1409. https://doi.org/10.3390/ph18091409
APA StyleGoycheva, P., Petkova-Parlapanska, K., Georgieva, E., Zheleva, N., Lazarova, M., Karamalakova, Y., & Nikolova, G. (2025). Effect of Combination Therapy with Probiotic Bulgarian Goat Yoghurt Enriched with Aronia melanocarpa Fruit Juice in Patients with Type 2 Diabetes Mellitus and Complication of Diabetic Nephropathy: A Pilot Study. Pharmaceuticals, 18(9), 1409. https://doi.org/10.3390/ph18091409









