Immunoregulation by ESAT-6: From Pathogenesis of Tuberculosis to Potential Anti-Inflammatory and Anti-Rejection Application
Abstract
1. Introduction
2. ESAT-6 Is a Virulence Determinant for Mtb
3. ESAT-6 Plays an Immunoregulatory Role
3.1. Effects of ESAT-6 on Innate Immunity
3.1.1. Macrophages
3.1.2. Dendritic Cells (DCs)
3.1.3. Neutrophils
4. Effects of ESAT-6 on Adaptive Immunity: T Cells
5. Anti-Inflammatory Effects of ESAT-6 and Its Potential to Suppress Allograft Rejection
6. Conclusions and Future Directions Beyond Tuberculosis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESAT-6 | early secreted antigenic target of 6 kDa |
| COX-2 | cyclooxygenase-2 |
| DC | dendritic cell |
| BMDM | bone marrow-derived macrophage |
| MCP-1 | monocyte chemoattractant protein-1 |
| Mtb | mycobacterium tuberculosis |
| NO | nitric oxide |
| ROS | reactive oxygen species |
References
- Harboe, M.; Oettinger, T.; Wiker, H.G.; Rosenkrands, I.; Andersen, P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 1996, 64, 16–22. [Google Scholar] [CrossRef]
- Mahairas, G.G.; Sabo, P.J.; Hickey, M.J.; Singh, D.C.; Stover, C.K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M-bovis. J. Bacteriol. 1996, 178, 1274–1282. [Google Scholar] [CrossRef]
- Behr, M.A.; Wilson, M.A.; Gill, W.P.; Salamon, H.; Schoolnik, G.K.; Rane, S.; Small, P.M. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 1999, 284, 1520–1523. [Google Scholar] [CrossRef]
- Brodin, P.; Eiglmeier, K.; Marmiesse, M.; Billault, A.; Garnier, T.; Niemann, S.; Cole, S.T.; Brosch, R. Bacterial artificial chromosome-based comparative genomic analysis identifies Mycobacterium microti as a natural ESAT-6 deletion mutant. Infect. Immun. 2002, 70, 5568–5578. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Xie, J.P. Roles and underlying mechanisms of ESAT-6 in the context of Mycobacterium tuberculosis-host interaction from a systems biology perspective. Cell. Signal. 2012, 24, 1841–1846. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Gey Van Pittius, N.C.; Champion, P.A.D.; Cox, J.; Luirink, J.; Vandenbroucke-Grauls, C.M.J.E.; Appelmelk, B.J.; Bitter, W. Type VII secretion—Mycobacteria show the way. Nat. Rev. Microbiol. 2007, 5, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Ghosh, T.S.; Mande, S.S. Computational Analysis of the ESX-1 Region of Mycobacterium tuberculosis: Insights into the Mechanism of Type VII Secretion System. PLoS ONE 2011, 6, e27980. [Google Scholar] [CrossRef] [PubMed]
- Teutschbein, J.; Schumann, G.; Möllmann, U.; Grabley, S.; Cole, S.T.; Munder, T. A protein linkage map of the ESAT-6 secretion system 1 (ESX-1) of Mycobacterium tuberculosis. Microbiol. Res. 2009, 164, 253–259. [Google Scholar] [CrossRef]
- Lewis, K.N.; Liao, R.L.; Guinn, K.M.; Hickey, M.J.; Smith, S.; Behr, M.A.; Sherman, D.R. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. J. Infect. Dis. 2003, 187, 117–123. [Google Scholar] [CrossRef]
- Pym, A.S.; Brodin, P.; Brosch, R.; Huerre, M.; Cole, S.T. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 2002, 46, 709–717. [Google Scholar] [CrossRef]
- Pym, A.S.; Brodin, P.; Majlessi, L.; Brosch, R.; Demangel, C.; Williams, A.; Griffiths, K.E.; Marchal, G.; Leclerc, C.; Cole, S.T. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 2003, 9, 533–539. [Google Scholar] [CrossRef]
- Hsu, T.; Hingley-Wilson, S.M.; Chen, B.; Chen, M.; Dai, A.Z.; Morin, P.M.; Marks, C.B.; Padiyar, J.; Goulding, C.; Gingery, M.; et al. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. USA 2003, 100, 12420–12425. [Google Scholar] [CrossRef]
- Guinn, K.M.; Hickey, M.J.; Mathur, S.K.; Zakel, K.L.; Grotzke, J.E.; Lewinsohn, D.M.; Smith, S.; Sherman, D.R. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2004, 51, 359–370. [Google Scholar] [CrossRef]
- Wong, K.W. The Role of ESX-1 in Mycobacterium tuberculosis Pathogenesis. Microbiol. Spectr. 2017, 5, 3. [Google Scholar] [CrossRef]
- Peng, X.; Sun, J. Mechanism of ESAT-6 membrane interaction and its roles in pathogenesis of Mycobacterium tuberculosis. Toxicon 2016, 116, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Bo, H.; Moure, U.A.E.; Yang, Y.; Pan, J.; Li, L.; Wang, M.; Ke, X.; Cui, H. Mycobacterium tuberculosis-macrophage interaction: Molecular updates. Front. Cell. Infect. Microbiol. 2023, 13, 1062963. [Google Scholar] [CrossRef] [PubMed]
- Passos, B.B.S.; Araujo-Pereira, M.; Vinhaes, C.L.; Amaral, E.P.; Andrade, B.B. The role of ESAT-6 in tuberculosis immunopathology. Front. Immunol. 2024, 15, 1383098. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zeng, Y.; Lin, J.; Liu, H.; Liang, C.L.; Chen, Y.; Qiu, F.; Bromberg, J.S.; Dai, Z. ESAT-6 protein suppresses allograft rejection by inducing CD4(+)Foxp3(+) regulatory T cells through IkappaBalpha/cRel pathway. Front. Immunol. 2024, 15, 1529226. [Google Scholar] [CrossRef]
- van der Wel, N.; Hava, D.; Houben, D.; Fluitsma, D.; van Zon, M.; Pierson, J.; Brenner, M.; Peters, P.J. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 2007, 129, 1287–1298. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.C.; Jiang, G.Z.; Liu, W.; Deng, Q.; Li, X.J.; Qian, W.; Ouellet, H.; Sun, J.J. EsxA membrane-permeabilizing activity plays a key role in mycobacterial cytosolic translocation and virulence: Effects of single-residue mutations at glutamine 5. Sci. Rep. 2016, 6, 32618. [Google Scholar] [CrossRef]
- Bao, Y.Q.; Wang, L.; Sun, J.J. Post-translational knockdown and post-secretional modification of EsxA determine contribution of EsxA membrane permeabilizing activity for mycobacterial intracellular survival. Virulence 2021, 12, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.; Karki, C.B.; Li, L.; Reyes, S.V.; Estevao, I.; Grajeda, B.I.; Zhang, Q.; Arico, C.D.; Ouellet, H.; Sun, J.J. Nα-Acetylation of the virulence factor EsxA is required for mycobacterial cytosolic translocation and virulence. J. Biol. Chem. 2020, 295, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G.; Cardona, P.J.; Kim, M.J.; Allain, S.; Altare, F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 2009, 10, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Jamwal, S.; Jain, R.; Verma, P.; Gokhale, R.; Rao, K.V.S. Mycobacterium tuberculosis-Driven Targeted Recalibration of Macrophage Lipid Homeostasis Promotes the Foamy Phenotype. Cell Host Microbe 2012, 12, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Peyron, P.; Vaubourgeix, J.; Poquet, Y.; Levillain, F.; Botanch, C.; Bardou, F.; Daffé, M.; Emile, J.F.; Marchou, B.; Cardona, P.J.; et al. Foamy Macrophages from Tuberculous Patients’ Granulomas Constitute a Nutrient-Rich Reservoir for M. tuberculosis Persistence. PLoS Pathog. 2008, 4, e1000204. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, C.; Chaudhary, V.K.; Rao, K.V.S.; Chatterjee, S. M. tuberculosis Secretory Protein ESAT-6 Induces Metabolic Flux Perturbations to Drive Foamy Macrophage Differentiation. Sci. Rep. 2015, 5, 12906. [Google Scholar] [CrossRef]
- Refai, A.; Gritli, S.; Barbouche, M.R.; Essafi, M. Mycobacterium tuberculosis Virulent Factor ESAT-6 Drives Macrophage Differentiation Toward the Pro-inflammatory M1 Phenotype and Subsequently Switches It to the Anti-inflammatory M2 Phenotype. Front. Cell. Infect. Microbiol. 2018, 8, 327. [Google Scholar] [CrossRef]
- Ma, J.; Jung, B.G.; Yi, N.; Samten, B. Early Secreted Antigenic Target of 6 kDa of Mycobacterium tuberculosis Stimulates Macrophage Chemoattractant Protein-1 Production by Macrophages and Its Regulation by p38 Mitogen-Activated Protein Kinases and Interleukin-4. Scand. J. Immunol. 2016, 84, 39–48. [Google Scholar] [CrossRef]
- Jung, B.G.; Wang, X.S.; Yi, N.; Ma, J.; Turner, J.; Samten, B. Early Secreted Antigenic Target of 6-kDa of Mycobacterium tuberculosis Stimulates IL-6 Production by Macrophages through Activation of STAT3. Sci. Rep. 2017, 7, 40984. [Google Scholar] [CrossRef]
- Jung, B.G.; Vankayalapati, R.; Samten, B. Mycobacterium tuberculosis stimulates IL-1β production by macrophages in an ESAT-6 dependent manner with the involvement of serum amyloid A3. Mol. Immunol. 2021, 135, 285–293. [Google Scholar] [CrossRef]
- Lin, J.H.; Jiang, Y.Y.; Liu, D.; Dai, X.T.; Wang, M.; Dai, Y.L. Early secreted antigenic target of 6-kDa of Mycobacterium tuberculosis induces transition of macrophages into epithelioid macrophages by downregulating iNOS/NO-mediated H3K27 trimethylation in macrophages. Mol. Immunol. 2020, 117, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Chang, Q.; Dai, X.T.; Liu, D.; Jiang, Y.Y.; Dai, Y.L. Early secreted antigenic target of 6-kDa of Mycobacterium tuberculosis promotes caspase-9/caspase-3-mediated apoptosis in macrophages. Mol. Cell. Biochem. 2019, 457, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Moura-Alves, P.; Sonawane, A.; Hacohen, N.; Griffiths, G.; Moita, L.F.; Anes, E. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell. Microbiol. 2010, 12, 1046–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, F.K.; Luo, J.; Xu, H.; Wang, Y.; Jiang, W.B.; Chang, K.; Deng, S.L.; Chen, M. Early secreted antigenic target 6-kDa from Mycobacterium tuberculosis enhanced the protective innate immunity of macrophages partially via HIF1α. Biochem. Biophys. Res. Commun. 2020, 522, 26–32. [Google Scholar] [CrossRef]
- Wong, K.W.; Jacobs, W.R. Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell. Microbiol. 2011, 13, 1371–1384. [Google Scholar] [CrossRef]
- Sreejit, G.; Ahmed, A.; Parveen, N.; Jha, V.; Valluri, V.L.; Ghosh, S.; Mukhopadhyay, S. The ESAT-6 Protein of Mycobacterium tuberculosis Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage. PLoS Pathog. 2014, 10, e1004446. [Google Scholar] [CrossRef]
- Seghatoleslam, A.; Hemmati, M.; Ebadat, S.; Movahedi, B.; Mostafavi-Pour, Z. Macrophage Immune Response Suppression by Recombinant Mycobacterium tuberculosis Antigens, the ESAT-6, CFP-10, and ESAT-6/CFP-10 Fusion Proteins. Iran. J. Med. Sci. 2016, 41, 296–304. [Google Scholar]
- Sun, F.; Li, J.B.; Cao, L.; Yan, C.Z. Mycobacterium tuberculosis virulence protein ESAT-6 influences M1/M2 polarization and macrophage apoptosis to regulate tuberculosis progression. Genes Genom. 2023, 46, 37–47. [Google Scholar] [CrossRef]
- Behura, A.; Mishra, A.; Chugh, S.; Mawatwal, S.; Kumar, A.; Manna, D.; Mishra, A.; Singh, R.; Dhiman, R. ESAT-6 modulates Calcimycin-induced autophagy through microRNA-30a in mycobacteria infected macrophages. J. Infect. 2019, 79, 139–152. [Google Scholar] [CrossRef]
- Liu, W.W.; Peng, Y.; Yin, Y.L.; Zhou, Z.H.; Zhou, W.D.; Dai, Y.L. The Involvement of NADPH Oxidase-Mediated ROS in Cytokine Secretion from Macrophages Induced by Mycobacterium tuberculosis ESAT-6. Inflammation 2014, 37, 880–892. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Grover, A.; Izzo, A.A. BAT3 Regulates Mycobacterium tuberculosis Protein ESAT-6-Mediated Apoptosis of Macrophages. PLoS ONE 2012, 7, e40836. [Google Scholar] [CrossRef]
- Yang, S.; Li, F.; Jia, S.; Zhang, K.; Jiang, W.; Shang, Y.; Chang, K.; Deng, S.; Chen, M. Early secreted antigen ESAT-6 of Mycobacterium Tuberculosis promotes apoptosis of macrophages via targeting the microRNA155-SOCS1 interaction. Cell. Physiol. Biochem. 2015, 35, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Choi, H.; Park, J.Y.; Abekura, F.; Lee, Y.C.; Kim, J.R.; Kim, C.H. Mycobacterium tuberculosis-Secreted Protein, ESAT-6, Inhibits Lipopolysaccharide-Induced MMP-9 Expression and Inflammation Through NF-κB and MAPK Signaling in RAW 264.7 Macrophage Cells. Inflammation 2020, 43, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, N.; Giang, P.H.; Gupta, C.; Basu, S.K.; Siddiqui, I.; Salunke, D.M.; Sharma, P. Mycobacterium tuberculosis secretory proteins CFP-10, ESAT-6 and the CFP10:ESAT6 complex inhibit lipopolysaccharide-induced NF-κB transactivation by downregulation of reactive oxidative species (ROS) production. Immunol. Cell Biol. 2008, 86, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.K.; Basu, S.; Basu, K.K.; Banerjee, A.; Pathak, S.; Bhattacharyya, A.; Kaisho, T.; Kundu, M.; Basu, J. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat. Immunol. 2007, 8, 610–618. [Google Scholar] [CrossRef]
- Ganguly, N.; Giang, P.H.; Basu, S.K.; Mir, F.A.; Siddiqui, I.; Sharma, P. Mycobacterium tuberculosis 6-kDa early secreted antigenic target (ESAT-6) protein downregulates lipopolysaccharide induced c-myc expression by modulating the extracellular signal regulated kinases 1/2. BMC Immunol. 2007, 8, 24. [Google Scholar] [CrossRef]
- Zonghai, C.; Tao, L.; Pengjiao, M.; Liang, G.; Rongchuan, Z.; Xinyan, W.; Wenyi, N.; Wei, L.; Yi, W.; Lang, B. Mycobacterium tuberculosis ESAT6 modulates host innate immunity by downregulating miR-222-3p target PTEN. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166292. [Google Scholar] [CrossRef]
- Belogorodtsev, S.N.; Nemkova, E.K.; Stavitskaya, N.V.; Schwartz, Y.S. Pathogenic Effects of M. tuberculosis-Specific Proteins ESAT-6 and CFP-10 in Macrophage Culture and in 3D-Granulemogenesis Model In Vitro. Bull. Exp. Biol. Med. 2021, 171, 656–660. [Google Scholar] [CrossRef]
- Jha, V.; Pal, R.; Kumar, D.; Mukhopadhyay, S. ESAT-6 Protein of Mycobacterium tuberculosis Increases Holotransferrin-Mediated Iron Uptake in Macrophages by Downregulating Surface Hemochromatosis Protein HFE. J. Immunol. 2020, 205, 3095–3106. [Google Scholar] [CrossRef]
- Jang, A.R.; Choi, J.H.; Shin, S.J.; Park, J.H. Mycobacterium tuberculosis ESAT6 induces IFN-β gene expression in Macrophages via TLRs-mediated signaling. Cytokine 2018, 104, 104–109. [Google Scholar] [CrossRef]
- Hu, D.; Wu, J.; Zhao, R.P.; Xu, X.W.; Mu, M.; Cai, R.; Xing, Y.R.; Ni, S.F.; Zhang, R.B. ESAT6 inhibits autophagy flux and promotes BCG proliferation through MTOR. Biochem. Biophys. Res. Commun. 2016, 477, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yabaji, S.M.; Dhamija, E.; Mishra, A.K.; Srivastava, K.K. ESAT-6 regulates autophagous response through SOD-2 and as a result induces intracellular survival of Mycobacterium bovis BCG. BBA-Proteins Proteom. 2020, 1868, 140470. [Google Scholar] [CrossRef]
- Kumar, A.S.; Bansal, K.; Holla, S.; Verma-Kumar, S.; Sharma, P.; Balaji, K.N. ESAT-6 induced COX-2 expression involves coordinated interplay between PI3K and MAPK signaling. Mol. Immunol. 2012, 49, 655–663. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dwivedi, V.P.; Singh, Y.; Siddiqui, I.; Sharma, P.; Van Kaer, L.; Chattopadhyay, D.; Das, G. Early Secreted Antigen ESAT-6 of Mycobacterium tuberculosis Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner. PLoS Pathog. 2011, 7, e1002378. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.R.; Kim, G.; Hong, J.J.; Kang, S.M.; Shin, S.J.; Park, J.H. Mycobacterium tuberculosis ESAT6 Drives the Activation and Maturation of Bone Marrow-Derived Dendritic Cells via TLR4-Mediated Signaling. Immune Netw. 2019, 19, e13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Barnes, P.F.; Huang, F.F.; Alvarez, I.B.; Neuenschwander, P.F.; Sherman, D.R.; Samten, B. Early Secreted Antigenic Target of 6-kDa Protein of Mycobacterium tuberculosis Primes Dendritic Cells To Stimulate Th17 and Inhibit Th1 Immune Responses. J. Immunol. 2012, 189, 3092–3103. [Google Scholar] [CrossRef]
- Francis, R.J.; Butler, R.E.; Stewart, G.R. Mycobacterium tuberculosis ESAT-6 is a leukocidin causing Ca2+ influx, necrosis and neutrophil extracellular trap formation. Cell Death Dis. 2014, 5, e1474. [Google Scholar] [CrossRef]
- Wang, X.S.; Barnes, P.F.; Dobos-Elder, K.M.; Townsend, J.C.; Chung, Y.T.; Shams, H.; Weis, S.E.; Samten, B. ESAT-6 Inhibits Production of IFN-γ by Mycobacterium tuberculosis-Responsive Human T Cells. J. Immunol. 2009, 182, 3668–3677. [Google Scholar] [CrossRef]
- Marei, A.; Ghaemmaghami, A.; Renshaw, P.; Wiselka, M.; Barer, M.; Carr, M.; Ziegler-Heitbrock, L. Superior T cell activation by ESAT-6 as compared with the ESAT-6-CFP-10 complex. Int. Immunol. 2005, 17, 1439–1446. [Google Scholar] [CrossRef]
- Li, L.; Wu, C.Y. CD4+CD25+ Treg cells inhibit human memory γδ T cells to produce IFN-γ in response to antigen ESAT-6. Blood 2008, 111, 5629–5636. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Grigsby, S.J.; Philips, J.A. Immune evasion and provocation by mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Busquets-Cortés, C.; Capó, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 Inhibitors as a Therapeutic Target in Inflammatory Diseases. Curr. Med. Chem. 2019, 26, 3225–3241. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Liu, S.Z.; Yao, S.J.; Yang, H.; Liu, S.J.; Wang, Y.J. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef] [PubMed]
- Behura, A.; Das, M.; Kumar, A.; Naik, L.; Mishra, A.; Manna, D.; Patel, S.; Mishra, A.; Singh, R.; Dhiman, R. ESAT-6 impedes IL-18 mediated phagosome lysosome fusion via microRNA-30a upon Calcimycin treatment in mycobacteria infected macrophages. Int. Immunopharmacol. 2021, 101, 108319. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. BBA-Mol. Cell Res. 2013, 1833, 3448–3459. [Google Scholar] [CrossRef]
- Simeone, R.; Bobard, A.; Lippmann, J.; Bitter, W.; Majlessi, L.; Brosch, R.; Enninga, J. Phagosomal Rupture by Mycobacterium tuberculosis Results in Toxicity and Host Cell Death. PLoS Pathog. 2012, 8, e1002507. [Google Scholar] [CrossRef]
- Yin, X.Y.; Chen, S.T.; Eisenbarth, S.C. Dendritic Cell Regulation of T Helper Cells. Annu. Rev. Immunol. 2021, 39, 759–790. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.H.; Strainic, M.G.; Liu, J.B.; Zhang, W.J.; Yi, Z.Z.; Medof, M.E.; Heeger, P.S. TLR-Induced Murine Dendritic Cell (DC) Activation Requires DC-Intrinsic Complement. J. Immunol. 2017, 199, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Morath, A.; Schamel, W.W. αβ and γδ T cell receptors: Similar but different. J. Leukoc. Biol. 2020, 107, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Konigshofer, Y.; Chien, Y.H. γδ T cells -: Innate immune lymphocytes? Curr. Opin. Immunol. 2006, 18, 527–533. [Google Scholar] [CrossRef]
- Chopp, L.B.; Gopalan, V.; Ciucci, T.; Ruchinskas, A.; Rae, Z.; Lagarde, M.; Gao, Y.Y.; Li, C.Y.; Bosticardo, M.; Pala, F.; et al. An Integrated Epigenomic and Transcriptomic Map of Mouse and Human αβ T Cell Development. Immunity 2020, 53, 1182–1201. [Google Scholar] [CrossRef]
- Peng, H.; Wang, X.S.; Barnes, P.F.; Tang, H.; Townsend, J.C.; Samten, B. The Mycobacterium tuberculosis Early Secreted Antigenic Target of 6 kDa Inhibits T Cell Interferon-γ Production through the p38 Mitogen-activated Protein Kinase Pathway. J. Biol. Chem. 2011, 286, 24508–24518. [Google Scholar] [CrossRef]
- Luan, X.; Fan, X.; Li, G.; Li, M.; Li, N.; Yan, Y.; Zhao, X.; Liu, H.; Wan, K. Exploring the immunogenicity of Rv2201-519: A T-cell epitope-based antigen derived from Mycobacterium tuberculosis AsnB with implications for tuberculosis infection detection and vaccine development. Int. Immunopharmacol. 2024, 129, 111542. [Google Scholar] [CrossRef]
- Rahman, A.H.; Taylor, D.K.; Turka, L.A. The contribution of direct TLR signaling to T cell responses. Immunol. Res. 2009, 45, 25–36. [Google Scholar] [CrossRef]
- Kabelitz, D. Expression and function of Toll-like receptors in T lymphocytes. Curr. Opin. Immunol. 2007, 19, 39–45. [Google Scholar] [CrossRef]
- Komai-Koma, M.; Jones, L.; Ogg, G.S.; Xu, D.; Liew, F.Y. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 3029–3034. [Google Scholar] [CrossRef]
- Reba, S.M.; Li, Q.; Onwuzulike, S.; Ding, X.; Karim, A.F.; Hernandez, Y.; Fulton, S.A.; Harding, C.V.; Lancioni, C.L.; Nagy, N.; et al. TLR2 engagement on CD4(+) T cells enhances effector functions and protective responses to Mycobacterium tuberculosis. Eur. J. Immunol. 2014, 44, 1410–1421. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, E.; Quintas-Granados, L.I.; Flores-Villalva, S.; Cantó-Alarcón, J.G.; Milián-Suazo, F. Application of antigenic biomarkers for Mycobacterium tuberculosis. J. Zhejiang Univ. Sci. B 2020, 21, 856–870. [Google Scholar] [CrossRef]
- Mustafa, A.S. Early secreted antigenic target of 6 kda-like proteins of mycobacterium tuberculosis: Diagnostic and vaccine relevance. Int. J. Mycobacteriology 2022, 11, 10–15. [Google Scholar] [CrossRef]
- Refai, A.; Haoues, M.; Othman, H.; Barbouche, M.R.; Moua, P.; Bondon, A.; Mouret, L.; Srairi-Abid, N.; Essafi, M. Two distinct conformational states of Mycobacterium tuberculosis virulent factor early secreted antigenic target 6 kDa are behind the discrepancy around its biological functions. FEBS J. 2015, 282, 4114–4129. [Google Scholar] [CrossRef]
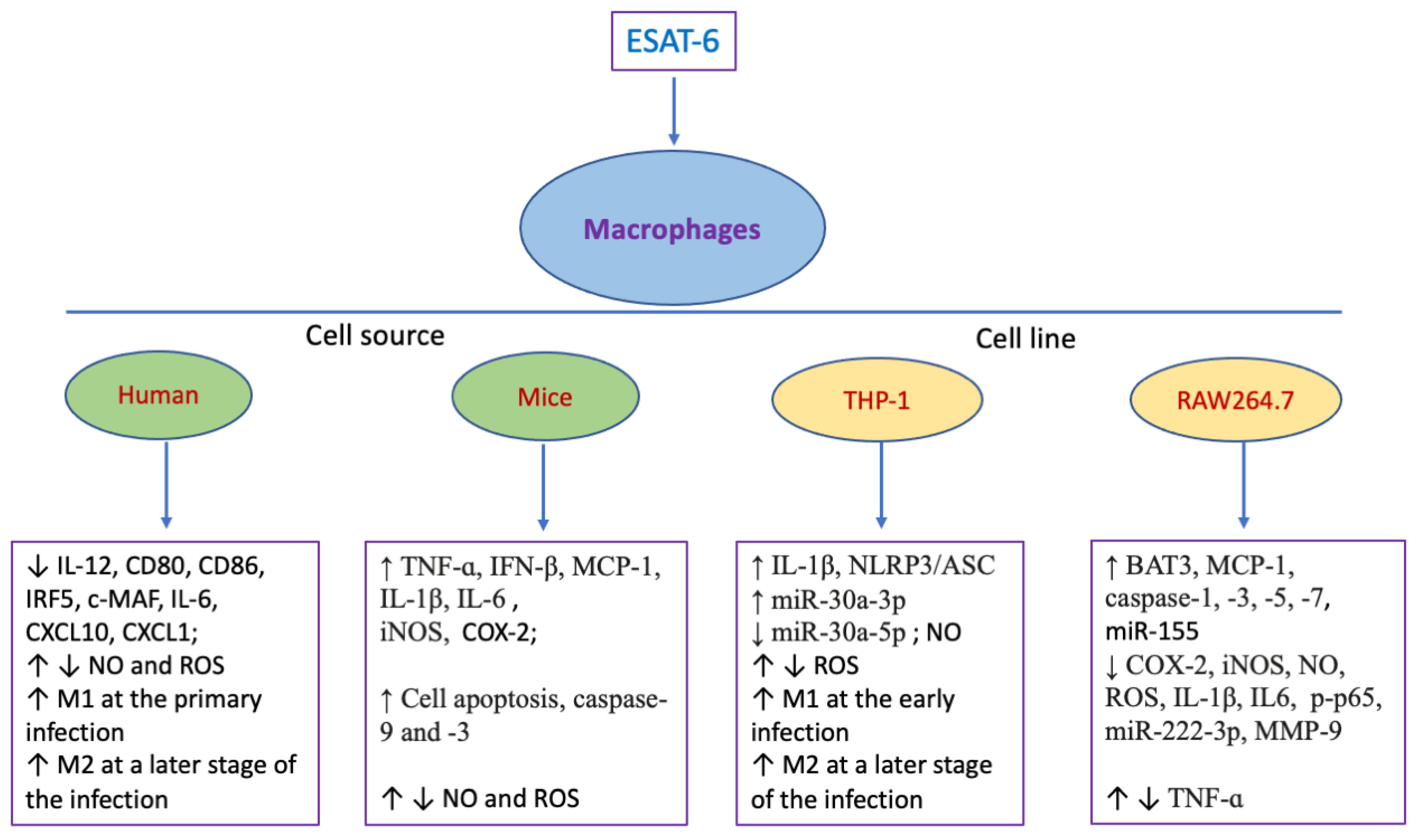
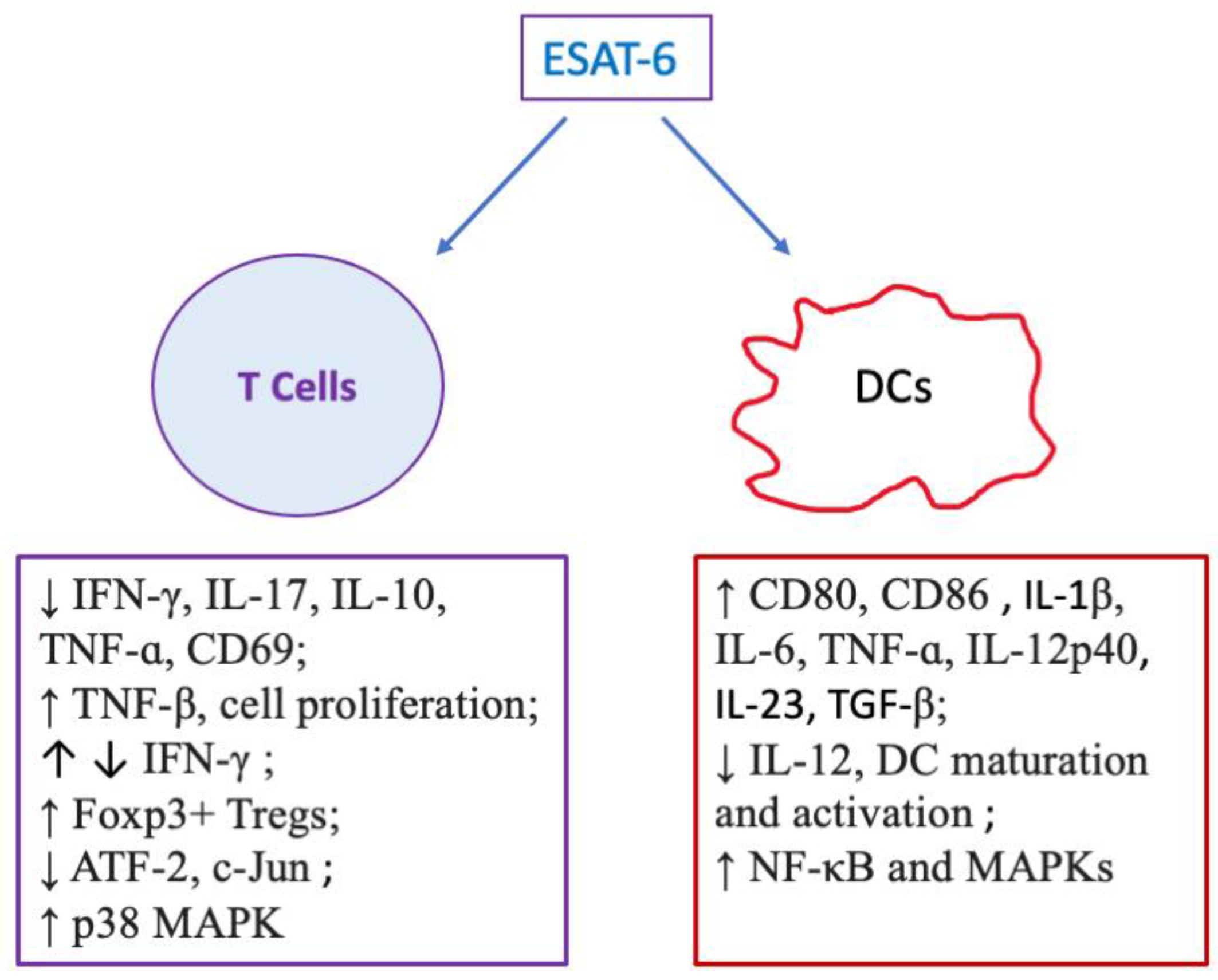
| Cell Type | Origin | Effects | Refs. |
|---|---|---|---|
| Macrophages | Human blood | ↑ Fusion of lysosomes and phagosomes, ↑ phagosome rupture | [19] |
| Human blood | ↓ IL-12, CD80, CD86, IRF5, c-MAF, IL-10, IL-6, CXCL10, CXCL1; ↑ M1 phenotype at the primo-infection, ↑ M2 phenotype at a later stage of the infection | [27] | |
| Murine bone marrow | ↑ TNF-ɑ, MCP-1, IL-1β, IL-6, STAT3 activation | [28,29,30] | |
| Murine bone marrow | ↑ iNOS/NO, E-cadherin, junction plakoglobin, ZO1, desmoplakin, desmoglein3 and catenin proteins; ↓ H3K27 trimethylation; ↑ epithelioid macrophages | [31] | |
| Murine bone marrow | ↑ Cell apoptosis, cleaved caspase-9 and -3, Bim activation, ROS generation, MAPKs phosphorylation | [32] | |
| THP-1 cells | ↑ IL-1β, glucose uptake, DHAP, AcCoA, lipid bodies, foamy macrophage, the activation of NLRP3/ASC inflammasome | [26,33] | |
| THP-1 cells | ↑ ROS, HIF1a, NLRP3 activation, phagocytosis activity, glucose metabolism, cell necrosis, lysosomal permeabilization | [34,35] | |
| THP-1 cells | ↓ NO, ROS, NO synthase activity, MHC-I-β2M complexes on cell surface, class I Ag presentation | [36,37] | |
| THP-1 cells | ↑ M1 polarization, activation of TLR4/MyD88/NF-κB pathway, cell apoptosis within 24h; ↑ M2 phenotype at 36h post-treatment, ↓ TLR4/MyD88/NF-κB pathway | [38] | |
| THP-1 cells | ↑ miR-30a-3p; ↓ miR-30a-5p, autophagy | [39] | |
| RAW264.7 cells | ↑ BAT3, TNF-ɑ, MCP-1, caspase-1, -3, -5, -7 and -8, miR-155, cell apoptosis, NAPDH-ROS-JNK/p38-NF-kB pathway | [40,41,42,43] | |
| RAW264.7 cells | ↓ COX-2, iNOS, NO, ROS, p65 transactivation, expression and enzyme activity of MMP-9; | [44,45] | |
| RAW264.7 cells | ↑ Activation of Akt; ↓ interaction between MyD88 and IRAK4, NF-ĸB activation | [46] | |
| RAW264.7 cells | ↑ ERK1/2 phosphorylation in the cytoplasm; ↓ ERK1/2 phosphorylation in the nucleus, C-myc | [47] | |
| RAW264.7 cells | ↓ IL-1β, IL6, TNFa, p-p65, miR-222-3p; ↑ PTEN | [48] | |
| Murine peritoneal cavity | ↓ NO and ROS; ↑ apoptosis and necrosis | [49] | |
| Murine peritoneal cavity | ↑ HFE in endoplasmic reticulum; ↓ HFE-TFR1 complex on cellular surface; ↑ holotransferrin-regulated iron uptake | [50] | |
| Murine bone marrow, peritoneal cavity, MH-S cells | ↑ IFN-β; ↑ activation of TBK1 and IRF3 | [51] | |
| Murine abdominal cavity, RAW264.7 cells | ↓ LC3II and SQSTM1 degradation; ↓ autophagy flux; ↑ mTOR activity | [52] | |
| J774 A.1 cell | ↑ Expression and activity of SOD-2 | [53] | |
| Murine peritoneal cavity | ↑ COX-2, PI3K and MAPK pathway | [54] |
| Cell Type | Origin | Effects | Ref. |
|---|---|---|---|
| DCs | Murine bone marrow | ↑ IL-6, TGF-β | [55] |
| Murine bone marrow | ↑ CD80, CD86 and MHC-II, IL-6, TNF-ɑ and IL-12p40, activation of NF-κB and MAPKs | [56] | |
| Human blood | ↓ IL-12, DC maturation and activation; ↑ IL-1β and IL-23 | [57] | |
| Neutrophils | Human blood | ↑ Intracellular Ca2+ overload, neutrophil extracellular traps, necrosis | [58] |
| T cells | Human blood | ↓ IFN-γ, IL-17, TNF-ɑ, CD69, ATF-2, c-Jun | [57] |
| Human blood | ↓ IFN-γ, IL-10, IL-17; ↑ activation of p38 MAPK | [59] | |
| Human blood | ↑ TNFβ in CD4+ T cells and IFN-γ in CD8+ T cells | [60] | |
| Human blood | ↑ Cell activation and proliferation | [61] | |
| Murine skin/spleen, and lymph nodes | ↓ CD3+ T cells in allografts, Th1, CD4+/CD8+ effector T cells in spleen and lymph nodes; ↑ Foxp3+ Treg in an allograft, spleen and lymph nodes; ↑ CD4+Foxp3+ Tregs differentiation, IĸBα/c-Rel signaling pathway in vitro; ↓ CD4+CD25− T cell proliferation in vitro | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.; Lin, J.; He, Y.; Yang, B.; Qiu, F.; Dai, Z. Immunoregulation by ESAT-6: From Pathogenesis of Tuberculosis to Potential Anti-Inflammatory and Anti-Rejection Application. Pharmaceuticals 2025, 18, 1408. https://doi.org/10.3390/ph18091408
Lu W, Lin J, He Y, Yang B, Qiu F, Dai Z. Immunoregulation by ESAT-6: From Pathogenesis of Tuberculosis to Potential Anti-Inflammatory and Anti-Rejection Application. Pharmaceuticals. 2025; 18(9):1408. https://doi.org/10.3390/ph18091408
Chicago/Turabian StyleLu, Weihui, Jingru Lin, Yuming He, Bin Yang, Feifei Qiu, and Zhenhua Dai. 2025. "Immunoregulation by ESAT-6: From Pathogenesis of Tuberculosis to Potential Anti-Inflammatory and Anti-Rejection Application" Pharmaceuticals 18, no. 9: 1408. https://doi.org/10.3390/ph18091408
APA StyleLu, W., Lin, J., He, Y., Yang, B., Qiu, F., & Dai, Z. (2025). Immunoregulation by ESAT-6: From Pathogenesis of Tuberculosis to Potential Anti-Inflammatory and Anti-Rejection Application. Pharmaceuticals, 18(9), 1408. https://doi.org/10.3390/ph18091408






