Skeletal Muscle Aging: Enhancing Skeletal Muscle Integrity and Function as a Potential Pharmacological Approach
Abstract
1. Introduction
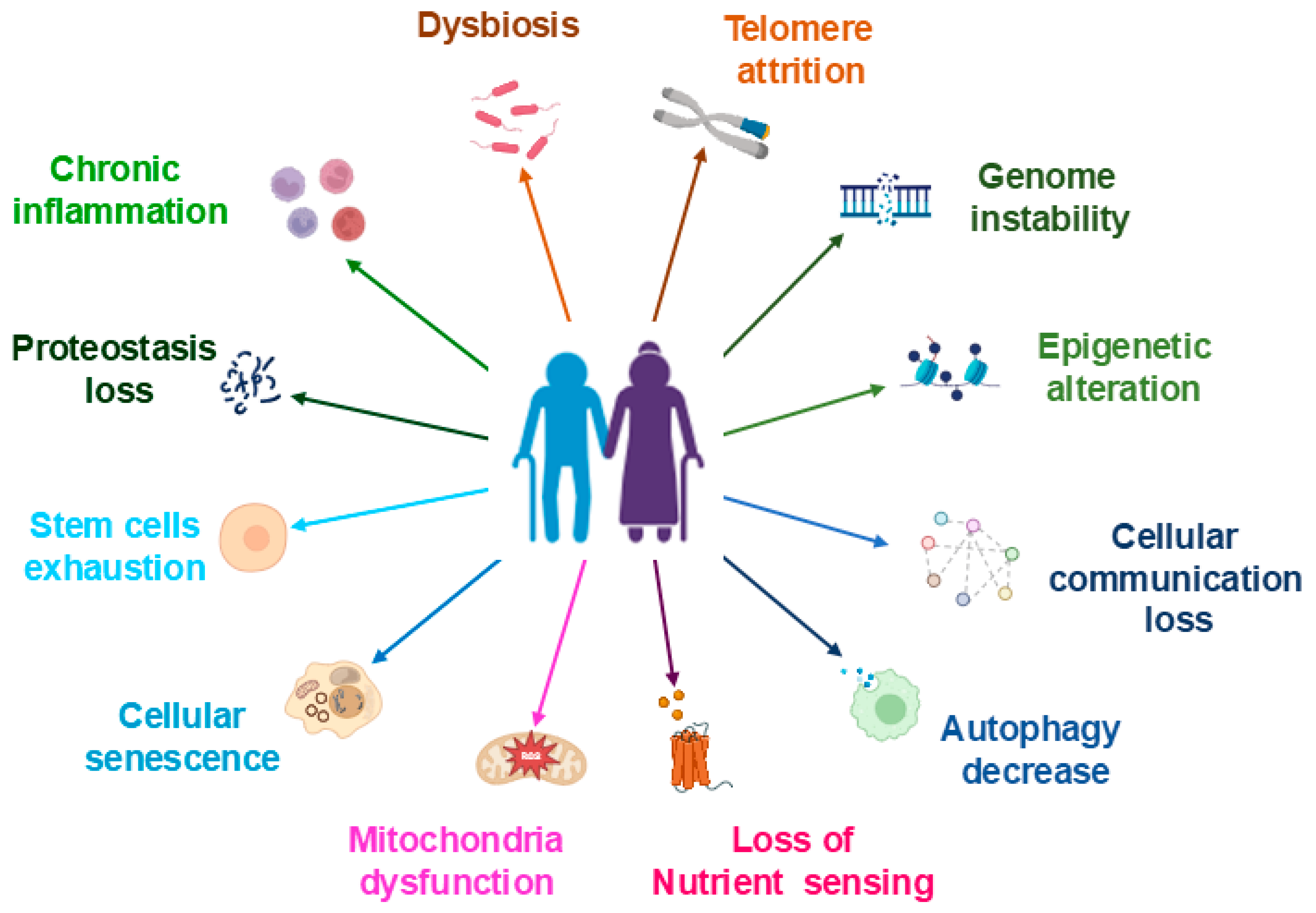
2. Biological Factors Contribute to Aging
2.1. Muscle Stem (Satellite) Cells (MSCs)
2.2. Protein Synthesis/Degradation
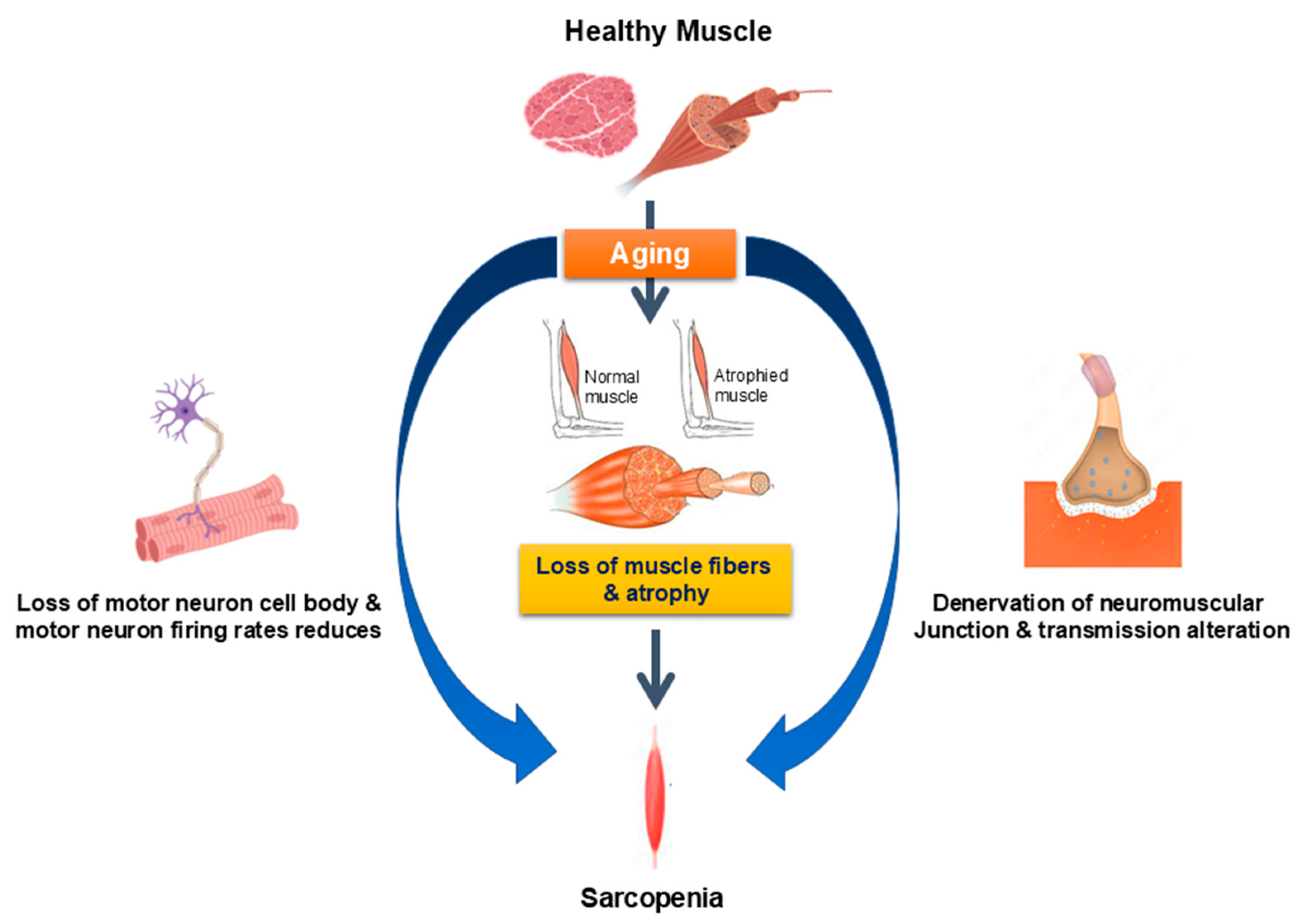
2.3. Neuromuscular System
2.4. Antioxidant Defense Systems
2.5. Hormones
2.6. Inflammation
2.7. Insulin Resistance
2.8. Mitochondrial Dysfunction
2.9. Other Biological Factors
3. Skeletal Muscle ECM Dynamics in Aging
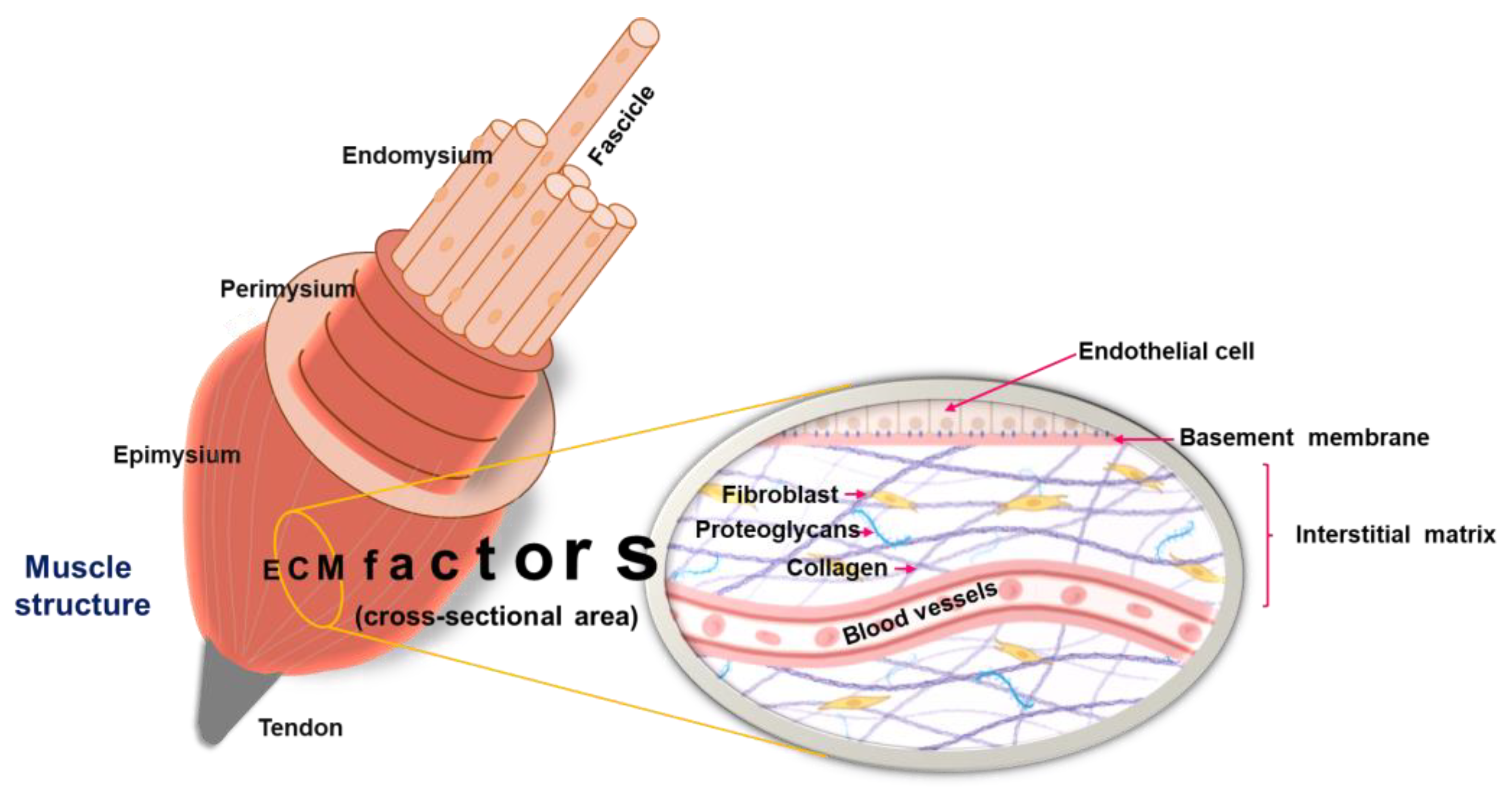
3.1. The ECM
3.2. Skeletal Muscle ECM
3.3. Effect of Aging on Skeletal Muscle ECM
4. Therapeutic Targets and Strategies for Managing Age-Related Skeletal Muscle Decline
5. Protein, Peptides, and Amino Acids as Therapeutic Agents
6. Natural Products and Bioactive Compounds as Therapeutic Agents
6.1. Natural Product Extract
6.2. Bioactive Compounds
6.2.1. Licochalcone A and B
6.2.2. Epicatechin
6.2.3. Curcumin
6.2.4. Succinic Acid
6.2.5. Echinacoside (ECH)
6.2.6. 8-Prenylnaringenin
6.2.7. Dieckol and 2,7-phloroglucinol-6,6′-bieckol
6.2.8. Dihydromyricetin (DM)
6.2.9. Ascorbic Acid (Vitamin C)
6.2.10. Silibinin
6.2.11. Omega-7 Palmitoleic Acids
6.2.12. Oleic Acid
6.2.13. 5,7-Dimethoxyflavone
6.2.14. Apigenin
6.2.15. Baicalin
6.2.16. Andrographolide
6.2.17. Luteolin
6.2.18. Sinensetin
6.2.19. β-Carotene (BC)
6.2.20. Morin
6.2.21. Quercetin
6.2.22. Genistein
6.2.23. α-Mangostin
6.2.24. Glabridin
6.2.25. Corylifol A
6.2.26. Melatonin
6.2.27. Daidzein
6.2.28. Sulforaphane (SFN)
6.2.29. Delphinidin
6.2.30. Eicosapentaenoic Acid (EPA)
6.2.31. Isobavachalcone (IBC)
6.2.32. Glyoxylic Acid
6.2.33. Oleocanthal
6.2.34. Panduratin A
6.2.35. Sophoranone
6.2.36. Diosmin
6.2.37. Ginsenoside Rg5
6.2.38. Resveratrol
6.2.39. Maslinic Acid (MA)
6.2.40. Ferulic Acid (FA)
6.2.41. Theaflavin
6.2.42. Paeoniflorin
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Feige, P.; Brun, C.E.; Ritso, M.; Rudnicki, M.A. Orienting Muscle Stem Cells for Regeneration in Homeostasis, Aging, and Disease. Cell Stem Cell 2018, 23, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Fukada, S.I. The roles of muscle stem cells in muscle injury, atrophy and hypertrophy. J. Biochem. 2018, 163, 353–358. [Google Scholar] [CrossRef]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 2018, 243, 118–128. [Google Scholar] [CrossRef]
- Aversa, Z.; Zhang, X.; Fielding, R.A.; Lanza, I.; LeBrasseur, N.K. The clinical impact and biological mechanisms of skeletal muscle aging. Bone 2019, 127, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Rodrigues, R.; Hoffmann, F.; Gasior, K.; Marin, B. Facts and Figures on Long-Term Care; Europe and North America; European Centre for Social Welfare Policy and Research: Vienna, Austria, 2009. [Google Scholar]
- Roubenoff, R. Sarcopenia and its implications for the elderly. Eur. J. Clin. Nutr. 2000, 54 (Suppl. S3), S40–S47. [Google Scholar] [CrossRef]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.; van Kranenburg, J.; Verdijk, L.B.; van Loon, L.J. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef]
- Gopinath, S.D.; Rando, T.A. Stem cell review series: Aging of the skeletal muscle stem cell niche. Aging Cell 2008, 7, 590–598. [Google Scholar] [CrossRef]
- Chini, C.C.S.; Peclat, T.R.; Warner, G.M.; Kashyap, S.; Espindola-Netto, J.M.; de Oliveira, G.C.; Gomez, L.S.; Hogan, K.A.; Tarrago, M.G.; Puranik, A.S.; et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat. Metab. 2020, 2, 1284–1304. [Google Scholar] [CrossRef]
- Kuswanto, W.; Burzyn, D.; Panduro, M.; Wang, K.K.; Jang, Y.C.; Wagers, A.J.; Benoist, C.; Mathis, D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 2016, 44, 355–367. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Ahmad, K.; Shaikh, S.; Lee, E.J.; Lee, Y.H.; Choi, I. Consequences of Dicarbonyl Stress on Skeletal Muscle Proteins in Type 2 Diabetes. Curr. Protein Pept. Sci. 2020, 21, 878–889. [Google Scholar] [CrossRef]
- Day, K.; Shefer, G.; Shearer, A.; Yablonka-Reuveni, Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev. Biol. 2010, 340, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, J.V.; Jones, K.M.; Basson, M.A.; Brack, A.S. The aged niche disrupts muscle stem cell quiescence. Nature 2012, 490, 355–360. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; van Loon, L.J. Satellite cells in human skeletal muscle; from birth to old age. Age 2014, 36, 545–547. [Google Scholar] [CrossRef]
- Sousa-Victor, P.; Gutarra, S.; Garcia-Prat, L.; Rodriguez-Ubreva, J.; Ortet, L.; Ruiz-Bonilla, V.; Jardi, M.; Ballestar, E.; Gonzalez, S.; Serrano, A.L.; et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014, 506, 316–321. [Google Scholar] [CrossRef]
- Carlson, B.M.; Dedkov, E.I.; Borisov, A.B.; Faulkner, J.A. Skeletal muscle regeneration in very old rats. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B224–B233. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Klose, A.; Forman, S.; Paris, N.D.; Wei-LaPierre, L.; Cortes-Lopez, M.; Tan, A.; Flaherty, M.; Miura, P.; Dirksen, R.T.; et al. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. eLife 2017, 6, e26464. [Google Scholar] [CrossRef]
- Mackey, A.L.; Karlsen, A.; Couppé, C.; Mikkelsen, U.R.; Nielsen, R.H.; Magnusson, S.P.; Kjaer, M. Differential satellite cell density of type I and II fibres with lifelong endurance running in old men. Acta Physiol. 2014, 210, 612–627. [Google Scholar] [CrossRef]
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.; Verdijk, L.B.; van Loon, L.J. Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLoS ONE 2015, 10, e0140903. [Google Scholar] [CrossRef]
- Fry, C.S.; Drummond, M.J.; Glynn, E.L.; Dickinson, J.M.; Gundermann, D.M.; Timmerman, K.L.; Walker, D.K.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet. Muscle 2011, 1, 11. [Google Scholar] [CrossRef]
- Koopman, R.; Walrand, S.; Beelen, M.; Gijsen, A.P.; Kies, A.K.; Boirie, Y.; Saris, W.H.; van Loon, L.J. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J. Nutr. 2009, 139, 1707–1713. [Google Scholar] [CrossRef]
- D’Hulst, G.; Masschelein, E.; De Bock, K. Resistance exercise enhances long-term mTORC1 sensitivity to leucine. Mol. Metab. 2022, 66, 101615. [Google Scholar] [CrossRef]
- Castets, P.; Lin, S.; Rion, N.; Di Fulvio, S.; Romanino, K.; Guridi, M.; Frank, S.; Tintignac, L.A.; Sinnreich, M.; Rüegg, M.A. Sustained activation of mTORC1 in skeletal muscle inhibits constitutive and starvation-induced autophagy and causes a severe, late-onset myopathy. Cell Metab. 2013, 17, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef]
- Smeuninx, B.; McKendry, J.; Wilson, D.; Martin, U.; Breen, L. Age-Related Anabolic Resistance of Myofibrillar Protein Synthesis Is Exacerbated in Obese Inactive Individuals. J. Clin. Endocrinol. Metab. 2017, 102, 3535–3545. [Google Scholar] [CrossRef] [PubMed]
- Grumati, P.; Coletto, L.; Sabatelli, P.; Cescon, M.; Angelin, A.; Bertaggia, E.; Blaauw, B.; Urciuolo, A.; Tiepolo, T.; Merlini, L.; et al. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat. Med. 2010, 16, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Gouspillou, G.; Sgarioto, N.; Kapchinsky, S.; Purves-Smith, F.; Norris, B.; Pion, C.H.; Barbat-Artigas, S.; Lemieux, F.; Taivassalo, T.; Morais, J.A.; et al. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J. 2014, 28, 1621–1633. [Google Scholar] [CrossRef]
- Drummond, M.J.; Addison, O.; Brunker, L.; Hopkins, P.N.; McClain, D.A.; LaStayo, P.C.; Marcus, R.L. Downregulation of E3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: A cross-sectional comparison. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1040–1048. [Google Scholar] [CrossRef]
- Garcia-Prat, L.; Munoz-Canoves, P.; Martinez-Vicente, M. Dysfunctional autophagy is a driver of muscle stem cell functional decline with aging. Autophagy 2016, 12, 612–613. [Google Scholar] [CrossRef]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy is required to maintain muscle mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef]
- Carnio, S.; LoVerso, F.; Baraibar, M.A.; Longa, E.; Khan, M.M.; Maffei, M.; Reischl, M.; Canepari, M.; Loefler, S.; Kern, H.; et al. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep. 2014, 8, 1509–1521. [Google Scholar] [CrossRef]
- Baechler, B.L.; Bloemberg, D.; Quadrilatero, J. Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation. Autophagy 2019, 15, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, M.; Ireland, A.; Stashuk, D.; Hamilton-Wright, A.; Jones, D.A.; McPhee, J.S. Age-related neuromuscular changes affecting human vastus lateralis. J. Physiol. 2016, 594, 4525–4536. [Google Scholar] [CrossRef]
- Sheth, K.A.; Iyer, C.C.; Wier, C.G.; Crum, A.E.; Bratasz, A.; Kolb, S.J.; Clark, B.C.; Burghes, A.H.M.; Arnold, W.D. Muscle strength and size are associated with motor unit connectivity in aged mice. Neurobiol. Aging 2018, 67, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R.; Roby, M.A.; Eason, M.K.; Harris, M.B. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp. Gerontol. 2010, 45, 389–393. [Google Scholar] [CrossRef]
- Bongers, K.S.; Fox, D.K.; Ebert, S.M.; Kunkel, S.D.; Dyle, M.C.; Bullard, S.A.; Dierdorff, J.M.; Adams, C.M. Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E907–E915. [Google Scholar] [CrossRef]
- Titova, A.; Bilyalov, A.; Filatov, N.; Perepechenov, S.; Kupriyanova, D.; Brovkin, S.; Shestakov, D.; Bodunova, N.; Gusev, O. Muscle Aging Heterogeneity: Genetic and Structural Basis of Sarcopenia Resistance. Genes 2025, 16, 948. [Google Scholar] [CrossRef]
- Wu, P.; Chawla, A.; Spinner, R.J.; Yu, C.; Yaszemski, M.J.; Windebank, A.J.; Wang, H. Key changes in denervated muscles and their impact on regeneration and reinnervation. Neural Regen. Res. 2014, 9, 1796–1809. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y.; Yang, Y.; Yu, J.; Wu, J.; Liao, Z.; Guo, A.; Sun, Y.; Zhao, Y.; Chen, J.; et al. Lysyl oxidase-like 2 inhibitor rescues D-galactose-induced skeletal muscle fibrosis. Aging Cell 2022, 21, e13659. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Sun, X.; Zhang, W.; Shi, X.; Xu, S. Di-(2-ethyl hexyl) phthalate induced oxidative stress promotes microplastics mediated apoptosis and necroptosis in mice skeletal muscle by inhibiting PI3K/AKT/mTOR pathway. Toxicology 2022, 474, 153226. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Kato, H.; Watanabe, H.; Imafuku, T.; Arimura, N.; Fujita, I.; Noguchi, I.; Tanaka, S.; Nakano, T.; Tokumaru, K.; Enoki, Y.; et al. Advanced oxidation protein products contribute to chronic kidney disease-induced muscle atrophy by inducing oxidative stress via CD36/NADPH oxidase pathway. J. Cachexia Sarcopenia Muscle 2021, 12, 1832–1847. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef] [PubMed]
- Palomero, J.; Vasilaki, A.; Pye, D.; McArdle, A.; Jackson, M.J. Aging increases the oxidation of dichlorohydrofluorescein in single isolated skeletal muscle fibers at rest, but not during contractions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R351–R358. [Google Scholar] [CrossRef] [PubMed]
- Broome, C.S.; Kayani, A.C.; Palomero, J.; Dillmann, W.H.; Mestril, R.; Jackson, M.J.; McArdle, A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J. 2006, 20, 1549–1551. [Google Scholar] [CrossRef]
- Sullivan-Gunn, M.J.; Lewandowski, P.A. Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia. BMC Geriatr. 2013, 13, 104. [Google Scholar] [CrossRef]
- Ryan, M.J.; Dudash, H.J.; Docherty, M.; Geronilla, K.B.; Baker, B.A.; Haff, G.G.; Cutlip, R.G.; Alway, S.E. Aging-dependent regulation of antioxidant enzymes and redox status in chronically loaded rat dorsiflexor muscles. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1015–1026. [Google Scholar] [CrossRef]
- Giannoulis, M.G.; Martin, F.C.; Nair, K.S.; Umpleby, A.M.; Sonksen, P. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr. Rev. 2012, 33, 314–377. [Google Scholar] [CrossRef]
- Lapauw, B.; Goemaere, S.; Zmierczak, H.; Van Pottelbergh, I.; Mahmoud, A.; Taes, Y.; De Bacquer, D.; Vansteelandt, S.; Kaufman, J.M. The decline of serum testosterone levels in community-dwelling men over 70 years of age: Descriptive data and predictors of longitudinal changes. Eur. J. Endocrinol. 2008, 159, 459–468. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Liem, A.Y.; South, S.; Weltman, A.; Weltman, J.; Clemmons, D.A.; Abbott, R.; Mulligan, T.; Johnson, M.L.; Pincus, S.; et al. Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J. Clin. Endocrinol. Metab. 1995, 80, 3209–3222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Enns, D.L.; Tiidus, P.M. The influence of estrogen on skeletal muscle: Sex matters. Sports Med. 2010, 40, 41–58. [Google Scholar] [CrossRef]
- Goodman-Gruen, D.; Barrett-Connor, E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am. J. Epidemiol. 1997, 145, 970–976. [Google Scholar] [CrossRef]
- Tidball, J.G. Mechanical signal transduction in skeletal muscle growth and adaptation. J. Appl. Physiol. 2005, 98, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 2010, 346, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Chapman, I.M.; Visvanathan, R.; Hammond, A.J.; Morley, J.E.; Field, J.B.; Tai, K.; Belobrajdic, D.P.; Chen, R.Y.; Horowitz, M. Effect of testosterone and a nutritional supplement, alone and in combination, on hospital admissions in undernourished older men and women. Am. J. Clin. Nutr. 2009, 89, 880–889. [Google Scholar] [CrossRef]
- Rolland, Y.; Czerwinski, S.; Abellan Van Kan, G.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2018, 23, 570. [Google Scholar] [CrossRef]
- Wu, J.; Lin, S.; Chen, W.; Lian, G.; Wu, W.; Chen, A.; Sagor, M.I.H.; Luo, L.; Wang, H.; Xie, L. TNF-alpha contributes to sarcopenia through caspase-8/caspase-3/GSDME-mediated pyroptosis. Cell Death Discov. 2023, 9, 76. [Google Scholar] [CrossRef]
- Liu, M.; Fu, X.; Yu, D.; Li, M.; Pan, Y.; Yang, C.; Sun, G. Mapping the causal associations of cytokines with sarcopenia and aging traits: Evidence from bidirectional Mendelian randomization. J. Cachexia Sarcopenia Muscle 2024, 15, 1121–1133. [Google Scholar] [CrossRef]
- Antuna, E.; Potes, Y.; Baena-Huerta, F.J.; Cachan-Vega, C.; Menendez-Coto, N.; Alvarez Darriba, E.; Fernandez-Fernandez, M.; Burgos Bencosme, N.; Bermudez, M.; Lopez Alvarez, E.M.; et al. NLRP3 Contributes to Sarcopenia Associated to Dependency Recapitulating Inflammatory-Associated Muscle Degeneration. Int. J. Mol. Sci. 2024, 25, 1439. [Google Scholar] [CrossRef]
- Van Long, N.; Chien, P.N.; Tung, T.X.; Van Anh, L.T.; Giang, N.N.; Nga, P.T.; Linh, L.T.T.; Nam, S.Y.; Heo, C.Y. Complementary combination of biomarkers for diagnosis of sarcopenia in C57BL/6J mice. Life Sci. 2023, 312, 121213. [Google Scholar] [CrossRef] [PubMed]
- Stowe, R.P.; Peek, M.K.; Cutchin, M.P.; Goodwin, J.S. Plasma cytokine levels in a population-based study: Relation to age and ethnicity. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 429–433. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Degens, H. The role of systemic inflammation in age-related muscle weakness and wasting. Scand. J. Med. Sci. Sports 2010, 20, 28–38. [Google Scholar] [CrossRef]
- Goodman, M.N. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am. J. Physiol. 1991, 260, E727–E730. [Google Scholar] [CrossRef] [PubMed]
- Mercier, S.; Breuille, D.; Mosoni, L.; Obled, C.; Patureau Mirand, P. Chronic inflammation alters protein metabolism in several organs of adult rats. J. Nutr. 2002, 132, 1921–1928. [Google Scholar] [CrossRef]
- Langen, R.C.; Van Der Velden, J.L.; Schols, A.M.; Kelders, M.C.; Wouters, E.F.; Janssen-Heininger, Y.M. Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J. 2004, 18, 227–237. [Google Scholar] [CrossRef]
- Shimizu, Y.; Hamada, K.; Guo, T.; Hasegawa, C.; Kuga, Y.; Takeda, K.; Yagi, T.; Koyama, H.; Takagi, H.; Aotani, D.; et al. Role of PPARalpha in inflammatory response of C2C12 myotubes. Biochem. Biophys. Res. Commun. 2024, 694, 149413. [Google Scholar] [CrossRef]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef]
- Wang, Y.; Wehling-Henricks, M.; Samengo, G.; Tidball, J.G. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell 2015, 14, 678–688. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. eBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- Ehrhardt, N.; Cui, J.; Dagdeviren, S.; Saengnipanthkul, S.; Goodridge, H.S.; Kim, J.K.; Lantier, L.; Guo, X.; Chen, Y.I.; Raffel, L.J.; et al. Adiposity-Independent Effects of Aging on Insulin Sensitivity and Clearance in Mice and Humans. Obesity 2019, 27, 434–443. [Google Scholar] [CrossRef]
- Huffman, D.M.; Barzilai, N. Role of visceral adipose tissue in aging. Biochim. Biophys. Acta 2009, 1790, 1117–1123. [Google Scholar] [CrossRef]
- Petersen, K.F.; Morino, K.; Alves, T.C.; Kibbey, R.G.; Dufour, S.; Sono, S.; Yoo, P.S.; Cline, G.W.; Shulman, G.I. Effect of aging on muscle mitochondrial substrate utilization in humans. Proc. Natl. Acad. Sci. USA 2015, 112, 11330–11334. [Google Scholar] [CrossRef]
- Shou, J.; Chen, P.J.; Xiao, W.H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef]
- Karakelides, H.; Irving, B.A.; Short, K.R.; O’Brien, P.; Nair, K.S. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes 2010, 59, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; de Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Trobec, K.; von Haehling, S.; Anker, S.D.; Lainscak, M. Growth hormone, insulin-like growth factor 1, and insulin signaling-a pharmacological target in body wasting and cachexia. J. Cachexia Sarcopenia Muscle 2011, 2, 191–200. [Google Scholar] [CrossRef]
- Proud, C.G. Regulation of protein synthesis by insulin. Biochem. Soc. Trans. 2006, 34, 213–216. [Google Scholar] [CrossRef]
- Jeong, I.; Cho, E.J.; Yook, J.S.; Choi, Y.; Park, D.H.; Kang, J.H.; Lee, S.H.; Seo, D.Y.; Jung, S.J.; Kwak, H.B. Mitochondrial Adaptations in Aging Skeletal Muscle: Implications for Resistance Exercise Training to Treat Sarcopenia. Life 2024, 14, 962. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Yoon, D.S.; Lim, I.K.; Yoon, S.H.; Chung, H.Y.; Rojo, M.; Malka, F.; Jou, M.J.; Martinou, J.C.; Yoon, G. Formation of elongated giant mitochondria in DFO-induced cellular senescence: Involvement of enhanced fusion process through modulation of Fis1. J. Cell. Physiol. 2006, 209, 468–480. [Google Scholar] [CrossRef]
- Ljubicic, V.; Hood, D.A. Diminished contraction-induced intracellular signaling towards mitochondrial biogenesis in aged skeletal muscle. Aging Cell 2009, 8, 394–404. [Google Scholar] [CrossRef]
- Conley, K.E.; Jubrias, S.A.; Esselman, P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000, 526 Pt 1, 203–210. [Google Scholar] [CrossRef]
- Chabi, B.; Ljubicic, V.; Menzies, K.J.; Huang, J.H.; Saleem, A.; Hood, D.A. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 2008, 7, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Song, W.; Jang, Y.C.; Liu, Y.; Sabia, M.; Richardson, A.; Van Remmen, H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1159–R1168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Guo, C.; Zou, Y.Y.; Feng, C.; Yang, D.X.; Sun, C.C.; Wen, W.; Jian, Z.J.; Zhao, Z.; Xiao, Q.; et al. Aerobic exercise enhances mitochondrial homeostasis to counteract D-galactose-induced sarcopenia in zebrafish. Exp. Gerontol. 2023, 180, 112265. [Google Scholar] [CrossRef]
- Membrez, M.; Migliavacca, E.; Christen, S.; Yaku, K.; Trieu, J.; Lee, A.K.; Morandini, F.; Giner, M.P.; Stiner, J.; Makarov, M.V.; et al. Trigonelline is an NAD+ precursor that improves muscle function during ageing and is reduced in human sarcopenia. Nat. Metab. 2024, 6, 433–447. [Google Scholar] [CrossRef]
- Stephenson, M.C.; Ho, J.X.M.; Migliavacca, E.; Kalimeri, M.; Karnani, N.; Banerji, S.; Totman, J.J.; Feige, J.N.; Merchant, R.A.; Tay, S.K.H. Evidence for inefficient contraction and abnormal mitochondrial activity in sarcopenia using magnetic resonance spectroscopy. J. Cachexia Sarcopenia Muscle 2023, 14, 1482–1494. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.; Toniolo, L.; Nagaraj, N.; Ciciliot, S.; Vindigni, V.; Schiaffino, S.; Reggiani, C.; Mann, M. Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep. 2017, 19, 2396–2409. [Google Scholar] [CrossRef]
- Deschenes, M.R. Effects of aging on muscle fibre type and size. Sports Med. 2004, 34, 809–824. [Google Scholar] [CrossRef]
- Christensen, K.; McGue, M.; Yashin, A.; Iachine, I.; Holm, N.V.; Vaupel, J.W. Genetic and environmental influences on functional abilities in Danish twins aged 75 years and older. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M446–M452. [Google Scholar] [CrossRef]
- Scott, D.; Blizzard, L.; Fell, J.; Ding, C.; Winzenberg, T.; Jones, G. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin. Endocrinol. 2010, 73, 581–587. [Google Scholar] [CrossRef]
- Tieland, M.; Brouwer-Brolsma, E.M.; Nienaber-Rousseau, C.; van Loon, L.J.; De Groot, L.C. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur. J. Clin. Nutr. 2013, 67, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- McKay, B.R.; Ogborn, D.I.; Bellamy, L.M.; Tarnopolsky, M.A.; Parise, G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 2012, 26, 2509–2521. [Google Scholar] [CrossRef] [PubMed]
- White, T.A.; LeBrasseur, N.K. Myostatin and sarcopenia: Opportunities and challenges—A mini-review. Gerontology 2014, 60, 289–293. [Google Scholar] [CrossRef]
- Wilhelmsen, A.; Stephens, F.B.; Bennett, A.J.; Karagounis, L.G.; Jones, S.W.; Tsintzas, K. Skeletal muscle myostatin mRNA expression is upregulated in aged human adults with excess adiposity but is not associated with insulin resistance and ageing. Geroscience 2024, 46, 2033–2049. [Google Scholar] [CrossRef]
- Muramatsu, H.; Kuramochi, T.; Katada, H.; Ueyama, A.; Ruike, Y.; Ohmine, K.; Shida-Kawazoe, M.; Miyano-Nishizawa, R.; Shimizu, Y.; Okuda, M.; et al. Novel myostatin-specific antibody enhances muscle strength in muscle disease models. Sci. Rep. 2021, 11, 2160. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.Y.; Ho Lim, J.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef]
- Wang, T.; Yu, M.; Li, H.; Qin, S.; Ren, W.; Ma, Y.; Bo, W.; Xi, Y.; Cai, M.; Tian, Z. FNDC5/Irisin Inhibits the Inflammatory Response and Mediates the Aerobic Exercise-Induced Improvement of Liver Injury after Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 4159. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J. Cachexia Sarcopenia Muscle 2023, 14, 391–405. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.M.; Sim, C.M.; Subramaniyam, N.; Ge, X.; Sharma, M.; Kambadur, R.; McFarlane, C. Irisin treatment improves healing of dystrophic skeletal muscle. Oncotarget 2017, 8, 98553–98566. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y.; Yu, J.; Zhang, Y.; Li, Y.; Fu, R.; Sun, Y.; Zhao, K.; Xiao, Q. Irisin ameliorates D-galactose-induced skeletal muscle fibrosis via the PI3K/Akt pathway. Eur. J. Pharmacol. 2023, 939, 175476. [Google Scholar] [CrossRef]
- Nishikawa, H.; Nakamura, S.; Miyazaki, T.; Kakimoto, K.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Inflammatory Bowel Disease and Sarcopenia: Its Mechanism and Clinical Importance. J. Clin. Med. 2021, 10, 4214. [Google Scholar] [CrossRef]
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Gerges Geagea, A.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2016, 160, 461–466. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Seo, M.H.; Kim, M.K.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Lee, W.Y.; Baek, K.H.; Song, K.H.; Kang, M.I.; Oh, K.W. The association between daily calcium intake and sarcopenia in older, non-obese Korean adults: The fourth Korea National Health and Nutrition Examination Survey (KNHANES IV) 2009. Endocr. J. 2013, 60, 679–686. [Google Scholar] [CrossRef]
- Waters, D.L.; Wayne, S.J.; Andrieu, S.; Cesari, M.; Villareal, D.T.; Garry, P.; Vellas, B. Sexually dimorphic patterns of nutritional intake and eating behaviors in community-dwelling older adults with normal and slow gait speed. J. Nutr. Health Aging 2014, 18, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.C.; Van Remmen, H. Age-associated alterations of the neuromuscular junction. Exp. Gerontol. 2011, 46, 193–198. [Google Scholar] [CrossRef]
- Gillon, A.; Nielsen, K.; Steel, C.; Cornwall, J.; Sheard, P. Exercise attenuates age-associated changes in motoneuron number, nucleocytoplasmic transport proteins and neuromuscular health. Geroscience 2018, 40, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Auyeung, T.W.; Kwok, T.; Lau, E.M.; Leung, P.C.; Woo, J. Associated factors and health impact of sarcopenia in older chinese men and women: A cross-sectional study. Gerontology 2007, 53, 404–410. [Google Scholar] [CrossRef]
- McMahon, C.D.; Chai, R.; Radley-Crabb, H.G.; Watson, T.; Matthews, K.G.; Sheard, P.W.; Soffe, Z.; Grounds, M.D.; Shavlakadze, T. Lifelong exercise and locally produced insulin-like growth factor-1 (IGF-1) have a modest influence on reducing age-related muscle wasting in mice. Scand. J. Med. Sci. Sports 2014, 24, e423–e435. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Morales, J.S.; Pareja-Galeano, H.; Izquierdo, M.; Emanuele, E.; de la Villa, P.; Lucia, A. Physical strategies to prevent disuse-induced functional decline in the elderly. Ageing Res. Rev. 2018, 47, 80–88. [Google Scholar] [CrossRef]
- Stearns-Reider, K.M.; D’Amore, A.; Beezhold, K.; Rothrauff, B.; Cavalli, L.; Wagner, W.R.; Vorp, D.A.; Tsamis, A.; Shinde, S.; Zhang, C.; et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 2017, 16, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, S.; Pei, Y.A.; Pei, M. Nidogen: A matrix protein with potential roles in musculoskeletal tissue regeneration. Genes Dis. 2022, 9, 598–609. [Google Scholar] [CrossRef]
- Du, F.; Shusta, E.V.; Palecek, S.P. Extracellular matrix proteins in construction and function of in vitro blood-brain barrier models. Front. Chem. Eng. 2023, 5, 1130127. [Google Scholar] [CrossRef]
- Loreti, M.; Sacco, A. The jam session between muscle stem cells and the extracellular matrix in the tissue microenvironment. npj Regen. Med. 2022, 7, 16. [Google Scholar] [CrossRef]
- Freitas-Rodriguez, S.; Folgueras, A.R.; Lopez-Otin, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Chen, W.J.; Lin, I.H.; Lee, C.W.; Chen, Y.F. Aged Skeletal Muscle Retains the Ability to Remodel Extracellular Matrix for Degradation of Collagen Deposition after Muscle Injury. Int. J. Mol. Sci. 2021, 22, 2123. [Google Scholar] [CrossRef]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef]
- Lukjanenko, L.; Jung, M.J.; Hegde, N.; Perruisseau-Carrier, C.; Migliavacca, E.; Rozo, M.; Karaz, S.; Jacot, G.; Schmidt, M.; Li, L.; et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat. Med. 2016, 22, 897–905. [Google Scholar] [CrossRef]
- Lee, E.J.; Jan, A.T.; Baig, M.H.; Ashraf, J.M.; Nahm, S.S.; Kim, Y.W.; Park, S.Y.; Choi, I. Fibromodulin: A master regulator of myostatin controlling progression of satellite cells through a myogenic program. FASEB J. 2016, 30, 2708–2719. [Google Scholar] [CrossRef]
- Lee, E.J.; Jan, A.T.; Baig, M.H.; Ahmad, K.; Malik, A.; Rabbani, G.; Kim, T.; Lee, I.K.; Lee, Y.H.; Park, S.Y.; et al. Fibromodulin and regulation of the intricate balance between myoblast differentiation to myocytes or adipocyte-like cells. FASEB J. 2018, 32, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Ahmad, S.; Lim, J.; Ahmad, K.; Shaikh, S.; Lee, Y.; Park, S.; Jin, J.; Lee, Y.; Choi, I. Interaction of fibromodulin and myostatin to regulate skeletal muscle aging: An opposite regulation in muscle aging, diabetes, and intracellular lipid accumulation. Cells 2021, 10, 2083. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Jan, A.T.; Baig, M.H.; Lee, E.J.; Choi, I. Matrix gla protein: An extracellular matrix protein regulates myostatin expression in the muscle developmental program. Life Sci. 2017, 172, 55–63. [Google Scholar] [CrossRef]
- Lee, E.-J.; Ahmad, K.; Pathak, S.; Lee, S.; Baig, M.H.; Jeong, J.-H.; Doh, K.-O.; Lee, D.-M.; Choi, I. Identification of Novel FNIN2 and FNIN3 Fibronectin-derived peptides that promote cell adhesion, proliferation and differentiation in primary cells and stem cells. Int. J. Mol. Sci. 2021, 22, 3042. [Google Scholar] [CrossRef]
- Kim, T.; Ahmad, K.; Shaikh, S.; Jan, A.T.; Seo, M.G.; Lee, E.J.; Choi, I. Dermatopontin in Skeletal Muscle Extracellular Matrix Regulates Myogenesis. Cells 2019, 8, 332. [Google Scholar] [CrossRef]
- Ahmad, K.; Shaikh, S.; Chun, H.J.; Ali, S.; Lim, J.H.; Ahmad, S.S.; Lee, E.J.; Choi, I. Extracellular matrix: The critical contributor to skeletal muscle regeneration—A comprehensive review. Inflamm. Regen. 2023, 43, 58. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Lim, J.H.; Shaikh, S.; Lee, E.J.; Choi, I. Therapeutic applications of biological macromolecules and scaffolds for skeletal muscle regeneration: A review. Int. J. Biol. Macromol. 2024, 267, 131411. [Google Scholar] [CrossRef]
- Cai, L.; Shi, L.; Peng, Z.; Sun, Y.; Chen, J. Ageing of skeletal muscle extracellular matrix and mitochondria: Finding a potential link. Ann. Med. 2023, 55, 2240707. [Google Scholar] [CrossRef]
- Stammers, M.; Ivanova, I.M.; Niewczas, I.S.; Segonds-Pichon, A.; Streeter, M.; Spiegel, D.A.; Clark, J. Age-related changes in the physical properties, cross-linking, and glycation of collagen from mouse tail tendon. J. Biol. Chem. 2020, 295, 10562–10571. [Google Scholar] [CrossRef]
- Sparavigna, A. Role of the extracellular matrix in skin aging and dedicated treatment-state of the art. Plast. Aesthetic Res. 2020, 7, 14. [Google Scholar] [CrossRef]
- Gao, J.; Guo, Z.; Zhang, Y.; Liu, Y.; Xing, F.; Wang, J.; Luo, X.; Kong, Y.; Zhang, G. Age-related changes in the ratio of Type I/III collagen and fibril diameter in mouse skin. Regen. Biomater. 2023, 10, rbac110. [Google Scholar] [CrossRef] [PubMed]
- Pavan, P.; Monti, E.; Bondi, M.; Fan, C.; Stecco, C.; Narici, M.; Reggiani, C.; Marcucci, L. Alterations of Extracellular Matrix Mechanical Properties Contribute to Age-Related Functional Impairment of Human Skeletal Muscles. Int. J. Mol. Sci. 2020, 21, 3992. [Google Scholar] [CrossRef]
- Wetzlich, B.; Nyakundi, B.B.; Yang, J. Therapeutic applications and challenges in myostatin inhibition for enhanced skeletal muscle mass and functions. Mol. Cell. Biochem. 2025, 480, 1535–1553. [Google Scholar] [CrossRef] [PubMed]
- Emmelot-Vonk, M.H.; Verhaar, H.J.; Nakhai Pour, H.R.; Aleman, A.; Lock, T.M.; Bosch, J.L.; Grobbee, D.E.; van der Schouw, Y.T. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: A randomized controlled trial. JAMA 2008, 299, 39–52. [Google Scholar] [CrossRef]
- Srinivas-Shankar, U.; Roberts, S.A.; Connolly, M.J.; O’Connell, M.D.; Adams, J.E.; Oldham, J.A.; Wu, F.C. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: A randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2010, 95, 639–650. [Google Scholar] [CrossRef]
- Basaria, S.; Coviello, A.D.; Travison, T.G.; Storer, T.W.; Farwell, W.R.; Jette, A.M.; Eder, R.; Tennstedt, S.; Ulloor, J.; Zhang, A.; et al. Adverse events associated with testosterone administration. N. Engl. J. Med. 2010, 363, 109–122. [Google Scholar] [CrossRef]
- Becker, C.; Lord, S.R.; Studenski, S.A.; Warden, S.J.; Fielding, R.A.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Padhi, D.; Higano, C.S.; Shore, N.D.; Sieber, P.; Rasmussen, E.; Smith, M.R. Pharmacological inhibition of myostatin and changes in lean body mass and lower extremity muscle size in patients receiving androgen deprivation therapy for prostate cancer. J. Clin. Endocrinol. Metab. 2014, 99, E1967–E1975. [Google Scholar] [CrossRef]
- Attie, K.M.; Borgstein, N.G.; Yang, Y.; Condon, C.H.; Wilson, D.M.; Pearsall, A.E.; Kumar, R.; Willins, D.A.; Seehra, J.S.; Sherman, M.L. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve 2013, 47, 416–423. [Google Scholar] [CrossRef]
- Rooks, D.; Praestgaard, J.; Hariry, S.; Laurent, D.; Petricoul, O.; Perry, R.G.; Lach-Trifilieff, E.; Roubenoff, R. Treatment of Sarcopenia with Bimagrumab: Results from a Phase II, Randomized, Controlled, Proof-of-Concept Study. J. Am. Geriatr. Soc. 2017, 65, 1988–1995. [Google Scholar] [CrossRef]
- Egerman, M.A.; Cadena, S.M.; Gilbert, J.A.; Meyer, A.; Nelson, H.N.; Swalley, S.E.; Mallozzi, C.; Jacobi, C.; Jennings, L.L.; Clay, I.; et al. GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration. Cell Metab. 2015, 22, 164–174. [Google Scholar] [CrossRef]
- Hammers, D.W.; Merscham-Banda, M.; Hsiao, J.Y.; Engst, S.; Hartman, J.J.; Sweeney, H.L. Supraphysiological levels of GDF11 induce striated muscle atrophy. EMBO Mol. Med. 2017, 9, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Zimmers, T.A.; Jiang, Y.; Wang, M.; Liang, T.W.; Rupert, J.E.; Au, E.D.; Marino, F.E.; Couch, M.E.; Koniaris, L.G. Exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting. Basic Res. Cardiol. 2017, 112, 48. [Google Scholar] [CrossRef] [PubMed]
- Biesemann, N.; Mendler, L.; Wietelmann, A.; Hermann, S.; Schafers, M.; Kruger, M.; Boettger, T.; Borchardt, T.; Braun, T. Myostatin regulates energy homeostasis in the heart and prevents heart failure. Circ. Res. 2014, 115, 296–310. [Google Scholar] [CrossRef]
- Rodgers, B.D.; Interlichia, J.P.; Garikipati, D.K.; Mamidi, R.; Chandra, M.; Nelson, O.L.; Murry, C.E.; Santana, L.F. Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling. J. Physiol. 2009, 587, 4873–4886. [Google Scholar] [CrossRef]
- Siriett, V.; Salerno, M.S.; Berry, C.; Nicholas, G.; Bower, R.; Kambadur, R.; Sharma, M. Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol. Ther. 2007, 15, 1463–1470. [Google Scholar] [CrossRef]
- Dewasi, G.; Nagda, P.; Bahl, G.; Jain, V.; Gupta, S.K. Myostatin-driven muscle hypertrophy: A double-edged sword in muscle physiology. J. Rare Dis. 2025, 4, 29. [Google Scholar] [CrossRef]
- Lee, E.J.; Shaikh, S.; Choi, D.; Ahmad, K.; Baig, M.H.; Lim, J.H.; Lee, Y.H.; Park, S.J.; Kim, Y.W.; Park, S.Y.; et al. Transthyretin Maintains Muscle Homeostasis Through the Novel Shuttle Pathway of Thyroid Hormones During Myoblast Differentiation. Cells 2019, 8, 1565. [Google Scholar] [CrossRef]
- Shaikh, S.; Ali, S.; Lim, J.H.; Chun, H.J.; Ahmad, K.; Ahmad, S.S.; Hwang, Y.C.; Han, K.S.; Kim, N.R.; Lee, E.J.; et al. Dipeptidyl peptidase-4 inhibitory potentials of Glycyrrhiza uralensis and its bioactive compounds licochalcone A and licochalcone B: An in silico and in vitro study. Front. Mol. Biosci. 2022, 9, 1024764. [Google Scholar] [CrossRef]
- Ali, S.; Ahmad, K.; Shaikh, S.; Chun, H.J.; Choi, I.; Lee, E.J. Mss51 protein inhibition serves as a novel target for type 2 diabetes: A molecular docking and simulation study. J. Biomol. Struct. Dyn. 2024, 42, 4862–4869. [Google Scholar] [CrossRef]
- Ali, S.; Ahmad, K.; Shaikh, S.; Lim, J.H.; Chun, H.J.; Ahmad, S.S.; Lee, E.J.; Choi, I. Identification and Evaluation of Traditional Chinese Medicine Natural Compounds as Potential Myostatin Inhibitors: An In Silico Approach. Molecules 2022, 27, 4303. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Shaikh, S.; Choi, I. Computational Identification of Dithymoquinone as a Potential Inhibitor of Myostatin and Regulator of Muscle Mass. Molecules 2021, 26, 5407. [Google Scholar] [CrossRef] [PubMed]
- Fatima Qadri, A.; Shaikh, S.; Chan Hwang, Y.; Ahmad, K.; Choi, I.; Ju Lee, E. Effect of Glycyrrhiza uralensis crude water extract on the expression of Nitric Oxide Synthase 2 gene during myogenesis. Heliyon 2024, 10, e34747. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Lim, J.H.; Choi, I.; Lee, E.J. Biocomputational screening of natural compounds targeting 15-hydroxyprostaglandin dehydrogenase to improve skeletal muscle during aging. Mol. Divers. 2024, 28, 4425–4439. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Chun, H.J.; Ahmad, K.; Choi, I. Therapeutic applications of ginseng for skeletal muscle-related disorder management. J. Ginseng Res. 2024, 48, 12–19. [Google Scholar] [CrossRef]
- Ahmad, K.; Shaikh, S.; Lim, J.H.; Ahmad, S.S.; Chun, H.J.; Lee, E.J.; Choi, I. Therapeutic application of natural compounds for skeletal muscle-associated metabolic disorders: A review on diabetes perspective. Biomed. Pharmacother. 2023, 168, 115642. [Google Scholar] [CrossRef]
- Shaikh, S.; Ahmad, K.; Lim, J.H.; Ahmad, S.S.; Lee, E.J.; Choi, I. Biological insights and therapeutic potential of Glycyrrhiza uralensis and its bioactive compounds: An updated review. Arch. Pharmacal Res. 2024, 47, 871–892. [Google Scholar] [CrossRef]
- Shaikh, S.; Lee, E.J.; Ahmad, K.; Choi, I. Therapeutic potential and action mechanisms of licochalcone B: A mini review. Front. Mol. Biosci. 2024, 11, 1440132. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Zhao, R.; Wang, Y.; Zhang, W.; Pang, W. Isoleucine increases muscle mass through promoting myogenesis and intramyocellular fat deposition. Food Funct. 2021, 12, 144–153. [Google Scholar] [CrossRef]
- Lee, E.J.; Shaikh, S.; Baig, M.H.; Park, S.Y.; Lim, J.H.; Ahmad, S.S.; Ali, S.; Ahmad, K.; Choi, I. MIF1 and MIF2 Myostatin Peptide Inhibitors as Potent Muscle Mass Regulators. Int. J. Mol. Sci. 2022, 23, 4222. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, S.; Cui, Y.; Tong, H.; Li, S.; Yan, Y. Podocan affects C2C12 myogenic differentiation by enhancing Wnt/beta-catenin signaling. J. Cell. Physiol. 2019, 234, 11130–11139. [Google Scholar] [CrossRef] [PubMed]
- Quintana, H.T.; Baptista, V.I.A.; Lazzarin, M.C.; Antunes, H.K.M.; Le Sueur-Maluf, L.; de Oliveira, C.A.M.; de Oliveira, F. Insulin Modulates Myogenesis and Muscle Atrophy Resulting From Skin Scald Burn in Young Male Rats. J. Surg. Res. 2021, 257, 56–68. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chang, C.F.; Angayarkanni, J.; Lin, W.T. Alcalase Potato Protein Hydrolysate-PPH902 Enhances Myogenic Differentiation and Enhances Skeletal Muscle Protein Synthesis under High Glucose Condition in C2C12 Cells. Molecules 2021, 26, 6577. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Baskaran, R.; Chang, C.F.; Mohammedsaleh, Z.M.; Lin, W.T. Decapeptide from Potato Hydrolysate Induces Myogenic Differentiation and Ameliorates High Glucose-Associated Modulations in Protein Synthesis and Mitochondrial Biogenesis in C2C12 Cells. Biomolecules 2022, 12, 565. [Google Scholar] [CrossRef]
- Zhou, M.; Wei, Y.; Feng, Y.; Zhang, S.; Ma, N.; Wang, K.; Tan, P.; Zhao, Y.; Zhao, J.; Ma, X. Arginine Regulates Skeletal Muscle Fiber Type Formation via mTOR Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 6184. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Zhou, C.; Wang, M.; Wang, H.; Ding, H.; Cheng, L.; Gan, L.; Wu, X.; Du, Z. Thioredoxin mitigates H(2) O(2) -induced inhibition of myogenic differentiation of rat bone marrow mesenchymal stem cells by enhancing AKT activation. FEBS Open Bio 2020, 10, 835–846. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Liu, Q.; Wang, Y.; Li, S.; Xu, S. Selenoprotein K protects skeletal muscle from damage and is required for satellite cells-mediated myogenic differentiation. Redox Biol. 2022, 50, 102255. [Google Scholar] [CrossRef]
- Jang, J.H.; Jung, H.H.; Oh, N.S. Enhanced protective effect of whey protein fermented with Lacticaseibacillus rhamnosus IM36 on dexamethasone-induced myotube atrophy. Food Sci. Biotechnol. 2024, 33, 2243–2254. [Google Scholar] [CrossRef]
- Vallecillo-Zuniga, M.L.; Rathgeber, M.F.; Poulson, P.D.; Hayes, S.; Luddington, J.S.; Gill, H.N.; Teynor, M.; Kartchner, B.C.; Valdoz, J.; Stowell, C.; et al. Treatment with galectin-1 improves myogenic potential and membrane repair in dysferlin-deficient models. PLoS ONE 2020, 15, e0238441. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Shaikh, S.; Ahmad, K.; Ahmad, S.S.; Lim, J.H.; Park, S.; Yang, H.J.; Cho, W.K.; Park, S.J.; Lee, Y.H.; et al. Isolation and Characterization of Compounds from Glycyrrhiza uralensis as Therapeutic Agents for the Muscle Disorders. Int. J. Mol. Sci. 2021, 22, 876. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.B.; Lee, H.S.; Hong, J.S.; Kim, D.H.; Moon, J.M.; Park, Y. Effects of tannase-converted green tea extract on skeletal muscle development. BMC Complement. Med. Ther. 2020, 20, 47. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, J.Y.; Kwon, Y.H.; Lee, S.H.; Park, C.; Choi, Y.H.; Kim, N.D. Effects of Rosemary Extract on C2C12 Myoblast Differentiation and 5-Aminoimidazole-4-carboxamide Ribonucleoside (AICAR)-Induced Muscle Cell Atrophy. Appl. Sci. 2023, 13, 986. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jang, Y.N.; Han, Y.M.; Kim, H.M.; Seo, H.S.; Kim, H.J.; Jung, T.W.; Jeong, J.H.; Abd El-Aty, A.M.; Jung, K.O. Aster glehni Extract, Including Caffeoylquinic Acids as the Main Constituents, Induces PPAR beta/delta-Dependent Muscle-Type Change and Myogenesis in Apolipoprotein E Knockout Mice. J. Med. Food 2024, 27, 521–532. [Google Scholar] [CrossRef]
- Kametaka, S.; Isobe, M.; Komata, K.; Morinaga, M.; Nagahata, K.; Lee-Hotta, S.; Uchiyama, Y.; Shibata, M.; Sugiura, H. Protective effects of hachimijiogan (HJG), a Japanese Kampo medicine, on cancer cachectic muscle wasting in mice. Biomed. Res. 2023, 44, 199–207. [Google Scholar] [CrossRef]
- Eun, S.Y.; Chung, C.H.; Cheon, Y.H.; Park, G.D.; Lee, C.H.; Kim, J.Y.; Lee, M.S. Saururus chinensis (Lour.) Baill. extract promotes skeletal muscle cell differentiation by positively regulating mitochondrial biogenesis and AKT/mTOR signaling in vitro. Mol. Med. Rep. 2024, 30, 125. [Google Scholar] [CrossRef]
- Sharma, B.; Dutt, V.; Kaur, N.; Mittal, A.; Dabur, R. Tinospora cordifolia protects from skeletal muscle atrophy by alleviating oxidative stress and inflammation induced by sciatic denervation. J. Ethnopharmacol. 2020, 254, 112720. [Google Scholar] [CrossRef]
- Cheon, Y.H.; Lee, C.H.; Chung, C.H.; Kim, J.Y.; Lee, M.S. Vigeo Promotes Myotube Differentiation and Protects Dexamethasone-Induced Skeletal Muscle Atrophy via Regulating the Protein Degradation, AKT/mTOR, and AMPK/Sirt-1/PGC1alpha Signaling Pathway In Vitro and In Vivo. Nutrients 2024, 16, 2687. [Google Scholar] [CrossRef]
- Kim, S.H.; Leem, Y.E.; Park, H.E.; Jeong, H.I.; Lee, J.; Kang, J.S. The Extract of Gloiopeltis tenax Enhances Myogenesis and Alleviates Dexamethasone-Induced Muscle Atrophy. Int. J. Mol. Sci. 2024, 25, 6806. [Google Scholar] [CrossRef]
- Yeo, C.; Kim, H.; Jeon, W.J.; Lee, J.; Hong, J.Y.; Kim, H.; Lee, Y.J.; Baek, S.H.; Ha, I.H. Protective effect of Luffa cylindrica Roemer against dexamethasone-induced muscle atrophy in primary rat skeletal muscle cells. J. Muscle Res. Cell Motil. 2024, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liang, L.; Zhang, W.; Liu, X.; Huo, G.; Liu, X.; Lv, X.; Zhao, J. Sea buckthorn oil regulates primary myoblasts proliferation and differentiation in vitro. Vitr. Cell. Dev. Biol. Anim. 2024, 60, 139–150. [Google Scholar] [CrossRef]
- Kang, Y.G.; Kwon, J.; Kwon, S.; Kim, A.R. Synergistic Effects of Korean Mistletoe and Apple Peel Extracts on Muscle Strength and Endurance. Nutrients 2024, 16, 3255. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, J.W.; Kim, J.H.; Kim, C.Y.; Ko, J.W.; Kim, T.W. Korean red ginseng suppresses mitochondrial apoptotic pathway in denervation-induced skeletal muscle atrophy. J. Ginseng Res. 2024, 48, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Han, X.; La, I.J.; Lee, S.J.; Oh, G.; Im, J.H.; Fu, X.; Lim, J.S.; Bae, K.S.; Seong, G.S.; et al. Ameliorative Effects of Fermented Red Ginseng Extract on Muscle Atrophy in Dexamethasone-Induced C2C12 Cell And Hind Limb-Immobilized C57BL/6J Mice. J. Med. Food 2024, 27, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.S.; Chang, B.Y.; Choi, Y.J.; Choi, J.S.; Kwon, H.Y.; Lee, J.Y.; Kim, S.Y.; Choung, S.Y. Ashwagandha Ethanol Extract Attenuates Sarcopenia-Related Muscle Atrophy in Aged Mice. Nutrients 2024, 16, 157. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.J.; Seo, D.; Lee, S.A.; Heo, J.B.; Baek, D.H.; Lee, W.; Song, G.Y. Steamed Ginseng Berry Powder Ameliorates Skeletal Muscle Atrophy via Myogenic Effects. J. Microbiol. Biotechnol. 2024, 34, 157–166. [Google Scholar] [CrossRef]
- Lee, H.; Heo, J.W.; Kim, A.R.; Kweon, M.; Nam, S.; Lim, J.S.; Sung, M.K.; Kim, S.E.; Ryu, J.H. Z-ajoene from Crushed Garlic Alleviates Cancer-Induced Skeletal Muscle Atrophy. Nutrients 2019, 11, 2724. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Lee, E.J.; Ali, S.; Han, K.S.; Hur, S.J.; Lim, J.H.; Choi, I. Licochalcone A and B enhance muscle proliferation and differentiation by regulating Myostatin. Phytomedicine 2024, 125, 155350. [Google Scholar] [CrossRef]
- Li, P.; Liu, A.; Liu, C.; Qu, Z.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Role and mechanism of catechin in skeletal muscle cell differentiation. J. Nutr. Biochem. 2019, 74, 108225. [Google Scholar] [CrossRef]
- Mirza, K.A.; Pereira, S.L.; Edens, N.K.; Tisdale, M.J. Attenuation of muscle wasting in murine C2C 12 myotubes by epigallocatechin-3-gallate. J. Cachexia Sarcopenia Muscle 2014, 5, 339–345. [Google Scholar] [CrossRef]
- Kim, A.R.; Kim, K.M.; Byun, M.R.; Hwang, J.H.; Park, J.I.; Oh, H.T.; Kim, H.K.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. Catechins activate muscle stem cells by Myf5 induction and stimulate muscle regeneration. Biochem. Biophys. Res. Commun. 2017, 489, 142–148. [Google Scholar] [CrossRef]
- Chang, W.-T.; Chen, C.-S.; Cheng, M.-C.; Wu, M.-F.; Cheng, F.-T.; Hsu, C.-L. Effects of resveratrol, epigallocatechin gallate, and epicatechin on mitochondrial functions in C2C12 myotubes. J. Funct. Foods 2017, 35, 507–512. [Google Scholar] [CrossRef]
- Hemdan, D.I.; Hirasaka, K.; Nakao, R.; Kohno, S.; Kagawa, S.; Abe, T.; Harada-Sukeno, A.; Okumura, Y.; Nakaya, Y.; Terao, J.; et al. Polyphenols prevent clinorotation-induced expression of atrogenes in mouse C2C12 skeletal myotubes. J. Med. Investig. 2009, 56, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Leem, Y.E.; Go, G.Y.; Choi, Y.; Song, Y.J.; Kim, I.; Kim, D.Y.; Kim, Y.K.; Seo, D.W.; Kang, J.S.; et al. Epicatechin elicits MyoD-dependent myoblast differentiation and myogenic conversion of fibroblasts. PLoS ONE 2017, 12, e0175271. [Google Scholar] [CrossRef]
- Wang, M.Y.; Yang, J.M.; Wu, Y.; Li, H.; Zhong, Y.B.; Luo, Y.; Xie, R.L. Curcumin-activated Wnt5a pathway mediates Ca2+ channel opening to affect myoblast differentiation and skeletal muscle regeneration. J. Cachexia Sarcopenia Muscle 2024, 15, 1834–1849. [Google Scholar] [CrossRef]
- Abalenikhina, Y.V.; Isayeva, M.O.; Mylnikov, P.Y.; Shchulkin, A.V.; Yakusheva, E.N. Mechanism of Stimulation of Myogenesis under the Action of Succinic Acid through the Succinate Receptor SUCNR1. Biochemistry 2024, 89, 1325–1335. [Google Scholar] [CrossRef]
- Haddish, K.; Yun, J.W. Echinacoside stimulates myogenesis and ATP-dependent thermogenesis in the skeletal muscle via the activation of D1-like dopaminergic receptors. Arch. Biochem. Biophys. 2024, 752, 109886. [Google Scholar] [CrossRef]
- Mukai, R.; Horikawa, H.; Lin, P.Y.; Tsukumo, N.; Nikawa, T.; Kawamura, T.; Nemoto, H.; Terao, J. 8-Prenylnaringenin promotes recovery from immobilization-induced disuse muscle atrophy through activation of the Akt phosphorylation pathway in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1022–R1031. [Google Scholar] [CrossRef]
- Mukai, R.; Horikawa, H.; Fujikura, Y.; Kawamura, T.; Nemoto, H.; Nikawa, T.; Terao, J. Prevention of disuse muscle atrophy by dietary ingestion of 8-prenylnaringenin in denervated mice. PLoS ONE 2012, 7, e45048. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.H.; Kang, N.; Kim, K.N.; Jeon, Y.J. The Effects of Marine Algal Polyphenols, Phlorotannins, on Skeletal Muscle Growth in C2C12 Muscle Cells via Smad and IGF-1 Signaling Pathways. Mar. Drugs 2021, 19, 266. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, K.; Ren, Q.; Yi, L.; Zhu, J.; Zhang, Q.; Mi, M. Dihydromyricetin Attenuates Dexamethasone-Induced Muscle Atrophy by Improving Mitochondrial Function via the PGC-1alpha Pathway. Cell. Physiol. Biochem. 2018, 49, 758–779. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yang, X.; Li, J.; Shu, Z.; Dai, J.; Liu, X.; Li, B.; Jia, S.; Kou, X.; Yang, Y.; et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Li, J.; Liu, X.; Yang, X.; Fan, J.; Chen, N. Ampelopsin attenuates the atrophy of skeletal muscle from d-gal-induced aging rats through activating AMPK/SIRT1/PGC-1alpha signaling cascade. Biomed. Pharmacother. 2017, 90, 311–320. [Google Scholar] [CrossRef]
- Scioli, M.G.; Coniglione, F.; Greggi, C.; Evangelista, L.; Fiorelli, E.; Savino, L.; Ferlosio, A.; Piccirilli, E.; Gasbarra, E.; Iundusi, R.; et al. Ascorbic acid reduces Ropivacaine-induced myotoxicity in cultured human osteoporotic skeletal muscle cells. BMC Musculoskelet Disord. 2023, 24, 576. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, X.; Tian, L.; Zhao, Y.; Yan, Y.; Li, S.; Li, S.; Tong, H. Vitamin C Promotes Muscle Development Mediated by the Interaction of CSRP3 with MyoD and MyoG. J. Agric. Food Chem. 2022, 70, 7158–7169. [Google Scholar] [CrossRef]
- Diao, Z.; Matsui, T.; Funaba, M. Stimulation of myogenesis by ascorbic acid and capsaicin. Biochem. Biophys. Res. Commun. 2021, 568, 83–88. [Google Scholar] [CrossRef]
- Shukla, S.K.; Dasgupta, A.; Mehla, K.; Gunda, V.; Vernucci, E.; Souchek, J.; Goode, G.; King, R.; Mishra, A.; Rai, I.; et al. Silibinin-mediated metabolic reprogramming attenuates pancreatic cancer-induced cachexia and tumor growth. Oncotarget 2015, 6, 41146–41161. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Yoshizaki, H.; Toriumi, A.; Kawaharada, R.; Ishida, C.; Hori, M.; Nakamura, A. Effects of omega-7 palmitoleic acids on skeletal muscle differentiation in a hyperglycemic condition. J. Vet. Med. Sci. 2021, 83, 1369–1377. [Google Scholar] [CrossRef]
- Watanabe, N.; Komiya, Y.; Sato, Y.; Watanabe, Y.; Suzuki, T.; Arihara, K. Oleic acid up-regulates myosin heavy chain (MyHC) 1 expression and increases mitochondrial mass and maximum respiration in C2C12 myoblasts. Biochem. Biophys. Res. Commun. 2020, 525, 406–411. [Google Scholar] [CrossRef]
- Komiya, Y.; Iseki, S.; Ochiai, M.; Takahashi, Y.; Yokoyama, I.; Suzuki, T.; Tatsumi, R.; Sawano, S.; Mizunoya, W.; Arihara, K. Dietary oleic acid intake increases the proportion of type 1 and 2X muscle fibers in mice. Sci. Rep. 2024, 14, 755. [Google Scholar] [CrossRef]
- Kim, C.; Hwang, J.K. The 5,7-Dimethoxyflavone Suppresses Sarcopenia by Regulating Protein Turnover and Mitochondria Biogenesis-Related Pathways. Nutrients 2020, 12, 1079. [Google Scholar] [CrossRef]
- Toda, K.; Hitoe, S.; Takeda, S.; Shimoda, H. Black ginger extract increases physical fitness performance and muscular endurance by improving inflammation and energy metabolism. Heliyon 2016, 2, e00115. [Google Scholar] [CrossRef] [PubMed]
- Shiota, C.; Abe, T.; Kawai, N.; Ohno, A.; Teshima-Kondo, S.; Mori, H.; Terao, J.; Tanaka, E.; Nikawa, T. Flavones Inhibit LPS-Induced Atrogin-1/MAFbx Expression in Mouse C2C12 Skeletal Myotubes. J. Nutr. Sci. Vitaminol. 2015, 61, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Son, H.J.; Jang, Y.J.; Ahn, J.; Jung, C.H.; Ha, T.Y. Apigenin Ameliorates the Obesity-Induced Skeletal Muscle Atrophy by Attenuating Mitochondrial Dysfunction in the Muscle of Obese Mice. Mol. Nutr. Food Res. 2017, 61, 1700218. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Jang, Y.J.; Son, H.J.; Ahn, J.; Jung, C.H.; Ha, T.Y. Apigenin inhibits sciatic nerve denervation-induced muscle atrophy. Muscle Nerve 2018, 58, 314–318. [Google Scholar] [CrossRef]
- Li, B.; Wan, L.; Li, Y.; Yu, Q.; Chen, P.; Gan, R.; Yang, Q.; Han, Y.; Guo, C. Baicalin, a component of Scutellaria baicalensis, alleviates anorexia and inhibits skeletal muscle atrophy in experimental cancer cachexia. Tumour Biol. 2014, 35, 12415–12425. [Google Scholar] [CrossRef]
- Emanuele, E.; Bertona, M.; Pareja-Galeano, H.; Fiuza-Luces, C.; Morales, J.S.; Sanchis-Gomar, F.; Lucia, A. Baicalin supplementation reduces serum biomarkers of skeletal muscle wasting and may protect against lean body mass reduction in cancer patients: Results from a pilot open-label study. Neuro Endocrinol. Lett. 2016, 37, 213–216. [Google Scholar]
- Wu, Z.; Xu, H.; Xu, Y.; Fan, W.; Yao, H.; Wang, Y.; Hu, W.; Lou, G.; Shi, Y.; Chen, X.; et al. Andrographolide promotes skeletal muscle regeneration after acute injury through epigenetic modulation. Eur. J. Pharmacol. 2020, 888, 173470. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, B.; Xu, Y.; Meng, S.; Wang, Y.; Jiang, Y. Luteolin reduces cancer-induced skeletal and cardiac muscle atrophy in a Lewis lung cancer mouse model. Oncol. Rep. 2018, 40, 1129–1137. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, S.M.; Ha, S.E.; Vetrivel, P.; Saralamma, V.V.G.; Kim, E.H.; Kim, G.S. Sinensetin regulates age-related sarcopenia in cultured primary thigh and calf muscle cells. BMC Complement. Altern. Med. 2019, 19, 287. [Google Scholar] [CrossRef]
- Toti, E.; Chen, C.O.; Palmery, M.; Villano Valencia, D.; Peluso, I. Non-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxidative Med. Cell. Longev. 2018, 2018, 4637861. [Google Scholar] [CrossRef]
- Ogawa, M.; Kariya, Y.; Kitakaze, T.; Yamaji, R.; Harada, N.; Sakamoto, T.; Hosotani, K.; Nakano, Y.; Inui, H. The preventive effect of beta-carotene on denervation-induced soleus muscle atrophy in mice. Br. J. Nutr. 2013, 109, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Aoi, W.; Abe, R.; Kobayashi, Y.; Wada, S.; Kuwahata, M.; Higashi, A. Combined intake of astaxanthin, beta-carotene, and resveratrol elevates protein synthesis during muscle hypertrophy in mice. Nutrition 2020, 69, 110561. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Oh, Y.; Kim, Y.S.; Shin, J.H.; Lee, Y.S.; Kim, Y. beta-carotene attenuates muscle wasting in cancer cachexia by regulating myogenesis and muscle atrophy. Oncol. Rep. 2024, 51, 1–12. [Google Scholar] [CrossRef]
- Yoshimura, T.; Saitoh, K.; Sun, L.; Wang, Y.; Taniyama, S.; Yamaguchi, K.; Uchida, T.; Ohkubo, T.; Higashitani, A.; Nikawa, T.; et al. Morin suppresses cachexia-induced muscle wasting by binding to ribosomal protein S10 in carcinoma cells. Biochem. Biophys. Res. Commun. 2018, 506, 773–779. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Lim, J.H.; Ahmad, K.; Chun, H.J.; Hur, S.J.; Lee, E.J.; Choi, I. Targeting myostatin using quercetin as a media supplement to improve myogenesis for cultured meat production: An in silico and in vitro study. Curr. Res. Food Sci. 2024, 8, 100678. [Google Scholar] [CrossRef]
- Hour, T.C.; Vo, T.C.T.; Chuu, C.P.; Chang, H.W.; Su, Y.F.; Chen, C.H.; Chen, Y.K. The Promotion of Migration and Myogenic Differentiation in Skeletal Muscle Cells by Quercetin and Underlying Mechanisms. Nutrients 2022, 14, 4106. [Google Scholar] [CrossRef]
- Chen, C.; Yang, J.S.; Lu, C.C.; Chiu, Y.J.; Chen, H.C.; Chung, M.I.; Wu, Y.T.; Chen, F.A. Effect of Quercetin on Dexamethasone-Induced C2C12 Skeletal Muscle Cell Injury. Molecules 2020, 25, 3267. [Google Scholar] [CrossRef]
- Otsuka, Y.; Egawa, K.; Kanzaki, N.; Izumo, T.; Rogi, T.; Shibata, H. Quercetin glycosides prevent dexamethasone-induced muscle atrophy in mice. Biochem. Biophys. Rep. 2019, 18, 100618. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, C.S.; Joe, Y.; Chung, H.T.; Ha, T.Y.; Yu, R. Quercetin Reduces Tumor Necrosis Factor Alpha-Induced Muscle Atrophy by Upregulation of Heme Oxygenase-1. J. Med. Food. 2018, 21, 551–559. [Google Scholar] [CrossRef]
- Rehfeldt, C.; Kalbe, C.; Nurnberg, G.; Mau, M. Dose-dependent effects of genistein and daidzein on protein metabolism in porcine myotube cultures. J. Agric. Food Chem. 2009, 57, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jiang, S.; Lin, Y.; Ma, X.; Xi, P.; Cao, T.; Wang, X. Effect of genistein on antioxidative defence system and membrane fluidity in chick skeletal muscle cells. Asian-Australas. J. Anim. Sci. 2008, 21, 1220–1225. [Google Scholar] [CrossRef]
- Gan, M.; Shen, L.; Liu, L.; Guo, Z.; Wang, S.; Chen, L.; Zheng, T.; Fan, Y.; Tan, Y.; Jiang, D.; et al. miR-222 is involved in the regulation of genistein on skeletal muscle fiber type. J. Nutr. Biochem. 2020, 80, 108320. [Google Scholar] [CrossRef]
- Shen, L.; Liao, T.; Chen, J.; Ma, J.; Wang, J.; Chen, L.; Zhang, S.; Zhao, Y.; Niu, L.; Zeng, C.; et al. Genistein Promotes Skeletal Muscle Regeneration by Regulating miR-221/222. Int. J. Mol. Sci. 2022, 23, 13482. [Google Scholar] [CrossRef]
- Lin, M.; Zhou, S.; Sakamoto, K. Alpha Mangostin promotes myogenic differentiation of C2C12 mouse myoblast cells. Biochem. Biophys. Res. Commun. 2020, 528, 193–198. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Yamashita, Y.; Kishida, H.; Nakagawa, K.; Ashida, H. Licorice flavonoid oil enhances muscle mass in KK-A(y) mice. Life Sci. 2018, 205, 91–96. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kubota, Y.; Samukawa, Y.; Yamashita, Y.; Ashida, H. Glabridin inhibits dexamethasone-induced muscle atrophy. Arch. Biochem. Biophys. 2019, 664, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lee, H.; Li, H.; Ryu, J.H. Corylifol A from Psoralea corylifolia L. Enhances Myogenesis and Alleviates Muscle Atrophy. Int. J. Mol. Sci. 2020, 21, 1571. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Tsai, C.H.; Chen, H.T.; Wu, Y.S.; Chang, J.W.; Yang, S.F.; Tang, C.H. Melatonin improves muscle injury and differentiation by increasing Pax7 expression. Int. J. Biol. Sci. 2023, 19, 1049–1062. [Google Scholar] [CrossRef]
- Lee, S.-J.; Vuong, T.A.; Go, G.-Y.; Song, Y.-J.; Lee, S.; Lee, S.-Y.; Kim, S.-W.; Lee, J.; Kim, Y.K.; Seo, D.-W. An isoflavone compound daidzein elicits myoblast differentiation and myotube growth. J. Funct. Foods 2017, 38, 438–446. [Google Scholar] [CrossRef]
- Zheng, W.; Hemker, M.L.; Xie, M.; Soukup, S.T.; Diel, P. Anabolic Activity of a Soy Extract and Three Major Isoflavones in C2C12 Myotubes. Planta Med. 2018, 84, 1022–1029. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, S.; Zhao, F.; Wang, W.; Liu, D.; Chen, L.; Bai, T.; Wu, Z.; Ji, L.; Zhang, J. Sulforaphane enhanced muscle growth by promoting lipid oxidation through modulating key signaling pathways. Biosci. Rep. 2024, 44, BSR20240084. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Nonaka, H.; Komatsu, S.; Goto, M.; Morozumi, M.; Yamada, S.; Lin, I.C.; Yamashita, S.; Tachibana, H. Delphinidin Prevents Muscle Atrophy and Upregulates miR-23a Expression. J. Agric. Food Chem. 2017, 65, 45–50. [Google Scholar] [CrossRef]
- Li, C.; Cao, H.; Ren, Y.; Jia, J.; Yang, G.; Jin, J.; Shi, X. Eicosapentaenoic acid-mediated activation of PGAM2 regulates skeletal muscle growth and development via the PI3K/AKT pathway. Int. J. Biol. Macromol. 2024, 268, 131547. [Google Scholar] [CrossRef]
- Kweon, M.; Lee, H.; Park, C.; Choi, Y.H.; Ryu, J.H. A Chalcone from Ashitaba (Angelica keiskei) Stimulates Myoblast Differentiation and Inhibits Dexamethasone-Induced Muscle Atrophy. Nutrients 2019, 11, 2419. [Google Scholar] [CrossRef]
- Hur, J.; Kim, M.; Choi, S.Y.; Jang, Y.; Ha, T.Y. Isobavachalcone attenuates myotube atrophy induced by TNF-alpha through muscle atrophy F-box signaling and the nuclear factor erythroid 2-related factor 2 cascade. Phytother. Res. 2019, 33, 403–411. [Google Scholar] [CrossRef]
- Norikura, T.; Sasaki, Y.; Kojima-Yuasa, A.; Kon, A. Glyoxylic Acid, an alpha-Keto Acid Metabolite Derived from Glycine, Promotes Myogenesis in C2C12 Cells. Nutrients 2023, 15, 1763. [Google Scholar] [CrossRef]
- De Stefanis, D.; Balestrini, A.; Costelli, P. Oleocanthal Protects C2C12 Myotubes against the Pro-Catabolic and Anti-Myogenic Action of Stimuli Able to Induce Muscle Wasting In Vivo. Nutrients 2024, 16, 1302. [Google Scholar] [CrossRef]
- Sa, B.K.; Kim, C.; Kim, M.B.; Hwang, J.K. Panduratin A Prevents Tumor Necrosis Factor-Alpha-Induced Muscle Atrophy in L6 Rat Skeletal Muscle Cells. J. Med. Food 2017, 20, 1047–1054. [Google Scholar] [CrossRef]
- Xia, Q.; Ling, X.; Wang, Z.; Shen, T.; Chen, M.; Mao, D.; Ma, X.; Ning, J.; Zhang, H.; Chen, D.; et al. Flavonoids Sophoranone Promotes Differentiation of C2C12 and Extraocular Muscle Satellite Cells. Ophthalmic Res. 2021, 64, 337–344. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Liu, Z.; Ma, X.; Li, H.; Shen, M.; Chen, J.; Liu, H. Diosmin Promotes Myogenesis via Activating the Akt/FOXO1 Pathway to Facilitate the Proliferation of C2C12 Myoblasts. J. Agric. Food Chem. 2023, 71, 19705–19716. [Google Scholar] [CrossRef]
- Kim, R.; Kim, J.W.; Choi, H.; Oh, J.E.; Kim, T.H.; Go, G.Y.; Lee, S.J.; Bae, G.U. Ginsenoside Rg5 promotes muscle regeneration via p38MAPK and Akt/mTOR signaling. J. Ginseng Res. 2023, 47, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Avital-Cohen, N.; Chapnik, N.; Froy, O. Resveratrol Induces Myotube Development by Altering Circadian Metabolism via the SIRT1-AMPK-PP2A Axis. Cells 2024, 13, 1069. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Wang, H.; Wang, B.; Mao, X.; Du, M. Resveratrol improves muscle regeneration in obese mice through enhancing mitochondrial biogenesis. J. Nutr. Biochem. 2021, 98, 108804. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, J.; Xu, Z.; Shao, W.; Pang, X.; Li, D.; Huang, X.; Luo, W.; Du, Z.; Li, Y.; et al. Dietary resveratrol improves the flesh quality of Siberian sturgeon (Acipenser baerii) by enhancing myofiber growth, nutrient accumulation and antioxidant capacity. BMC Genom. 2024, 25, 514. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Ferdousi, F.; Fukumitsu, S.; Isoda, H. Maslinic Acid Attenuates Denervation-Induced Loss of Skeletal Muscle Mass and Strength. Nutrients 2021, 13, 2950. [Google Scholar] [CrossRef]
- Yin, X.; Liu, W.; Chen, H.; Qi, C.; Chen, H.; Niu, H.; Yang, J.; Kwok, K.W.H.; Dong, W. Effects of ferulic acid on muscle development and intestinal microbiota of zebrafish. J. Anim. Physiol. Anim. Nutr. 2022, 106, 429–440. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, C.; Li, P.; Xiong, W.; Zeng, Z.; Liu, A.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Theaflavin Promotes Myogenic Differentiation by Regulating the Cell Cycle and Surface Mechanical Properties of C2C12 Cells. J. Agric. Food Chem. 2020, 68, 9978–9992. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, C.; Gu, X.; Wang, X.; Zhang, G.; Fan, M.; Zhao, Y.; Liu, X.; Zhang, X. Paeoniflorin alleviated muscle atrophy in cancer cachexia through inhibiting TLR4/NF-kappaB signaling and activating AKT/mTOR signaling. Toxicol. Appl. Pharmacol. 2024, 484, 116846. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, L.L.; Lee, J.O.; Lee, H.Y.; Kim, Y.A.; Go, H.R. 6′-Sialyllactose Enhances Exercise Performance via Increased Muscle Mass and Strength. Nutrients 2024, 16, 2600. [Google Scholar] [CrossRef]
- Lee, J.H.; Peng, D.Q.; Jin, X.C.; Smith, S.B.; Lee, H.G. Vitamin D3 decreases myoblast fusion during the growth and increases myogenic gene expression during the differentiation phase in muscle satellite cells from Korean native beef cattle. J. Anim. Sci. 2023, 101, skad192. [Google Scholar] [CrossRef] [PubMed]
| Protein, Peptides/Amino Acid | Concentration/Dose | Mechanism | Object/Model | Reference |
|---|---|---|---|---|
| MIF1 and MIF2 | 1000 nM | MSTN (↓), proliferation and differentiation (↑) | Cell line (mouse C2C12) | [178] |
| 1.125 mM | muscle regeneration | Animal (mouse, cardiotoxin-induced C57BL/6 male mice) | ||
| Isoleucine | 25 g/L | muscle and fat mass (↑) | Animal (C57BL/6J mice fed a high-fat diet) | [177] |
| Potato peptide hydrolysate | 2.5, 5, and 10 µg/mL | Akt/mTOR signaling (↑) | Cell line (mouse C2C12) | [181] |
| Whey protein | 100, 500, and 1000 µg/mL | IGF-1 (↑); myotube formation (↑) | Cell line (mouse C2C12) | [186] |
| Thioredoxin | 2 µg/L | myogenic differentiation (↑) | Primary cells (rat bone marrow mesenchymal stem cells) | [184] |
| Selenoprotein K | - | regulates ER stress and oxidative stress | Cell line (mouse C2C12) | [185] |
| Galectin-1 | 0.054, 0.11, and 0.22 μM | MyoG (↑), MyoD (↑), and MyH (↑) | Human-Murine myoblasts H2K A/J−/− | [187] |
| Insulin | 5 UI/Kg | muscle regeneration (↑) | Animal (rat, burn-injured animals) | [180] |
| Podocan | 1.5 mg/mL | myogenic development (↑) | Cell line (mouse C2C12) | [179] |
| Arginine | Diet with 0.5%, or 1.0% arginine | MyH I (↑), MyH IIA (↑), PGC-1α (↑), NRF1 gene expression (↑), mTOR pathway (↑) | Animals (C57BL/6J male mice) | [183] |
| 1.2 mM or 3.6 mM | mitochondrial genes (PGC-1α, TFAM, MEF2C, and NRF1) (↑) | Cell line (mouse C2C12) |
| Natural Resources (Compounds and Extract) | Concentration/Dose | Mechanism | Object/Model | References |
|---|---|---|---|---|
| G. uralensis | 100 µg/mL | MSTN (↓), myogenesis (↑) | Cell line (mouse C2C12) | [188] |
| Green tea extract | 10 µg/mL | MyoG (↑), Myf5 (↑), and MyoD (↑) | Cell line (mouse C2C12) | [189] |
| Rosemary extract | 20 µg/mL | MyoG (↑), and MyoD (↑) | Cell line (mouse C2C12) | [190] |
| Aster glehni extract | - | MSTN (↓), MyoD (↑), myoglobin (↑), myosin (↑), and ATP generation (↑) | Cell line (mouse C2C12) | [191] |
| Gloiopeltis tenax aqueous extract | 10 µg/mL | myogenic differentiation (↑); Atrogin/MuRF1 pathway (↓) | Cell line (mouse C2C12) | [196] |
| 20 mg/kg | mitochondrial biogenesis and function (↑) | Animal (mouse, DXM-induced atrophy) | ||
| Hachimijiogan | 0.1 mg/mL | MyoG (↑) | Cell line (mouse C2C12) | [192] |
| muscle wasting (↓) | Animal (cancer-cachectic mice) | |||
| Tinospora cordifolia extract | 0.05 mg/mL | myogenic differentiation (↑) | Cell line (mouse C2C12) | [194] |
| Sea buckthorn oil | - | PCNA (↑), Cyclin D1/CDK4 (↑), and MyoG (↑) | Primary cells (sheep myoblasts) | [198] |
| Korean mistletoe and apple peel extracts | 100 mg/kg or 200 mg/kg | muscular strength and endurance (↑) | Animal (mice) | [199] |
| Luffa cylindrica Roemer | 200 and 400 μg/ml | myotube number and diameter (↑) | Primary cells (rat skeletal myotubes) | [197] |
| Saururus chinensis extract | 10 ng/ml | MyH (↑) | Cell line (mouse C2C12) | [193] |
| Withania somnifera extract | 200 mg/kg | muscle mass (↑) | Animal (aged mice) | [202] |
| 200 and 300 mg/kg | TNF-α and IL-1β (↓) | Animal (12-month-old mouse model) | ||
| Korean red ginseng | 100 and 400 mg/kg | SM growth (↑) | Animal (mice) | [200] |
| Ginseng Berry Powder | 25 or 50 mg/kg | myotube diameter (↑) | Animal (mouse, sarcopenia model) | [203] |
| Vigeo | 10, 25, and 50 µg/mL | myotube width, length, and fusion (↑); MyH (↑), MyoD (↑), and MyoG (↑) | Cell line (mouse C2C12) | [195] |
| Sialyllactose | 100 mg/kg | exercise performance (↑) | Animal (mice) | [277] |
| Ajoene | 5 mg/kg or 10 mg/kg | muscle degradation (↓) | Animal (mouse, BALB/c, CT26 tumor) | [204] |
| 100 nM | Myogenesis (↑) | Cell line (mouse C2C12) | ||
| Licochalcone A and Licochalcone B | 1 ng/mL, and 3 mg/kg | MSTN (↓); Atrogin1 (↓), and MuRF1 (↓) | Cell line (mouse C2C12) & Animal (mouse, C57BL/6) | [205] |
| Epicatechin | 10 µM, and 20 µM | myogenic differentiation (↑) | Cell line (mouse C2C12) | [206] |
| Curcumin | 80 mg/L | MyoD (↑), MYOG (↑), Myf5 (↑), and MyH (↑) | Cell line (mouse C2C12) | [212] |
| Succinic Acid | 10–1000 µM | MyoD (↑), and MyoG (↑) | Cell line (mouse C2C12) | [213] |
| Echinacoside | 1, 5, and 10 µM | Myogenesis (↑) | Cell line (mouse C2C12) | [214] |
| 8-Prenylnaringenin | - | muscle recovery (↑) | mice | [215] |
| Dieckol and 2,7-phloroglucinol-6,6′-bieckol | 5 nm, 10 nm, and 20 nm | Smad signaling (↓), and IGF-1 signaling (↑) | Cell line (mouse C2C12) | [217] |
| Dihydromyricetin | 100 mg/kg, and 200 mg/kg | fiber cross-sectional area (↑) | Animal (mouse, DXM-induced atrophy) | [218] |
| Ascorbic acid | 6 g/L through drinking water | Differentiation (↑), and muscle regeneration (↑) | Animal (injured mouse muscle) | [222] |
| Silibinin | 200 mg/kg | grip strength (↑), MuRF1 (↓), Atrogin-1 (↓), IL-6 (↓), and TNF-α (↓) | Animal (cachectic mice) | [224] |
| Omega-7 palmitoleic acids | 100 µM | Pax3 (↑), and ROS (↓) | Cell line (L6 myoblast) | [225] |
| Oleic acid | Diet supplemented with 10% (w/w) oleic acid | running endurance (↑) | Animal (mice) | [226,227] |
| 5,7-Dimethoxyflavone | 25 or 50 mg/kg | exercise capacity (↑), grip strength (↑), and muscular mass (↑) | Animal (18-month-old C57BL/6J mice) | [228] |
| Apigenin | 0.1% apigenin-containing diet | muscle atrophy (↓); MyH and MyH type IIb (↑) | Animal (mouse, denervation model) | [232] |
| Baicalin | 50 and 150 mg/kg | TNF-α and IL-6 (↓); MuRF1 and Atrogin-1 (↓) | Animal (mouse, cancer cachexia model) | [233] |
| Andrographolide | 10 mg/kg | SM regeneration (↑) | Animal (mouse, cardiotoxin-induced injury model | [235] |
| Luteolin | 20 mg/kg | TNF-α and IL-6 (↓) | Animal (cancer-induced muscle wasting in vivo) | [236] |
| Sinensetin | 50 and 100 μM | MSC differentiation (↑) | Animal (aged rats) | [237] |
| Vitamins A | 100 nM | muscle growth (↑) | Primary cells (bovine MSC) | [278] |
| β-carotene | 4 or 8 mg/kg | tumor development (↓); muscle weight and strength (↑) | Animal (mouse model of cancer cachexia) | [241] |
| Quercetin | 1, 10, and 100 nM | MSTN (↓); MSC differentiation and myotube formation (↑) | Cell line (mouse C2C12) | [243] |
| Genistein | 10 µM, and 10 mg/kg | MSC differentiation (↑) | in vitro and in vivo | [251] |
| α-Mangostin | 3, and 6 μM | MyoD and MyoG protein (↑) | Cell line (mouse C2C12) | [252] |
| Glabridin | 0.1, 1, and 10 μM | DXM-stimulated protein degradation (↓) | Cell line (mouse C2C12) | [254] |
| Corylifol A | 10, 50 and 100 nM | muscle atrophy (↓); myoblast (↑) | Cell line (mouse C2C12) | [255] |
| Daidzein | 50 nM | MyoD (↑), MyH (↑), myotube diameter and number (↑) | Cell line (mouse C2C12) | [257] |
| Sulforaphane | 1 mg/kg | body weight (↑) | Animal (mouse, C57BL/6J) | [259] |
| Delphinidin | 20 mg/kg | MuRF1 (↓) | Animal (mouse, C57BL/6J) | [260] |
| Isobavachalcone | 100 pM | MyH protein (↑) | Cell line (mouse C2C12) | [262] |
| Glyoxylic acid | 0.2, 0.4, and 0.8 mM | MyH II (↑), MyoD (↑), and MyoG (↑) | Cell line (mouse C2C12) | [264] |
| Oleocanthal | 10 µM | muscle wasting (↓) | Cell line (mouse C2C12) | [265] |
| Panduratin A | 1–40 µM | myotube diameter (↑) | Cell line (TNF-α-treated L6 SM cells) | [266] |
| Sophoranone | 10 μM | creatine kinase levels (↑) | Animal (rabbit model of acute medial rectus muscle injury) | [267] |
| Diosmin | 50 μM | proliferation (↑) | Cell line (mouse C2C12) | [268] |
| Ginsenoside Rg5 | 100 nM | p38MAPK phosphorylation and MyoD/E2A heterodimerization (↑) | Cell line (mouse C2C12) | [269] |
| Resveratrol | Diet supplemented with 0.4% (w/w) resveratrol | MSCs proliferation and differentiation (↑) | Animal (injured obese mice) | [271] |
| Maslinic Acid | Diet supplemented with 0.27% (w/w) maslinic acid | denervation-induced muscle atrophy (↓) | Animal (mouse gastrocnemius muscle) | [273] |
| Ferulic acid | MyoD (↑), MyoG (↑), and Myf5 (↑) | Zebrafish (adult male) | [274] | |
| Theaflavin | 20 μM | myogenesis (↑) | Cell line (mouse C2C12) | [275] |
| Paeoniflorin | 50 mg/kg | weight loss and muscle fiber atrophy (↓) | Animal (C26 tumor-bearing mice) | [276] |
| 10, 30, and 100 μM | IL-6 production (↓) | Cell line (C26 cancer cells) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, S.; Ahmad, K.; Lim, J.H.; Ahmad, S.S.; Lee, E.J.; Choi, I. Skeletal Muscle Aging: Enhancing Skeletal Muscle Integrity and Function as a Potential Pharmacological Approach. Pharmaceuticals 2025, 18, 1407. https://doi.org/10.3390/ph18091407
Shaikh S, Ahmad K, Lim JH, Ahmad SS, Lee EJ, Choi I. Skeletal Muscle Aging: Enhancing Skeletal Muscle Integrity and Function as a Potential Pharmacological Approach. Pharmaceuticals. 2025; 18(9):1407. https://doi.org/10.3390/ph18091407
Chicago/Turabian StyleShaikh, Sibhghatulla, Khurshid Ahmad, Jeong Ho Lim, Syed Sayeed Ahmad, Eun Ju Lee, and Inho Choi. 2025. "Skeletal Muscle Aging: Enhancing Skeletal Muscle Integrity and Function as a Potential Pharmacological Approach" Pharmaceuticals 18, no. 9: 1407. https://doi.org/10.3390/ph18091407
APA StyleShaikh, S., Ahmad, K., Lim, J. H., Ahmad, S. S., Lee, E. J., & Choi, I. (2025). Skeletal Muscle Aging: Enhancing Skeletal Muscle Integrity and Function as a Potential Pharmacological Approach. Pharmaceuticals, 18(9), 1407. https://doi.org/10.3390/ph18091407








