Lipoprotein(a)/CD36 Interaction Drives IL-6/RhoA-GTP Signaling and miRNA Epigenetic Regulation in Coronary Artery Spasm
Abstract
1. Introduction
2. Results
2.1. Study Cohort Baseline Characteristics
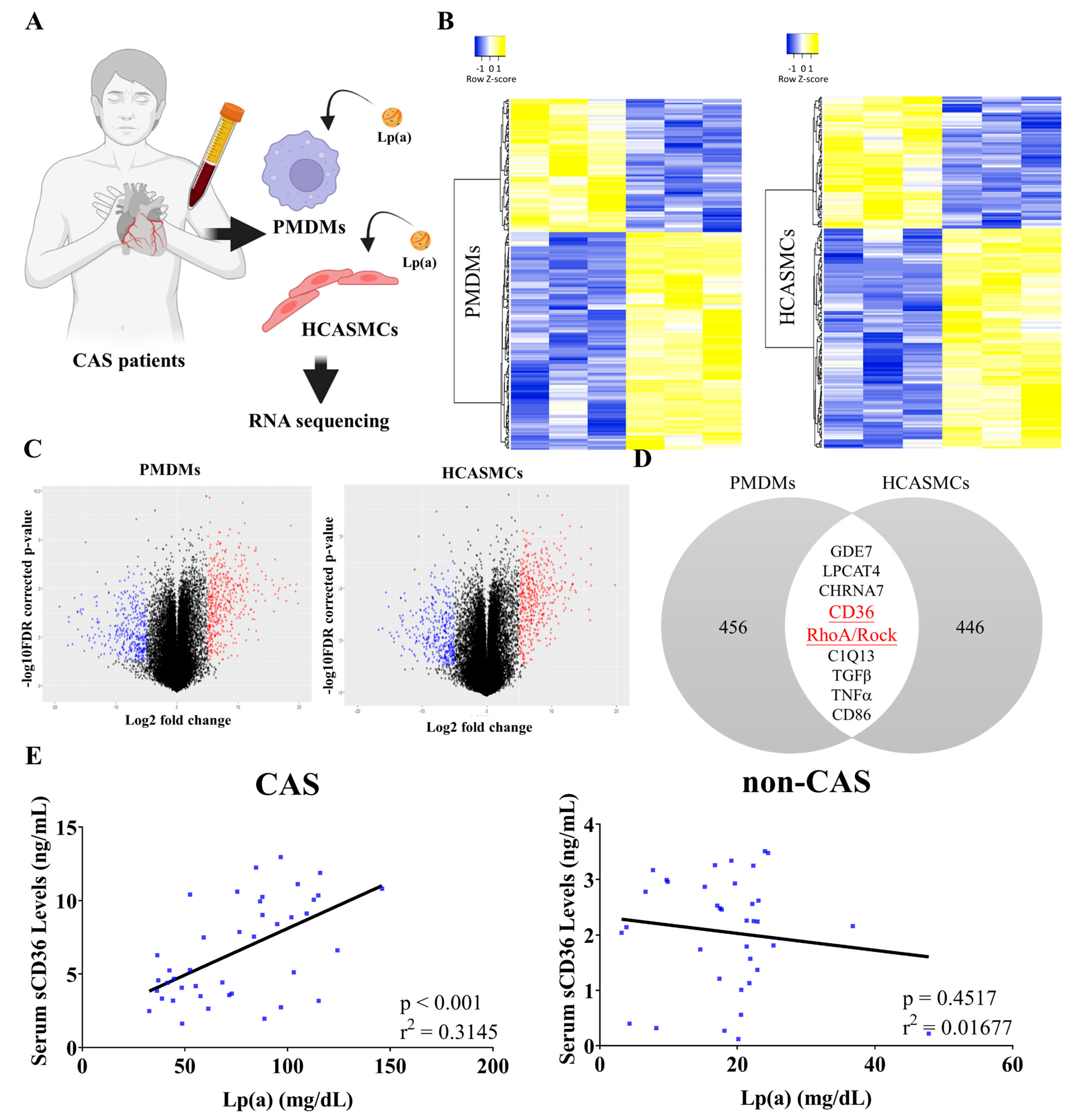
2.2. Correlation of Lp(a) with sCD36
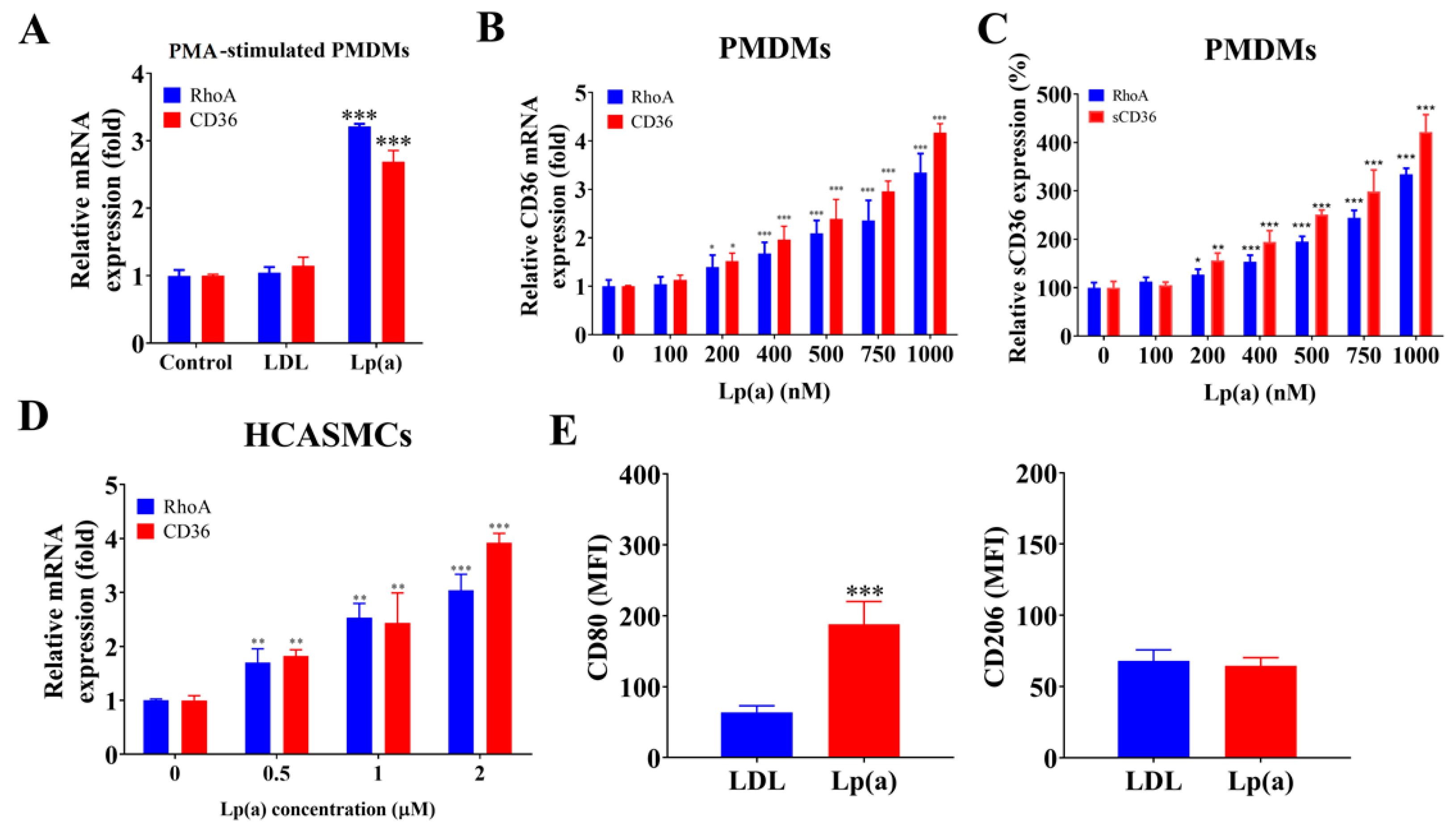
2.3. Lp(a) Stimulated CD36 and RhoA Expression in PMDMs, HCASMCs, and Preferentially Induced PMDM M1 Polarization
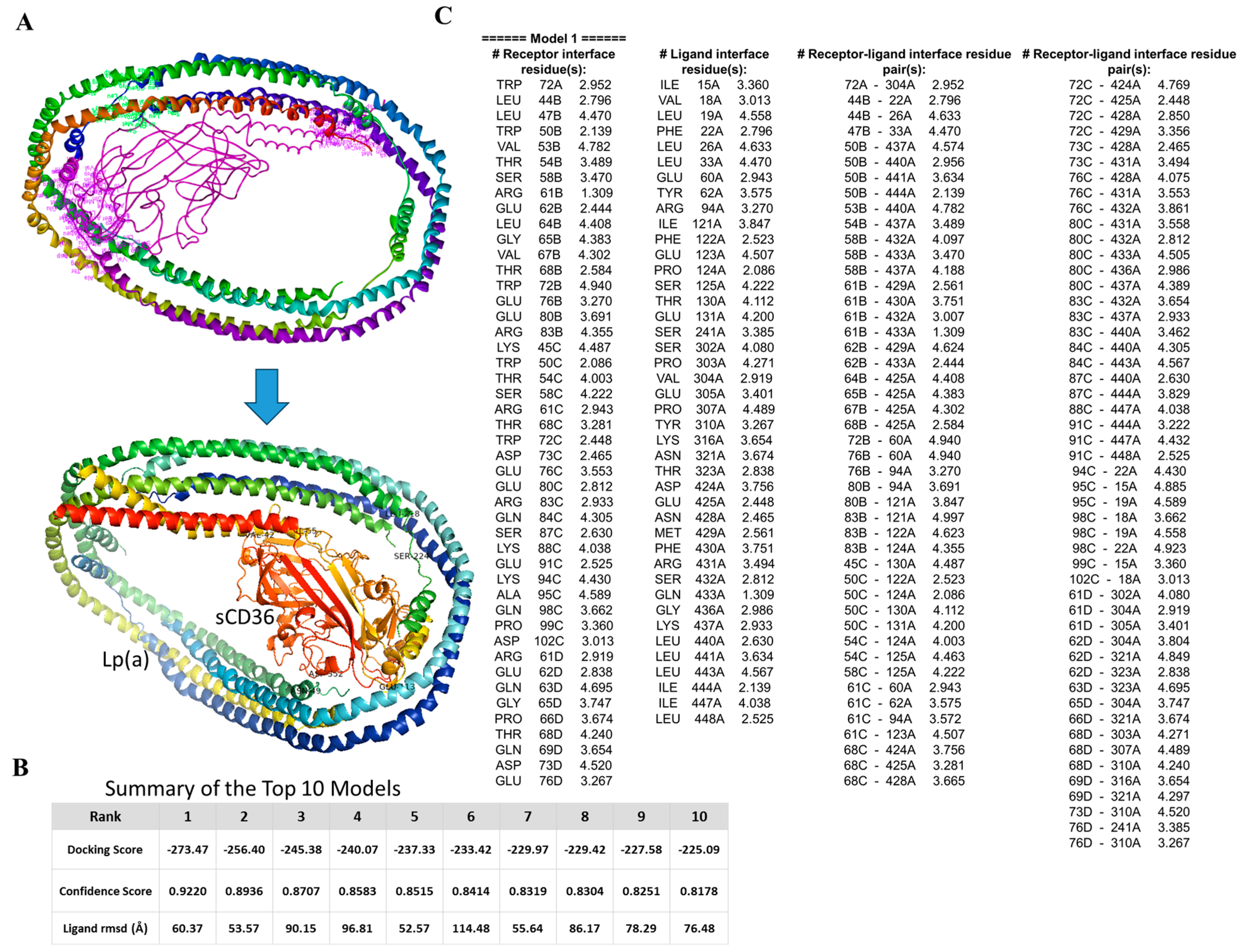
2.4. In Silico Molecular Docking to Examine Lp(a)/sCD36 Binding Interactions
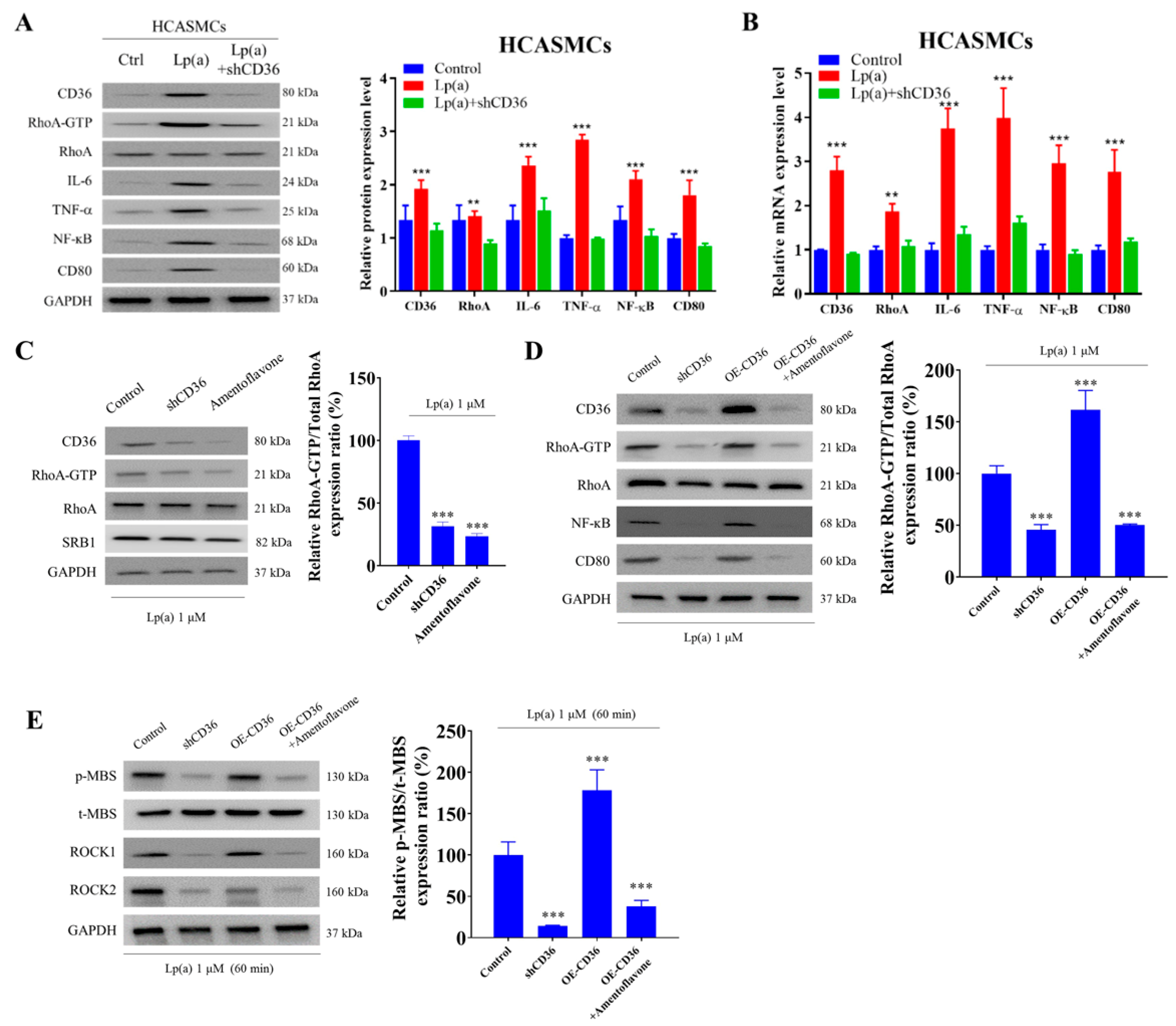
2.5. CD36 Knockdown Reduced Lp(a)-Induced Proinflammatory Signaling in HCASMCs
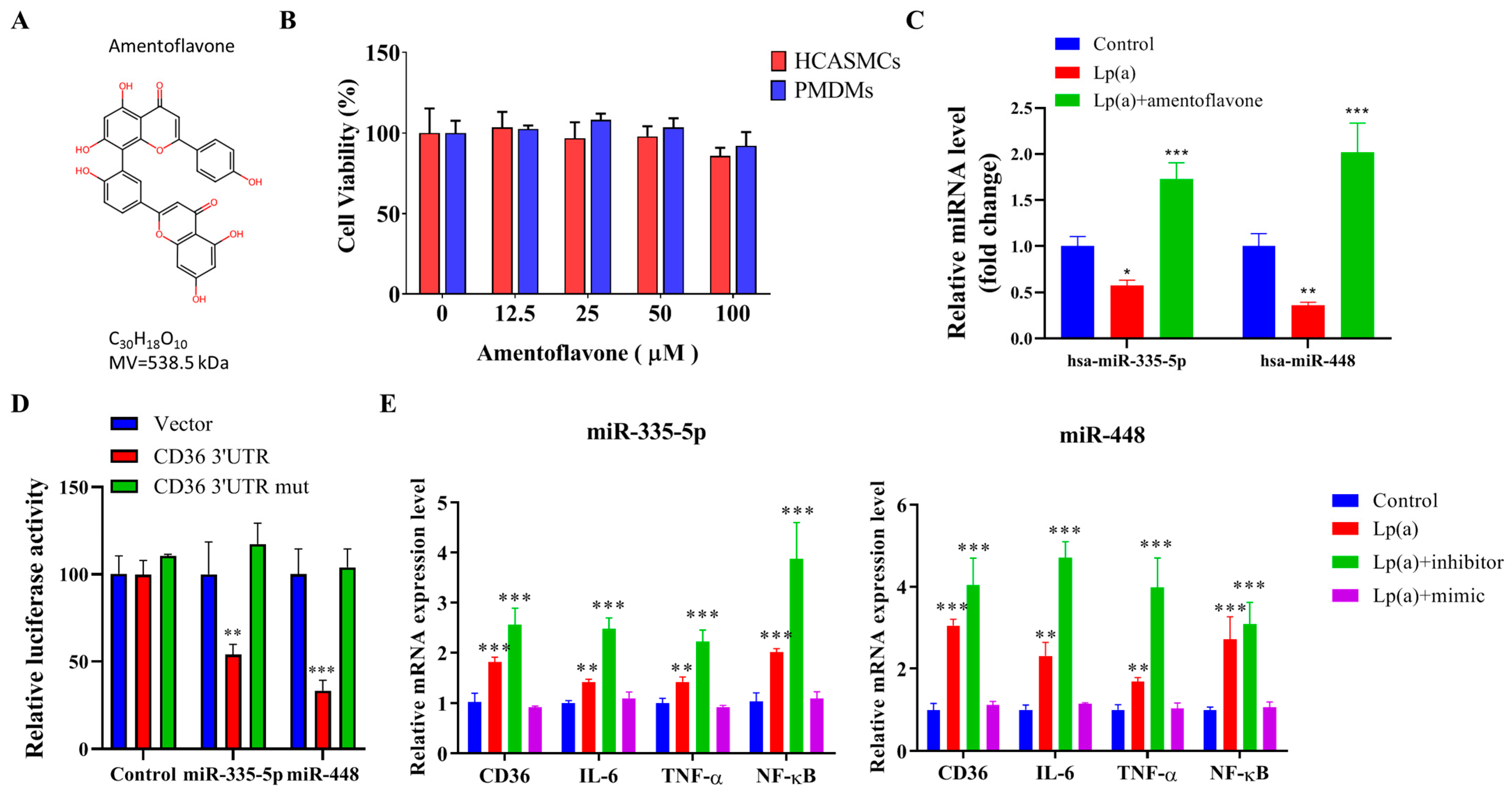
2.6. Epigenetic Regulation of Lp(a)-Triggered CD36, IL-6, TNF-α, and NF-κB Expression in HCASMCs
3. Discussion
4. Materials and Methods
4.1. Cells, Compounds, and Reagents
4.2. Study Population
4.3. Patient Data Collection
4.4. Spasm Provocation Test Protocol
4.5. Monocyte Isolation from Human Peripheral Blood
4.6. Monocyte Differentiation into Macrophages
4.7. HCASMCs Culture
4.8. Lp(a) Assay
4.9. CD36 Expression Analysis in HCASMCs
4.10. RNA Processing and Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) for CD36, RhoA, and miRNA
4.11. Molecular Docking
4.12. Western Blot Analysis
4.13. Flow Cytometry Analysis
4.14. Cell Viability Assessment After Amentoflavone Treatment
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Writing Committee Members; Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Cardiovasc. Comput. Tomogr. 2022, 16, 54–122. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-J.; Hsu, K.-H.; Chang, N.-C.; Hung, M.-Y. Increased Numbers of Coronary Events in Winter and Spring Due to Coronary Artery Spasm: Effect of Age, Sex, Smoking, and Inflammation. J. Am. Coll. Cardiol. 2015, 65, 2047–2048. [Google Scholar] [CrossRef]
- Hung, M.-J.; Kuo, L.-T.; Cheng, C.W.; Chang, C.-P.; Cherng, W.J. Comparison of peripheral monocyte counts in patients with and without coronary spasm and without fixed coronary narrowing. Am. J. Cardiol. 2004, 93, 620–624. [Google Scholar] [CrossRef]
- Hung, M.-J.; Hsu, K.-H.; Hu, W.-S.; Chang, N.-C.; Hung, M.-Y. C-reactive protein for predicting prognosis and its gender-specific associations with diabetes mellitus and hypertension in the development of coronary artery spasm. PLoS ONE 2013, 8, e77655. [Google Scholar] [CrossRef]
- Hung, M.-J.; Cherng, W.-J.; Cheng, C.-W.; Li, L.-F. Comparison of serum levels of inflammatory markers in patients with coronary vasospasm without significant fixed coronary artery disease versus patients with stable angina pectoris and acute coronary syndromes with significant fixed coronary artery disease. Am. J. Cardiol. 2006, 97, 1429–1434. [Google Scholar] [CrossRef]
- Hung, M.-Y.; Wu, Y.H.; Bamodu, O.A.; Chen, X.; Lin, Y.K.; Hu, P.; Chang, N.C.; Pang, J.S.; Yeh, C.T. Activation of the monocytic α7 nicotinic acetylcholine receptor modulates oxidative stress and inflammation-associated development of coronary artery spasm via a p38 MAP-kinase signaling-dependent pathway. Free Radic. Biol. Med. 2018, 120, 266–276. [Google Scholar] [CrossRef]
- Kugiyama, K.; Yasue, H.; Okumura, K.; Ogawa, H.; Fujimoto, K.; Nakao, K.; Yoshimura, M.; Motoyama, T.; Inobe, Y.; Kawano, H. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation 1996, 94, 266–271. [Google Scholar] [CrossRef]
- Miwa, K.; Nakagawa, K.; Yoshida, N.; Taguchi, Y.; Inoue, H. Lipoprotein(a) is a risk factor for occurrence of acute myocardial infarction in patients with coronary vasospasm. J. Am. Coll. Cardiol. 2000, 35, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-Y.; Witztum, J.L.; Tsimikas, S. New therapeutic targets for calcific aortic valve stenosis: The lipoprotein(a)-lipoprotein-associated phospholipase A2-oxidized phospholipid axis. J. Am. Coll. Cardiol. 2014, 63, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, C.; Dewan, A.; Orsoni, A.; Merki, E.; Miller, E.R.; Shin, M.J.; Binder, C.J.; Hörkkö, S.; Krauss, R.M.; Chapman, M.J.; et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 2008, 49, 2230–2239. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Yeh, C.-T.; Kuo, K.-T.; Fong, I.-H.; Yadav, V.-K.; Kounis, N.G.; Hu, P.; Hung, M.-Y. Apolipoprotein (a)/Lipoprotein(a)-Induced Oxidative-Inflammatory α7-nAChR/p38 MAPK/IL-6/RhoA-GTP Signaling Axis and M1 Macrophage Polarization Modulate Inflammation-Associated Development of Coronary Artery Spasm. Oxidative Med. Cell. Longev. 2022, 2022, 9964689. [Google Scholar] [CrossRef]
- Hung, M.-J.; Cherng, W.-J.; Hung, M.-Y.; Kuo, L.-T.; Cheng, C.-W.; Wang, C.-H.; Yang, N.-I.; Liao, J.K. Increased leukocyte Rho-associated coiled-coil containing protein kinase activity predicts the presence and severity of coronary vasospastic angina. Atherosclerosis 2012, 221, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Puchałowicz, K.; Rać, M.E. The Multifunctionality of CD36 in Diabetes Mellitus and Its Complications-Update in Pathogenesis, Treatment and Monitoring. Cells 2020, 9, 1877. [Google Scholar] [CrossRef]
- Kaewmalee, J.; Ontawong, A.; Duangjai, A.; Tansakul, C.; Rukachaisirikul, V.; Muanprasat, C.; Srimaroeng, C. High-Efficacy α,β-Dehydromonacolin S Improves Hepatic Steatosis and Suppresses Gluconeogenesis Pathway in High-Fat Diet-Induced Obese Rats. Pharmaceuticals 2021, 14, 375. [Google Scholar] [CrossRef]
- Parra-Reyna, B.; Padilla-Gutiérrez, J.R.; Aceves-Ramírez, M.; García-Garduño, T.C.; Martínez-Fernández, D.E.; Jacobo-García, J.J.; Valdés-Alvarado, E.; Valle, Y. Genetic variants, gene expression, and soluble CD36 analysis in acute coronary syndrome: Differential protein concentration between ST-segment elevation myocardial infarction and unstable angina. J. Clin. Lab. Anal. 2022, 36, e24529. [Google Scholar] [CrossRef]
- Bodart, V.; Febbraio, M.; Demers, A.; McNicoll, N.; Pohankova, P.; Perreault, A.; Sejlitz, T.; Escher, E.; Silverstein, R.L.; Lamontagne, D.; et al. CD36 mediates the cardiovascular action of growth hormone-releasing peptides in the heart. Circ. Res. 2002, 90, 844–849. [Google Scholar] [CrossRef]
- Hung, M.-Y.; Kounis, N.G.; Lu, M.-Y.; Hu, P. Myocardial Ischemic Syndromes, Heart Failure Syndromes, Electrocardiographic Abnormalities, Arrhythmic Syndromes and Angiographic Diagnosis of Coronary Artery Spasm: Literature Review. Int. J. Med. Sci. 2020, 17, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Thorand, B.; Löwel, H.; Schneider, A.; Kolb, H.; Meisinger, C.; Fröhlich, M.; Koenig, W. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: Results from the MONICA Augsburg cohort study, 1984–1998. Arch. Intern. Med. 2003, 163, 93–99. [Google Scholar] [CrossRef]
- Mansor, L.S.; Sousa Fialho, M.D.L.; Yea, G.; Coumans, W.A.; West, J.A.; Kerr, M.; Carr, C.A.; Luiken, J.J.F.P.; Glatz, J.F.C.; Evans, R.D.; et al. Inhibition of sarcolemmal FAT/CD36 by sulfo-N-succinimidyl oleate rapidly corrects metabolism and restores function in the diabetic heart following hypoxia/reoxygenation. Cardiovasc. Res. 2017, 113, 737–748. [Google Scholar] [CrossRef]
- Glatz, J.F.; Angin, Y.; Steinbusch, L.K.; Schwenk, R.W.; Luiken, J.J. CD36 as a target to prevent cardiac lipotoxicity and insulin resistance. Prostagland. Leukot. Essent. Fat. Acids 2013, 88, 71–77. [Google Scholar] [CrossRef]
- Kang, K.W.; Choi, B.G.; Rha, S.W. Impact of Insulin Resistance on Acetylcholine-Induced Coronary Artery Spasm in Non-Diabetic Patients. Yonsei Med. J. 2018, 59, 1057–1063. [Google Scholar] [CrossRef]
- Hung, M.-J.; Chang, N.-C.; Hu, P.; Chen, T.-H.; Mao, C.-T.; Yeh, C.-T.; Hung, M.-Y. Association between Coronary Artery Spasm and the risk of incident Diabetes: A Nationwide population-based Cohort Study. Int. J. Med. Sci. 2021, 18, 2630–2640. [Google Scholar] [CrossRef]
- Handberg, A.; Lopez-Bermejo, A.; Bassols, J.; Vendrell, J.; Ricart, W.; Fernandez-Real, J.M. Circulating soluble CD36 is associated with glucose metabolism and interleukin-6 in glucose-intolerant men. Diab. Vasc. Dis. Res. 2009, 6, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.; Abe, T.; Hochrainer, K.; Shimamura, M.; Anrather, J.; Racchumi, G.; Zhou, P.; Iadecola, C. Nuclear factor-kappaB activation and postischemic inflammation are suppressed in CD36-null mice after middle cerebral artery occlusion. J. Neurosci. 2008, 28, 1649–1658. [Google Scholar] [CrossRef]
- Tong, L.; Tergaonkar, V. Rho protein GTPases and their interactions with NFκB: Crossroads of inflaowiojldflmmation and matrix biology. Biosci. Rep. 2014, 34, e00115. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.-W.; Wu, M.-S.; Liu, Y.; Lu, M.; Guo, J.-D.; Meng, Y.-H.; Zhou, Y.-H. SIRT1-mediated deacetylation of NF-κB inhibits the MLCK/MLC2 pathway and the expression of ET-1, thus alleviating the development of coronary artery spasm. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H458–H468. [Google Scholar] [CrossRef]
- Netea, M.G.; de Bont, N.; Demacker, P.N.; Kullberg, B.J.; Jacobs, L.E.; Verver-Jansen, T.J.; Stalenhoef, A.F.; Van der Meer, J.W. Lipoprotein(a) inhibits lipopolysaccharide-induced tumor necrosis factor alpha production by human mononuclear cells. Infect. Immun. 1998, 66, 2365–2367. [Google Scholar] [CrossRef] [PubMed]
- Kvietys, P.R.; Granger, D.N. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic. Biol. Med. 2012, 52, 556–592. [Google Scholar] [CrossRef]
- Li, C.; Ma, Q.; Toan, S.; Wang, J.; Zhou, H.; Liang, J. SERCA overexpression reduces reperfusion-mediated cardiac microvascular damage through inhibition of the calcium/MCU/mPTP/necroptosis signaling pathways. Redox Biol. 2020, 36, 101659. [Google Scholar] [CrossRef]
- Mathis, D.; Shoelson, S.E. Immunometabolism: An emerging frontier. Nat. Rev. Immunol. 2011, 11, 81. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Cui, W.; Silverstein, R.L. CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 2022, 219, e20211314. [Google Scholar] [CrossRef]
- Huh, H.Y.; Pearce, S.F.; Yesner, L.M.; Schindler, J.L.; Silverstein, R.L. Regulated expression of CD36 during monocyte-to-macrophage differentiation: Potential role of CD36 in foam cell formation. Blood 1996, 87, 2020–2028. [Google Scholar] [CrossRef]
- Tontonoz, P.; Nagy, L.; Alvarez, J.G.; Thomazy, V.A.; Evans, R.M. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998, 93, 241–252. [Google Scholar] [CrossRef]
- Yesner, L.M.; Huh, H.Y.; Pearce, S.F.; Silverstein, R.L. Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arter. Thromb. Vasc. Biol. 1996, 16, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Podrez, E.A.; Byzova, T.V.; Febbraio, M.; Salomon, R.G.; Ma, Y.; Valiyaveettil, M.; Poliakov, E.; Sun, M.; Finton, P.J.; Curtis, B.R.; et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 2007, 13, 1086–1095. [Google Scholar] [CrossRef]
- Zhan, J.; Jin, K.; Ding, N.; Zhou, Y.; Hu, G.; Yuan, S.; Xie, R.; Wen, Z.; Chen, C.; Li, H.; et al. Positive feedback loop of miR-320 and CD36 regulates the hyperglycemic memory-induced diabetic diastolic cardiac dysfunction. Mol. Ther. Nucleic Acids 2022, 31, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Cain, A.E.; Tanner, D.M.; Khalil, R.A. Endothelin-1–induced enhancement of coronary smooth muscle contraction via MAPK-dependent and MAPK-independent [Ca2+]i sensitization pathways. Hypertension 2002, 39, 543–549. [Google Scholar] [CrossRef]
- Bomfim, G.F.; Dos Santos, R.A.; Oliveira, M.A.; Giachini, F.R.; Akamine, E.H.; Tostes, R.C.; Fortes, Z.B.; Webb, R.C.; Carvalho, M.H. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin. Sci. 2012, 122, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Hubert, A.; Seitz, A.; Pereyra, V.M.; Bekeredjian, R.; Sechtem, U.; Ong, P. Coronary Artery Spasm: The Interplay Between Endothelial Dysfunction and Vascular Smooth Muscle Cell Hyperreactivity. Eur. Cardiol. 2020, 15, e12. [Google Scholar] [CrossRef]
- Baghdadi, A.; Dunn, W.R.; Ralevic, V. Involvement of purinergic signalling in the vasomotor response to hypochlorous acid in porcine coronary artery. Purinergic Signal. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Langsted, A.; Kamstrup, P.R.; Nordestgaard, B.G. Lipoprotein(a): Fasting and nonfasting levels, inflammation, and cardiovascular risk. Atherosclerosis 2014, 234, 95–101. [Google Scholar] [CrossRef]
- Podrez, E.A.; Poliakov, E.; Shen, Z.; Zhang, R.; Deng, Y.; Sun, M.; Finton, P.J.; Shan, L.; Febbraio, M.; Hajjar, D.P.; et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 2002, 277, 38517–38523. [Google Scholar] [CrossRef]
- Freeman, D.J.; Griffin, B.A.; Murray, E.; Lindsay, G.M.; Gaffney, D.; Packard, C.J.; Shepherd, J. Smoking and plasma lipoproteins in man: Effects on low density lipoprotein cholesterol levels and high density lipoprotein subfraction distribution. Eur. J. Clin. Investig. 1993, 23, 630–640. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Benn, M.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: The Copenhagen City Heart Study. Circulation 2008, 117, 176–184. [Google Scholar] [CrossRef]
- Missala, I.; Kassner, U.; Steinhagen-Thiessen, E. A Systematic Literature Review of the Association of Lipoprotein(a) and Autoimmune Diseases and Atherosclerosis. Int. J. Rheumatol. 2012, 2012, 480784. [Google Scholar] [CrossRef]
- Koutroubakis, I.E.; Malliaraki, N.; Vardas, E.; Ganotakis, E.; Margioris, A.N.; Manousos, O.N.; Kouroumalis, E.A. Increased levels of lipoprotein (a) in Crohn’s disease: A relation to thrombosis? Eur. J. Gastroenterol. Hepatol. 2001, 13, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, T.; Wanner, C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Abe, A.; Seishima, M.; Makino, K.; Noma, A.; Kawade, M. Transient changes of serum lipoprotein(a) as an acute phase protein. Atherosclerosis 1989, 78, 145–150. [Google Scholar] [CrossRef]
- Boffa, M.B.; Koschinsky, M.L. Lipoprotein (a): Truly a direct prothrombotic factor in cardiovascular disease? J. Lipid Res. 2016, 57, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Patthy, L.; Trexler, M.; Váli, Z.; Bányai, L.; Váradi, A. Kringles: Modules specialized for protein binding. Homology of the gelatin-binding region of fibronectin with the kringle structures of proteases. FEBS Lett. 1984, 171, 131–136. [Google Scholar] [CrossRef]
- McCormick, S.P.A.; Schneider, W.J. Lipoprotein(a) catabolism: A case of multiple receptors. Pathology 2019, 51, 155–164. [Google Scholar] [CrossRef]
- Grainger, D.J.; Kemp, P.R.; Liu, A.C.; Lawn, R.M.; Metcalfe, J.C. Activation of transforming growth factor-beta is inhibited in transgenic apolipoprotein(a) mice. Nature 1994, 370, 460–462. [Google Scholar] [CrossRef]
- Xie, X.; Niu, Z.; Wang, L.; Zhou, X.; Yu, X.; Jing, H.; Yang, Y. Humanized CD36 (hCD36) mouse model supports the preclinical evaluation of therapeutic candidates targeting CD36. Exp. Anim. 2023, 72, 535–545. [Google Scholar] [CrossRef]
- Makino, K.; Scanu, A.M. Lipoprotein(a): Nonhuman primate models. Lipids 1991, 26, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Enkhmaa, B.; Abbuthalha, A.; Anuurad, E.; Zhang, W.; Tarantal, A.F.; Berglund, L. Rhesus monkey (Macaca mulatta) lipoprotein(a) and apolipoprotein(a): High frequency of small size apolipoprotein(a) isoforms. J. Med. Primatol. 2015, 44, 117–124. [Google Scholar] [CrossRef]
- Holm, S.; Oma, I.; Hagve, T.A.; Saatvedt, K.; Brosstad, F.; Mikkelsen, K.; Rydningen, H.; Risnes, I.; Almdahl, S.M.; Ueland, T.; et al. Levels of Lipoprotein (a) in patients with coronary artery disease with and without inflammatory rheumatic disease: A cross-sectional study. BMJ Open 2019, 9, e030651. [Google Scholar] [CrossRef]
- Berthold, H.K.; Laudes, M.; Krone, W.; Gouni-Berthold, I. Association between the interleukin-6 promoter polymorphism -174G/C and serum lipoprotein(a) concentrations in humans. PLoS ONE 2011, 6, e24719. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Schulte, D.M.; Türk, K.; Freitag-Wolf, S.; Hampe, J.; Zeuner, R.; Schröder, J.O.; Gouni-Berthold, I.; Berthold, H.K.; Krone, W.; et al. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J. Lipid Res. 2015, 56, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Scavini, M.; Orlando, R.A.; Murata, G.H.; Servilla, K.S.; Tzamaloukas, A.H.; Schrader, R.; Bedrick, E.J.; Burge, M.R.; Abumrad, N.A.; et al. Increased CD36 expression signals monocyte activation among patients with type 2 diabetes. Diabetes Care 2010, 33, 2065–2067. [Google Scholar] [CrossRef]
- Yue, H.; Febbraio, M.; Klenotic, P.A.; Kennedy, D.J.; Wu, Y.; Chen, S.; Gohara, A.F.; Li, O.; Belcher, A.; Kuang, B.; et al. CD36 Enhances Vascular Smooth Muscle Cell Proliferation and Development of Neointimal Hyperplasia. Arter. Thromb. Vasc. Biol. 2019, 39, 263–275. [Google Scholar] [CrossRef]
- Yuasa-Kawase, M.; Masuda, D.; Yamashita, T.; Kawase, R.; Nakaoka, H.; Inagaki, M.; Nakatani, K.; Tsubakio-Yamamoto, K.; Ohama, T.; Matsuyama, A.; et al. Patients with CD36 deficiency are associated with enhanced atherosclerotic cardiovascular diseases. J. Atheroscler. Thromb. 2012, 19, 263–275. [Google Scholar] [CrossRef]
- Kilian, L.S.; Voran, J.; Frank, D.; Rangrez, A.Y. RhoA: A dubious molecule in cardiac pathophysiology. J. Biomed. Sci. 2021, 28, 33. [Google Scholar] [CrossRef]
- Segain, J.P.; Raingeard de la Blétière, D.; Sauzeau, V.; Bourreille, A.; Hilaret, G.; Cario-Toumaniantz, C.; Pacaud, P.; Galmiche, J.P.; Loirand, G. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: Evidence in Crohn’s disease and experimental colitis. Gastroenterology 2003, 124, 1180–1187. [Google Scholar] [CrossRef]
- Shimokawa, H.; Sunamura, S.; Satoh, K. RhoA/Rho-Kinase in the Cardiovascular System. Circ. Res. 2016, 118, 352–366. [Google Scholar] [CrossRef]
- Wiendl, H.; Mitsdoerffer, M.; Schneider, D.; Chen, L.; Lochmüller, H.; Melms, A.; Weller, M. Human muscle cells express a B7-related molecule, B7-H1, with strong negative immune regulatory potential: A novel mechanism of counterbalancing the immune attack in idiopathic inflammatory myopathies. FASEB J. 2003, 17, 1892–1894. [Google Scholar] [CrossRef]
- Zhang, P.; Manes, T.D.; Pober, J.S.; Tellides, G. Human vascular smooth muscle cells lack essential costimulatory molecules to activate allogeneic memory T cells. Arter. Thromb. Vasc. Biol. 2010, 30, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Scotton, C.J.; Martinez, F.O.; Smelt, M.J.; Sironi, M.; Locati, M.; Mantovani, A.; Sozzani, S. Transcriptional profiling reveals complex regulation of the monocyte IL-1 beta system by IL-13. J. Immunol. 2005, 174, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, M.; Huang, W.; Chen, W.; Zhao, Y.; Schulte, M.L.; Volberding, P.; Gerbec, Z.; Zimmermann, M.T.; Zeighami, A.; et al. Mitochondrial Metabolic Reprogramming by CD36 Signaling Drives Macrophage Inflammatory Responses. Circ. Res. 2019, 125, 1087–1102. [Google Scholar] [CrossRef]
- Voss, K.; Hong, H.S.; Bader, J.E.; Sugiura, A.; Lyssiotis, C.A.; Rathmell, J.C. A guide to interrogating immunometabolism. Nat. Rev. Immunol. 2021, 21, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef]
- van der Valk, F.M.; Bekkering, S.; Kroon, J.; Yeang, C.; Van den Bossche, J.; van Buul, J.D.; Ravandi, A.; Nederveen, A.J.; Verberne, H.J.; Scipione, C.; et al. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation 2016, 134, 611–624. [Google Scholar] [CrossRef]
- Aicher, A.; Heeschen, C.; Mohaupt, M.; Cooke, J.P.; Zeiher, A.M.; Dimmeler, S. Nicotine strongly activates dendritic cell-mediated adaptive immunity: Potential role for progression of atherosclerotic lesions. Circulation 2003, 107, 604–611. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Locati, M. Macrophage polarization comes of age. Immunity 2005, 23, 344–346. [Google Scholar] [CrossRef]
- Lu, C.-H.; Lai, C.-Y.; Yeh, D.-W.; Liu, Y.-L.; Su, Y.-W.; Hsu, L.-C.; Chang, C.-H.; Catherine Jin, S.-L.; Chuang, T.-H. Involvement of M1 Macrophage Polarization in Endosomal Toll-Like Receptors Activated Psoriatic Inflammation. Mediat. Inflamm. 2018, 2018, 3523642. [Google Scholar] [CrossRef]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna. J. Med. Biotechnol. 2010, 2, 161–179. [Google Scholar] [PubMed]
- Park, C.S.; Kim, I.; Oh, G.C.; Han, J.K.; Yang, H.M.; Park, K.W.; Cho, H.J.; Kang, H.J.; Koo, B.K.; Chung, W.Y.; et al. Diagnostic Utility and Pathogenic Role of Circulating MicroRNAs in Vasospastic Angina. J. Clin. Med. 2020, 9, 1313. [Google Scholar] [CrossRef]
- Zhang, J.W.; Pan, H.T. microRNA profiles of serum exosomes derived from children with nonalcoholic fatty liver. Genes Genom. 2022, 44, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Mizuno, Y.; Harada, E.; Kashiwagi, Y.; Yoshimura, M.; Murohara, T.; Yasue, H. Pioglitazone, a peroxisome proliferator-activated receptor γ activator, suppresses coronary spasm. Coron. Artery Dis. 2014, 25, 671–677. [Google Scholar] [CrossRef][Green Version]
- Zeng, H.; Qin, H.; Liao, M.; Zheng, E.; Luo, X.; Xiao, A.; Li, Y.; Chen, L.; Wei, L.; Zhao, L.; et al. CD36 promotes de novo lipogenesis in hepatocytes through INSIG2-dependent SREBP1 processing. Mol. Metab. 2022, 57, 101428. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, G.; Lou, Z. Role of the Sterol Regulatory Element Binding Protein Pathway in Tumorigenesis. Front. Oncol. 2020, 10, 1788. [Google Scholar] [CrossRef]
- Zhang, R.; Sui, L.; Hong, X.; Yang, M.; Li, W. MiR-448 promotes vascular smooth muscle cell proliferation and migration in through directly targeting MEF2C. Environ. Sci. Pollut. Res. Int. 2017, 24, 22294–22300. [Google Scholar] [CrossRef]
- Ueno, M.; Nakagawa, T.; Nagai, Y.; Nishi, N.; Kusaka, T.; Kanenishi, K.; Onodera, M.; Hosomi, N.; Huang, C.; Yokomise, H.; et al. The expression of CD36 in vessels with blood-brain barrier impairment in a stroke-prone hypertensive model. Neuropathol. Appl. Neurobiol. 2011, 37, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Gareri, C.; Iaconetti, C.; Sorrentino, S.; Covello, C.; De Rosa, S.; Indolfi, C. miR-125a-5p Modulates Phenotypic Switch of Vascular Smooth Muscle Cells by Targeting ETS-1. J. Mol. Biol. 2017, 429, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, D.; Rayner, K.J. MicroRNAs in cardiovascular health: From order to disorder. Endocrinology 2013, 154, 4000–4009. [Google Scholar] [CrossRef]
- Abplanalp, W.T.; Fischer, A.; John, D.; Zeiher, A.M.; Gosgnach, W.; Darville, H.; Montgomery, R.; Pestano, L.; Allée, G.; Paty, I.; et al. Efficiency and Target Derepression of Anti-miR-92a: Results of a First in Human Study. Nucleic Acid. Ther. 2020, 30, 335–345. [Google Scholar] [CrossRef]
- Viereck, J.; Thum, T. Development of a Mechanism-Based Next-Generation Therapeutic for Heart Failure Derived from the Dark Genome. JACC Basic Transl. Sci. 2023, 8, 1595–1598. [Google Scholar] [CrossRef]
- Sakai, H.; Otogoto, S.; Chiba, Y.; Abe, K.; Misawa, M. Involvement of p42/44 MAPK and RhoA protein in augmentation of ACh-induced bronchial smooth muscle contraction by TNF-alpha in rats. J. Appl. Physiol. (1985) 2004, 97, 2154–2159. [Google Scholar] [CrossRef]
- Zhuang, J.L.; Liu, Y.Y.; Li, Z.Z.; Zhuang, Q.Z.; Tang, W.Z.; Xiong, Y.; Huang, X.Z. Amentoflavone prevents ox-LDL-induced lipid accumulation by suppressing the PPARγ/CD36 signal pathway. Toxicol. Appl. Pharmacol. 2021, 431, 115733. [Google Scholar] [CrossRef]
- Xiong, X.; Tang, N.; Lai, X.; Zhang, J.; Wen, W.; Li, X.; Li, A.; Wu, Y.; Liu, Z. Insights into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021, 12, 768708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ban, Y.M.; Li, D.M.; Wang, G.; Gu, J.; Zhu, L. Amentoflavone for treating cardiocerebrovascular diseases and neurological disorders. Front. Pharmacol. 2024, 15, 1406510. [Google Scholar] [CrossRef]
- Li, W.W.; Li, D.; Qin, Y.; Sun, C.X.; Wang, Y.L.; Gao, L.; Ling-Hu, L.; Zhang, F.; Cai, W.; Zhu, L.; et al. Cardioprotective effects of Amentoflavone by suppression of apoptosis and inflammation on an in vitro and vivo model of myocardial ischemia-reperfusion injury. Int. Immunopharmacol. 2021, 101, 108296. [Google Scholar] [CrossRef]
- Fang, G.; Li, X.; Yang, F.; Huang, T.; Qiu, C.; Peng, K.; Wang, Z.; Yang, Y.; Lan, C. Amentoflavone mitigates doxorubicin-induced cardiotoxicity by suppressing cardiomyocyte pyroptosis and inflammation through inhibition of the STING/NLRP3 signalling pathway. Phytomedicine 2023, 117, 154922. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.L.; Jang, E.H.; Lee, D.E.; Bang, C.; Kang, H.; Kim, S.; Yoon, S.Y.; Lee, D.H.; Na, J.H.; Lee, S.; et al. Amentoflavone, active compound of Selaginella tamariscina, inhibits in vitro and in vivo TGF-β-induced metastasis of human cancer cells. Arch. Biochem. Biophys. 2020, 687, 108384. [Google Scholar] [CrossRef]
- Pei, J.S.; Liu, C.C.; Hsu, Y.N.; Lin, L.L.; Wang, S.C.; Chung, J.G.; Bau, D.T.; Lin, S.S. Amentoflavone induces cell-cycle arrest and apoptosis in MCF-7 human breast cancer cells via mitochondria-dependent pathway. In Vivo 2012, 26, 963–970. [Google Scholar]
- Guruvayoorappan, C.; Kuttan, G. Amentoflavone stimulates apoptosis in B16F-10 melanoma cells by regulating bcl-2, p53 as well as caspase-3 genes and regulates the nitric oxide as well as proinflammatory cytokine production in B16F-10 melanoma cells, tumor associated macrophages and peritoneal macrophages. J. Exp. Ther. Oncol. 2008, 7, 207–218. [Google Scholar]
- Gibbons, R.J.; Chatterjee, K.; Daley, J.; Douglas, J.S.; Fihn, S.D.; Gardin, J.M.; Grunwald, M.A.; Levy, D.; Lytle, B.W.; O’Rourke, R.A.; et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: Executive summary and recommendations. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina). Circulation 1999, 99, 2829–2848. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): Digest version. Circ. J. 2010, 74, 1745–1762. [Google Scholar] [CrossRef] [PubMed]
- Fichtlscherer, S.; Breuer, S.; Schächinger, V.; Dimmeler, S.; Zeiher, A.M. C-reactive protein levels determine systemic nitric oxide bioavailability in patients with coronary artery disease. Eur. Heart J. 2004, 25, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Mahmood, T.; Yang, P.C. Western blot: Technique, theory and trouble shooting. N. Am. J. Med. Sci. 2014, 6, 160. [Google Scholar] [CrossRef]

| Controls (n = 36) | CAS (n = 41) | p-Value | ||||
|---|---|---|---|---|---|---|
| Age, years | 56.6 ± 15.4 | 56.3 ± 11.9 | 0.94 | |||
| Male sex, n (%) | 12 (33) | 23 (56) | 0.045 | |||
| Body mass index, kg/m2 | 24.5 ± 4.1 | 26.0 ± 4.5 | 0.12 | |||
| Current smoker, n (%) | 4 (11) | 15 (41) | 0.004 | |||
| Diabetes mellitus, n (%) | 4 (11) | 2 (5) | 0.38 | |||
| Hypertension, n (%) | 5 (14) | 9 (24) | 0.26 | |||
| Systolic blood pressure, mmHg | 113 ± 14 | 115 ± 17 | 0.56 | |||
| Diastolic blood pressure, mmHg | 67 ± 10 | 71 ± 10 | 0.10 | |||
| Heart rate, beats/min | 67 ± 9 | 71 ± 14 | 0.24 | |||
| Left ventricular ejection fraction, % | 65 ± 5 | 65 ± 7 | 0.98 | |||
| Total cholesterol, mg/dL | 171 ± 35 | 166 ± 33 | 0.56 | |||
| Triglyceride, mg/dL | 85 ± 51 | 99 ± 54 | 0.26 | |||
| HDL cholesterol, mg/dL | 53 ± 12 | 47 ± 12 | 0.034 | |||
| LDL cholesterol, mg/dL | 95 ± 29 | 98 ± 27 | 0.66 | |||
| Lipoprotein(a), mg/dL | 18.6 ± 8.7 | 75.9 ± 29.4 | 0.001 | |||
| sCD36 | 2.1 ± 1 | 6.6 ± 3.3 | 0.001 | |||
| Peripheral leukocytes, /mm3 | 6006 ± 1459 | 6786 ± 2182 | 0.088 | |||
| Monocytes, /mm3 | 458 ± 159 | 551 ± 178 | 0.027 | |||
| Macrophage, /mm3 | 113 ± 36 | 411 ± 113 | 0.001 | |||
| Lymphocytes, /mm3 | 1725 ± 643 | 1669 ± 866 | 0.76 | |||
| Hemoglobin, g/dL | 13.4 ± 1.5 | 14.1 ± 1.3 | 0.03 | |||
| Hematocrit, % | 39.0 ± 5.0 | 41.3 ± 3.6 | 0.026 | |||
| Platelets, ×103/mm3 | 222 ± 57 | 241 ± 61 | 0.18 | |||
| hs-CRP, mg/L * | 0.63 (0.26–1.40) | 0.70 (0.21–0.98) | 0.31 | |||
| Provoked coronary artery | ||||||
| Left anterior descending artery, n (%) | 10 (24) | |||||
| Left circumflex artery, n (%) | 1 (2) | |||||
| Right coronary artery, n (%) | 32 (78) | |||||
| Number of spastic arteries | ||||||
| One-vessel spasm, n (%) | 35 (90) | |||||
| Two-vessel spasm, n (%) | 4 (10) | |||||
| Three-vessel spasm, n (%) | 0 (0) | |||||
| Medications | A | D | A | D | A | D |
| Aspirin, n (%) | 31 (86) | 33 (81) | 31 (89) | 36 (88) | 0.51 | 0.92 |
| β-blockers, n (%) | 34 (94) | 34 (83) | 18 (51) | 7 (17) | 0.12 | 0.001 |
| Calcium channel blockers, n (%) | 4 (11) | 11 (27) | 17 (49) | 39 (95) | 0.08 | <0.001 |
| Diuretics, n (%) | 0 (0) | 1 (2) | 1 (3) | 1 (2) | 0.35 | 0.91 |
| Angiotensin receptor blockers, n (%) | 7 (19) | 12 (29) | 7 (20) | 11 (27) | 0.32 | 0.49 |
| Nitrates, n (%) | 1 (3) | 1 (2) | 1 (3) | 0 (0) | 0.93 | 0.28 |
| Statins, n (%) | 10 (28) | 20 (49) | 11 (31) | 22 (54) | 0.06 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-K.; Hsieh, T.-H.; Yeh, C.-T.; Yadav, V.K.; Fong, I.-H.; Kuo, K.-T.; Kounis, N.G.; Hu, P.; Hung, M.-Y. Lipoprotein(a)/CD36 Interaction Drives IL-6/RhoA-GTP Signaling and miRNA Epigenetic Regulation in Coronary Artery Spasm. Pharmaceuticals 2025, 18, 1384. https://doi.org/10.3390/ph18091384
Lin Y-K, Hsieh T-H, Yeh C-T, Yadav VK, Fong I-H, Kuo K-T, Kounis NG, Hu P, Hung M-Y. Lipoprotein(a)/CD36 Interaction Drives IL-6/RhoA-GTP Signaling and miRNA Epigenetic Regulation in Coronary Artery Spasm. Pharmaceuticals. 2025; 18(9):1384. https://doi.org/10.3390/ph18091384
Chicago/Turabian StyleLin, Yen-Kuang, Tsung-Han Hsieh, Chi-Tai Yeh, Vijesh Kumar Yadav, Iat-Hang Fong, Kuang-Tai Kuo, Nicholas G. Kounis, Patrick Hu, and Ming-Yow Hung. 2025. "Lipoprotein(a)/CD36 Interaction Drives IL-6/RhoA-GTP Signaling and miRNA Epigenetic Regulation in Coronary Artery Spasm" Pharmaceuticals 18, no. 9: 1384. https://doi.org/10.3390/ph18091384
APA StyleLin, Y.-K., Hsieh, T.-H., Yeh, C.-T., Yadav, V. K., Fong, I.-H., Kuo, K.-T., Kounis, N. G., Hu, P., & Hung, M.-Y. (2025). Lipoprotein(a)/CD36 Interaction Drives IL-6/RhoA-GTP Signaling and miRNA Epigenetic Regulation in Coronary Artery Spasm. Pharmaceuticals, 18(9), 1384. https://doi.org/10.3390/ph18091384











