Small Molecule Protease Inhibitors as Model Peptidomimetics
Abstract
1. Introduction
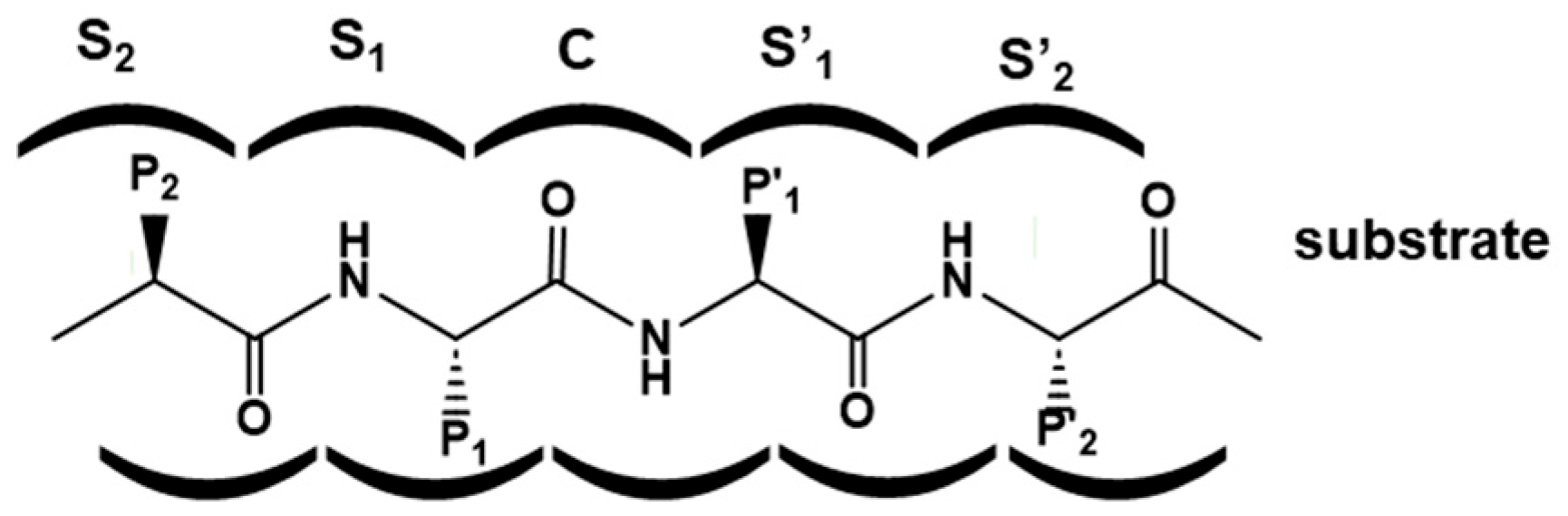
2. Designing Protease Inhibitors
3. Peptide Bond Isostere Replacement
4. Protease Inhibitor Design Case Studies
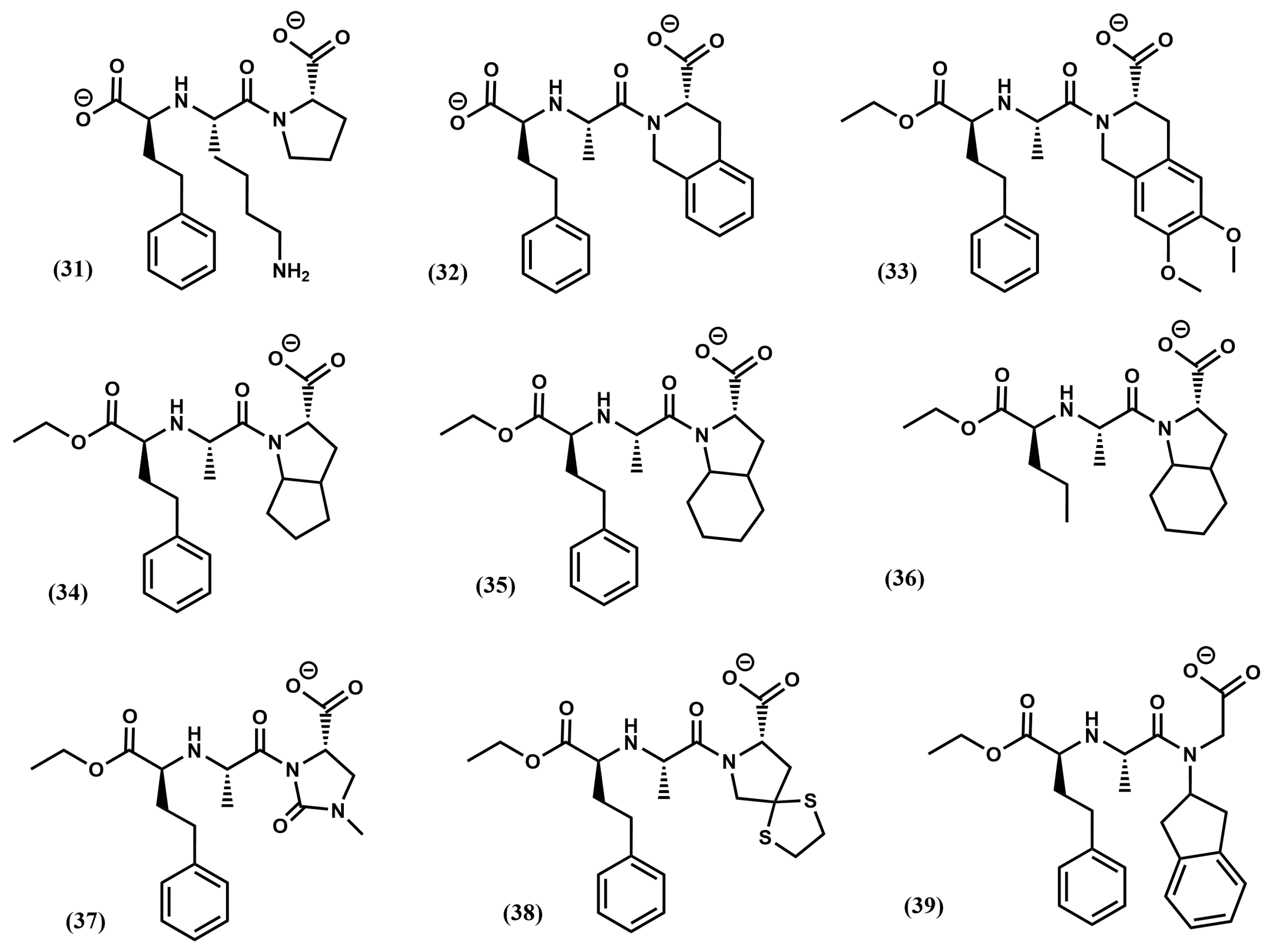
4.1. Angiotensin-1 Converting Enzyme Inhibitors
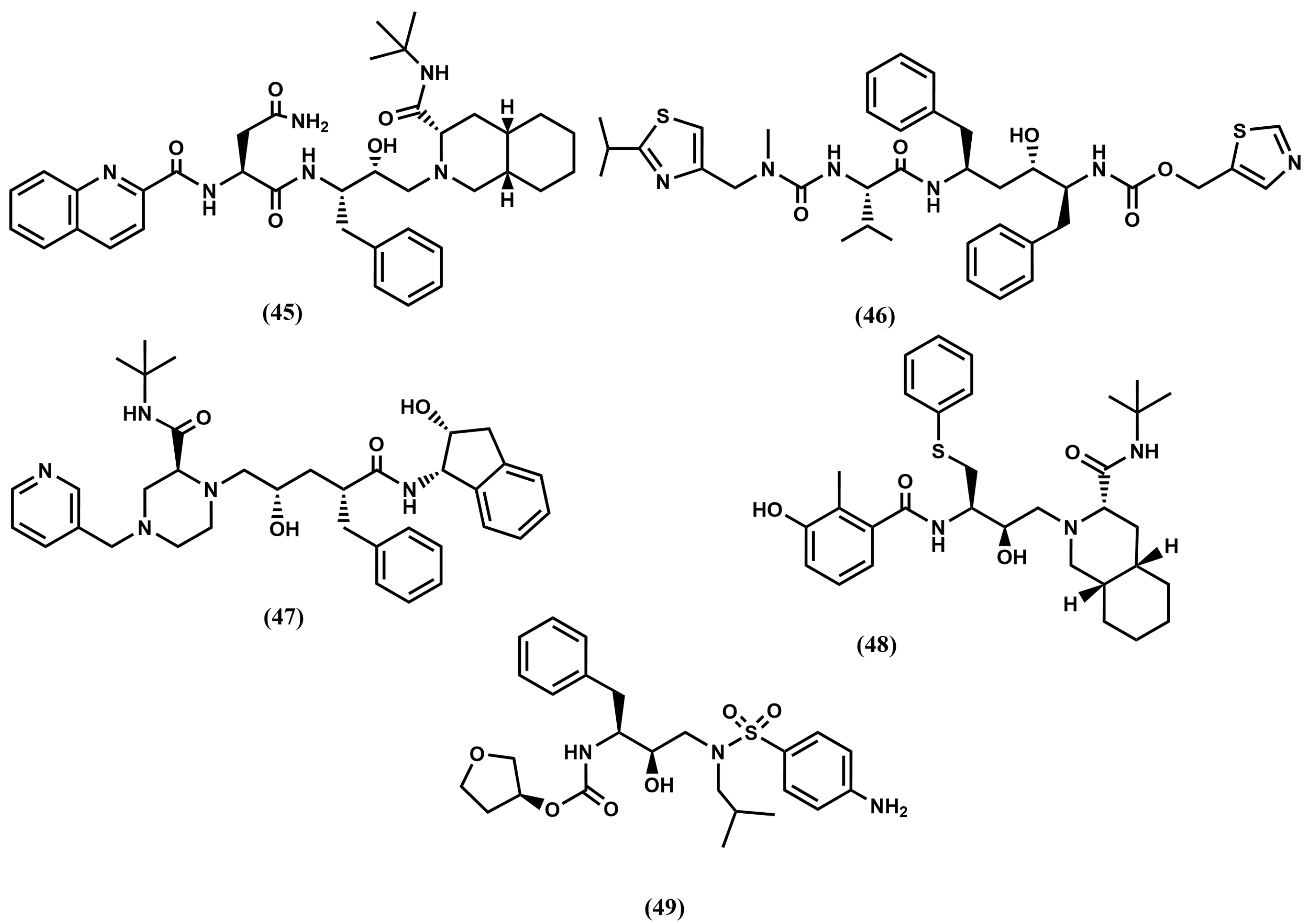
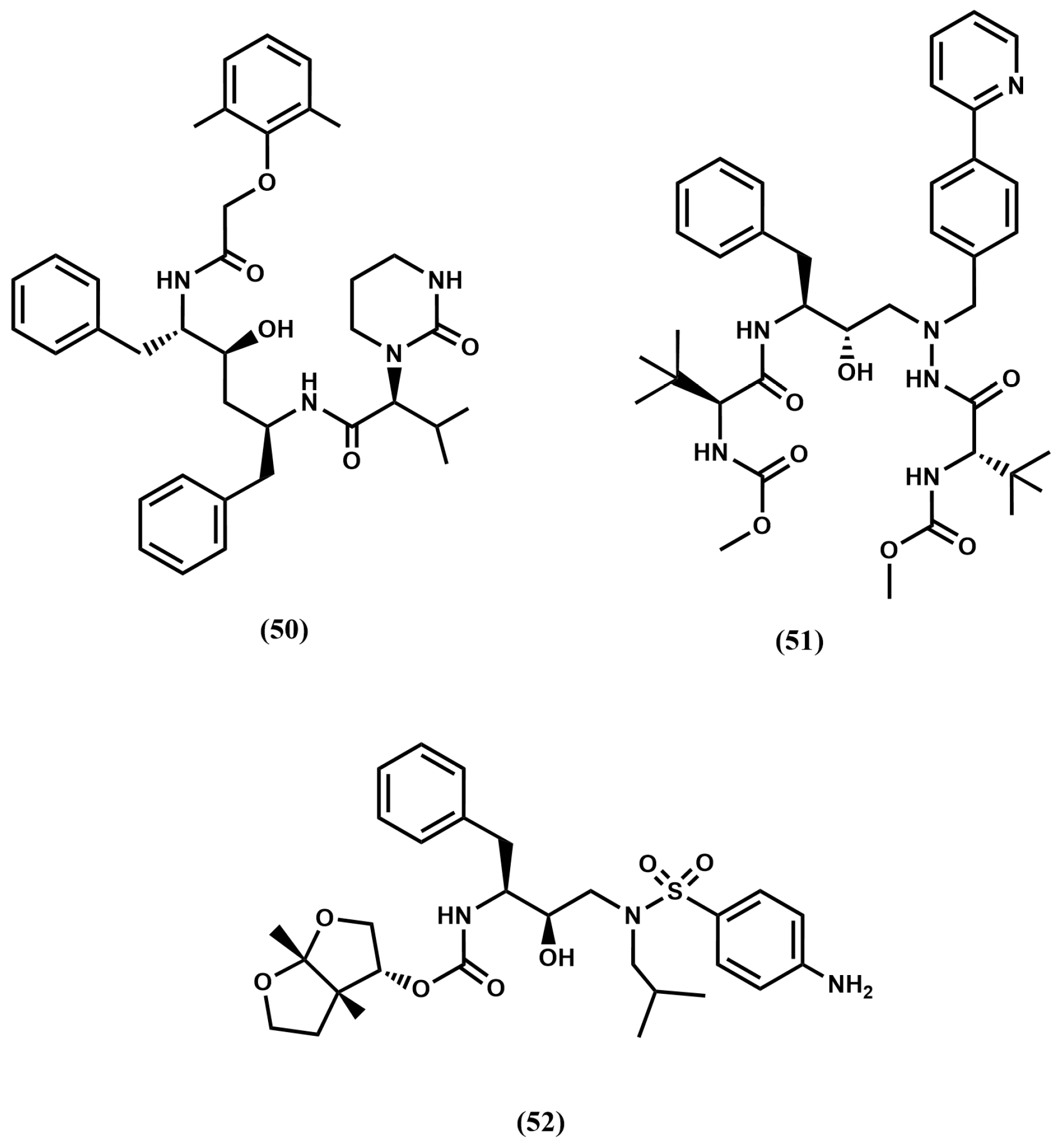
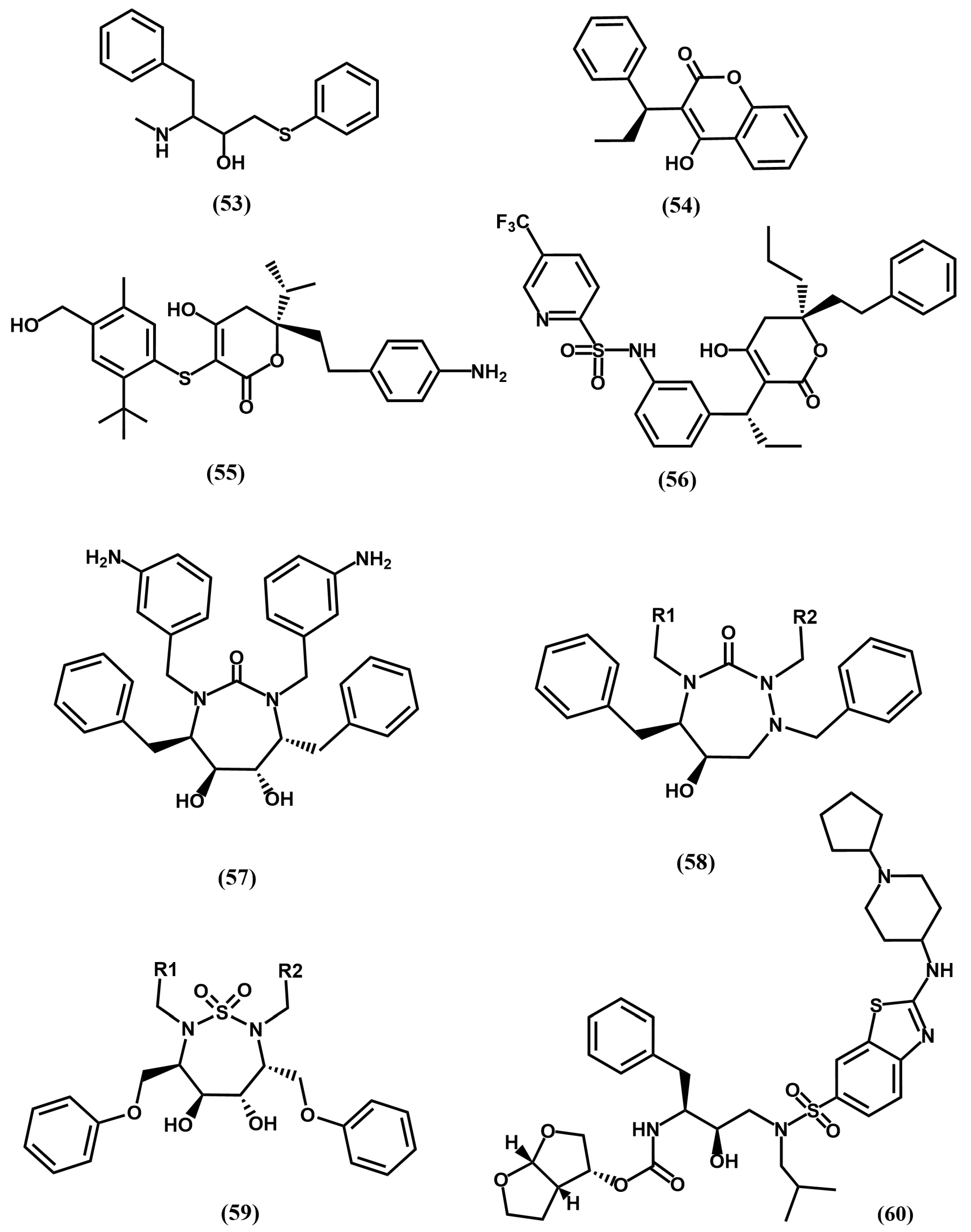
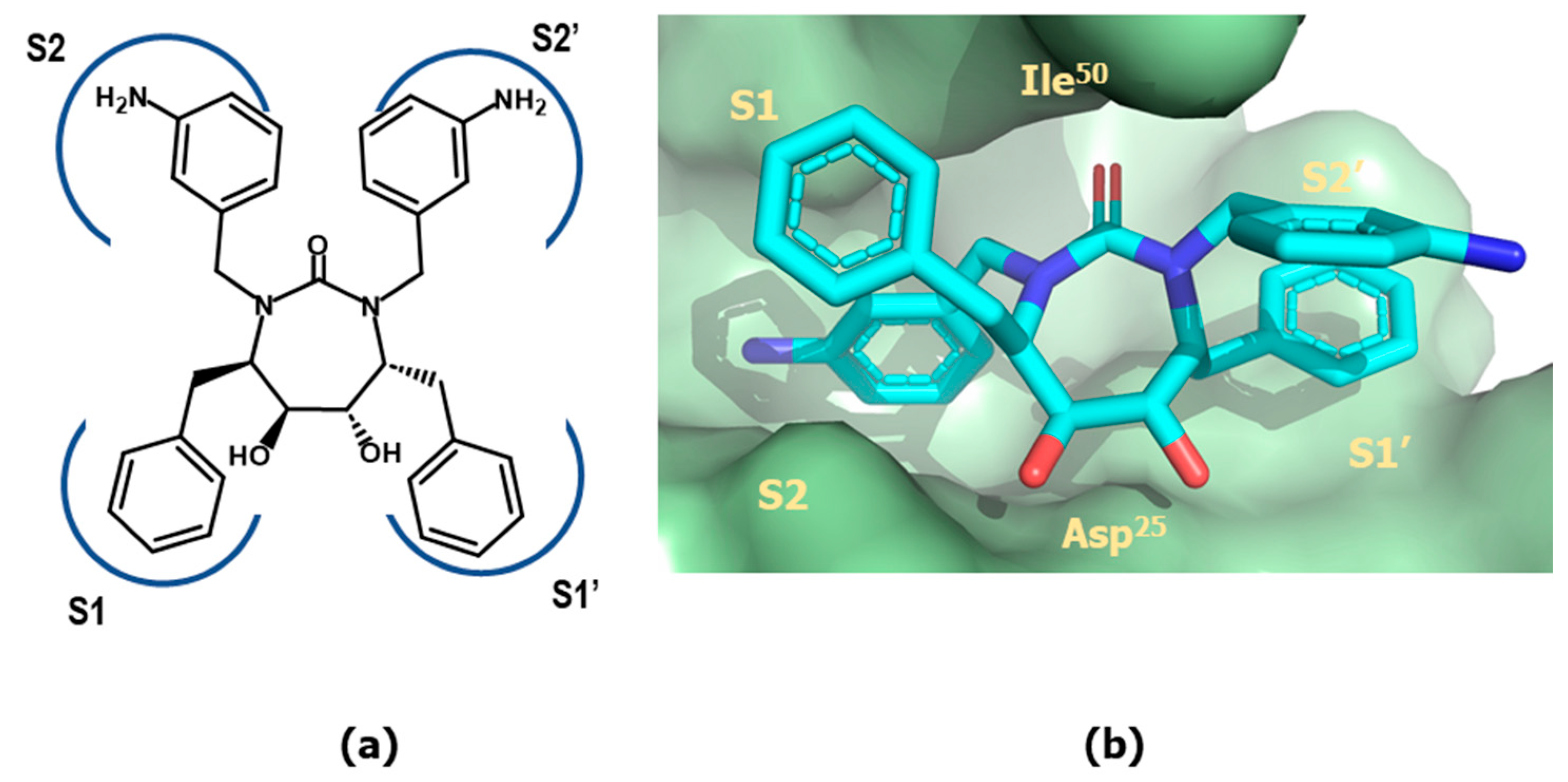
4.2. HIV-1 Protease Inhibitors
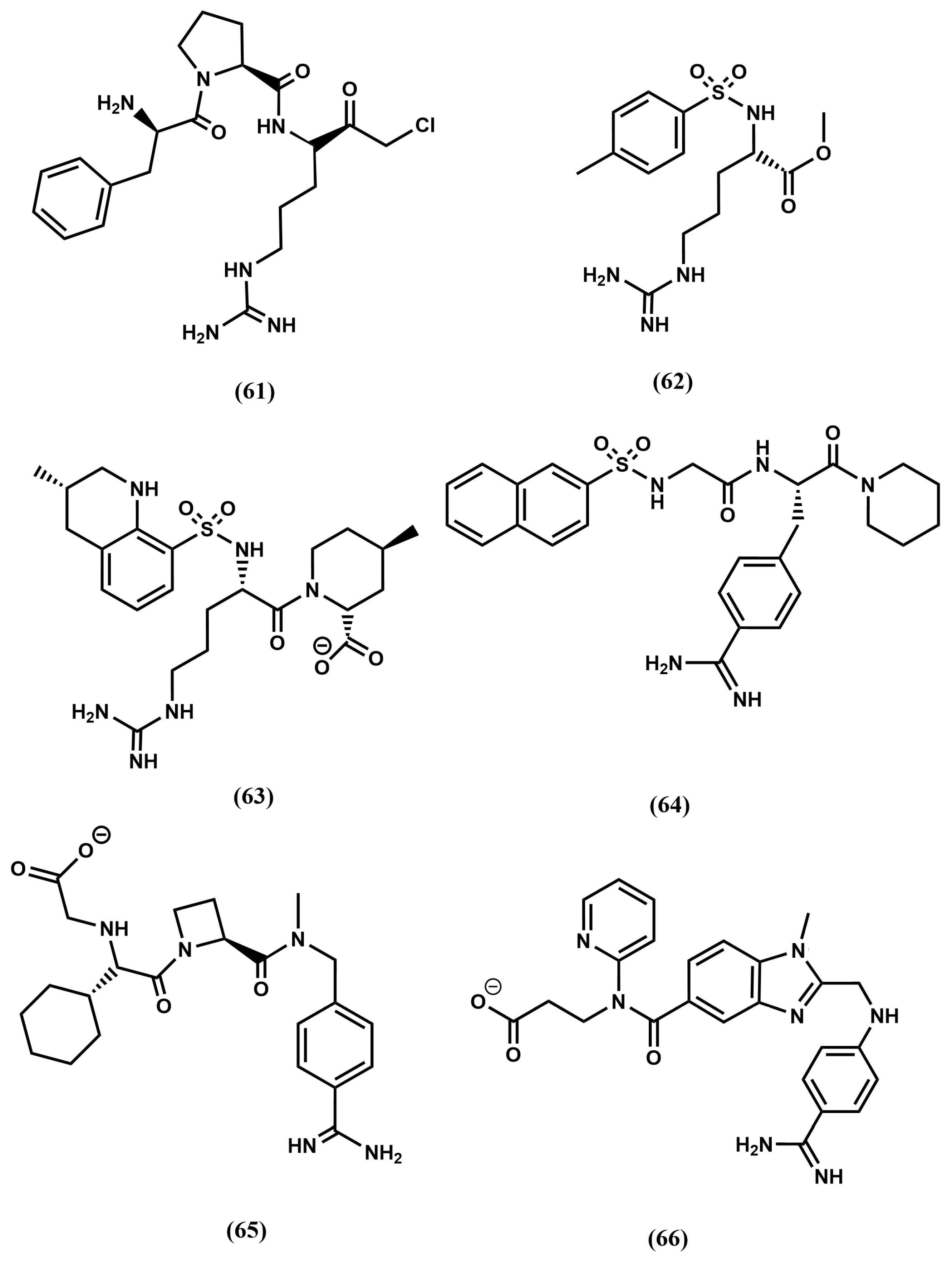
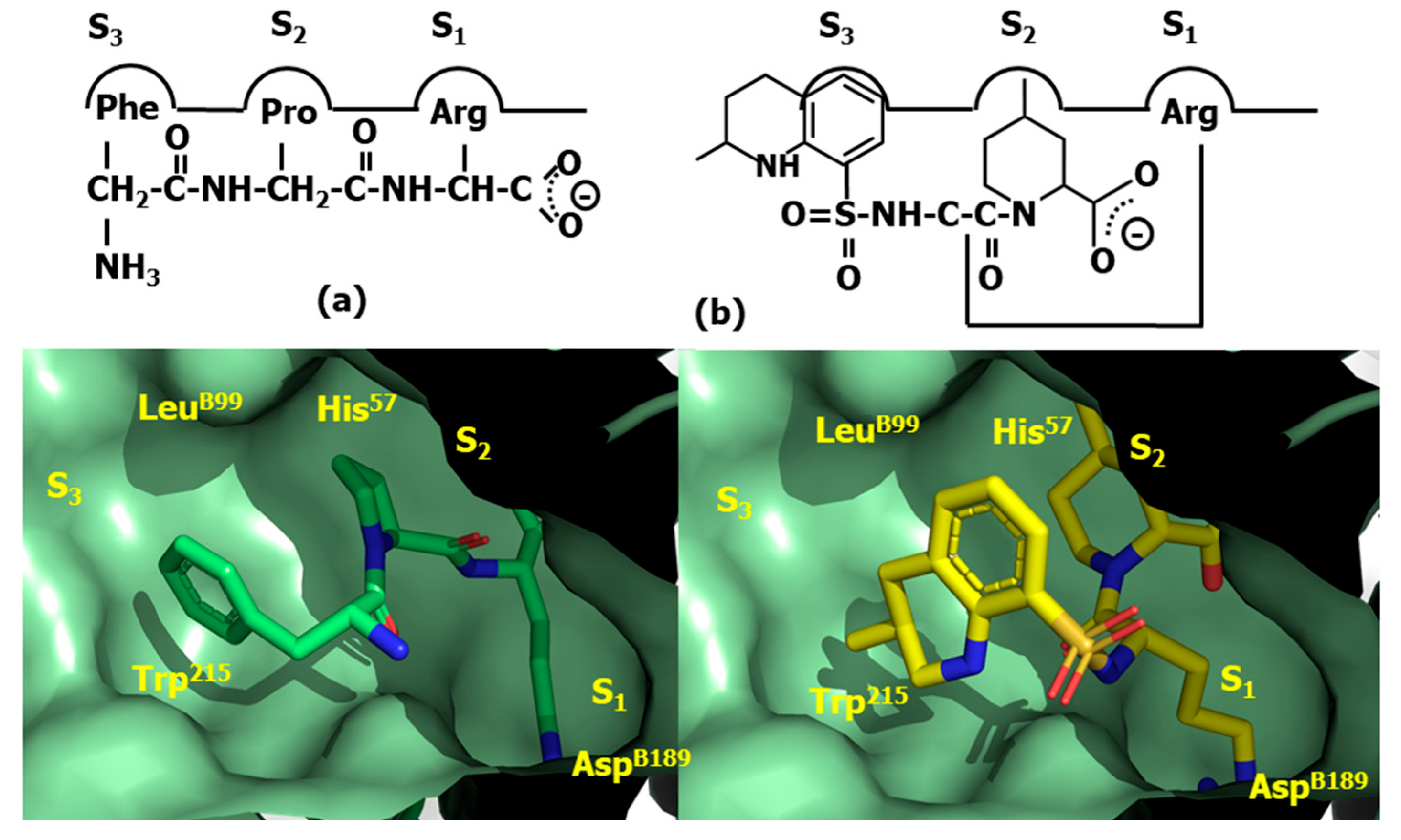
4.3. Thrombin Inhibitors
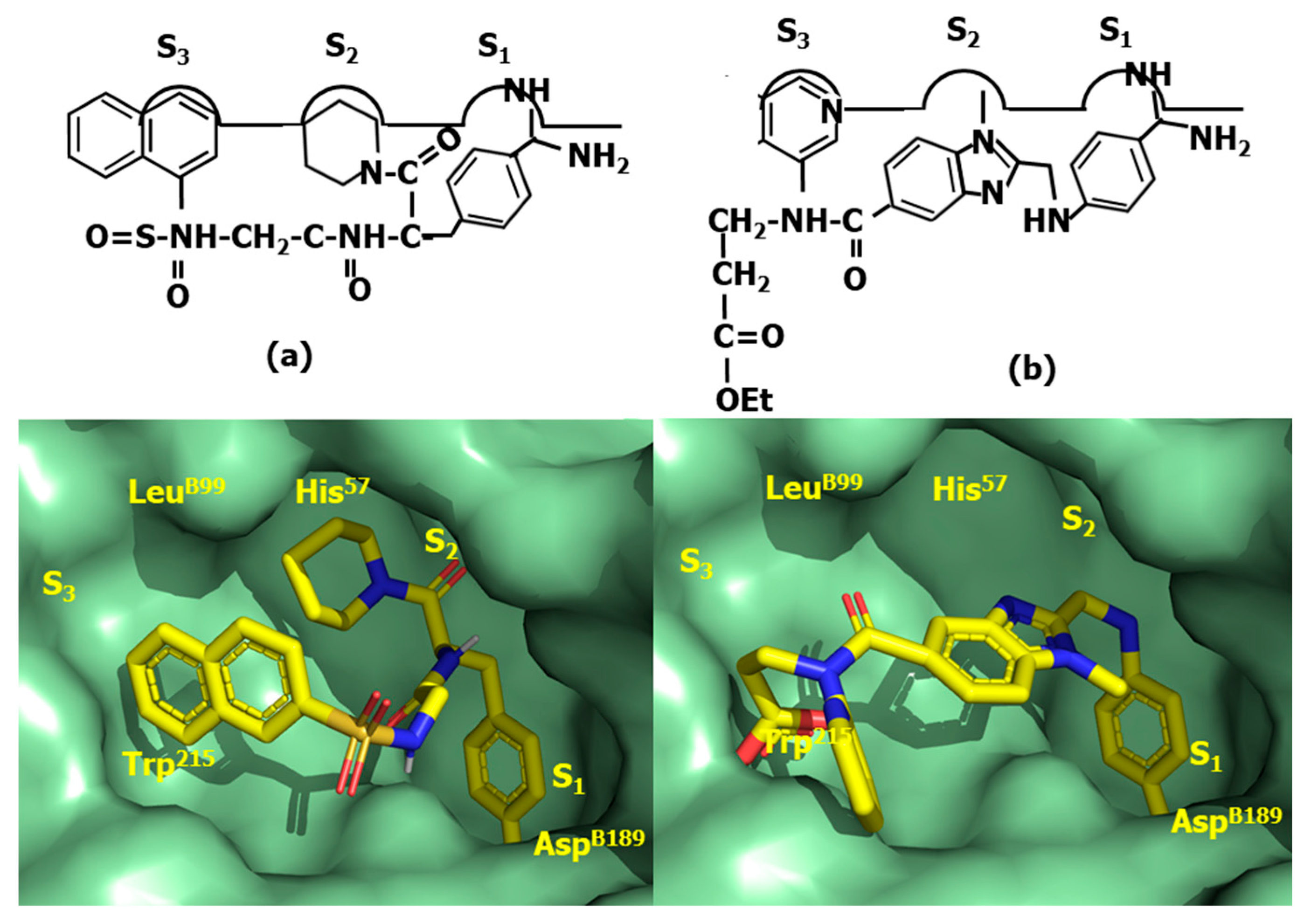
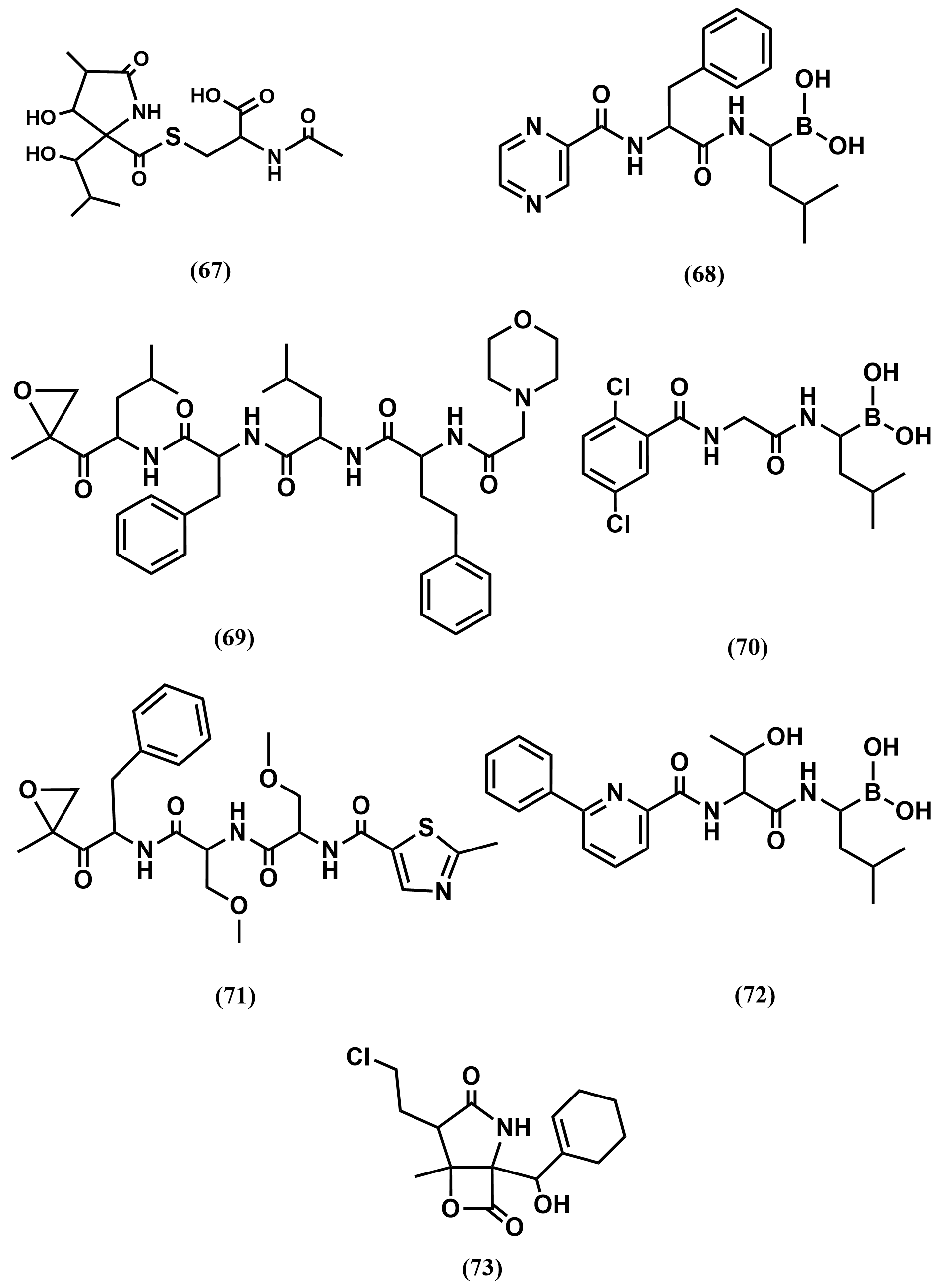
4.4. Proteasome Inhibitors
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puente, X.S.; Sanchez, L.M.; Overall, C.M.; Lopez-Otin, C. Human and mouse proteases: A comparative genomic approach. Nat. Rev. Genet. 2003, 4, 544–558. [Google Scholar] [CrossRef]
- Dikic, I. Proteasomal and autophagic degradation systems. Annu. Rev. Biochem. 2017, 86, 193–224. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Bond, J.S. Proteases: Multifunctional enzymes in life and disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef]
- Fear, G.; Komarnytsky, S.; Raskin, I. Protease inhibitors and their peptidomimetic derivatives as potential drugs. Pharmacol. Ther. 2007, 113, 354–368. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Tolle, D.P.; Barrett, A.J. Evolutionary families of peptidase inhibitors. Biochem. J. 2004, 378, 705–716. [Google Scholar] [CrossRef]
- Leung, D.; Abbenante, G.; Fairlie, D.P. Protease inhibitors: Current status and future prospects. J. Med. Chem. 2000, 43, 305–341. [Google Scholar] [CrossRef]
- Turk, B. Targeting proteases: Successes, failures and future prospects. Nat. Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef]
- Drag, M.; Salvesen, G.S. Emerging principles in protease-based drug discovery. Nat. Rev. Drug Discov. 2010, 9, 690–701. [Google Scholar] [CrossRef]
- Schechter, I.; Berger, A. On the Size of the Active Site in Proteases. I. Papain, Biochem. Biophys. Res. Commun. 1967, 27, 157–162. [Google Scholar] [CrossRef]
- Das, A.A.; Sharma, O.P.; Kumar, M.S.; Krishna, R.; Mathur, P.P. PepBind: A comprehensive database and computational tool for analysis of protein-peptide interactions. Genom. Proteom. Bioinform. 2013, 11, 241–246. [Google Scholar] [CrossRef]
- Timmer, J.C.; Zhu, W.; Pop, C.; Regan, T.; Snipas, S.J.; Eroshkin, A.M.; Riedl, S.J.; Salvesen, G.S. Structural and kinetic determinants of protease substrates. Nat. Struct. Mol. Biol. 2009, 16, 1101–1109. [Google Scholar] [CrossRef]
- Prabu-Jeyabalan, M.; Nalivaika, E.A.; Romano, K.; Schiffer, C.A. Mechanism of substrate recognition by drug-resistant human immunodeficiency virus type 1 protease variants revealed by a novel structural intermediate. J. Virol. 2006, 80, 3607–3616. [Google Scholar] [CrossRef]
- Katz, B.A.; Sprengeler, P.A.; Luong, C.; Verner, E.; Elrod, K.; Kirtley, M.; Janc, J.; Spencer, J.R.; Breitenbucher, J.G.; Hui, H.; et al. Engineering inhibitors highly selective for the S1 sites of Ser190 trypsin-like serine protease drug targets. Chem. Biol. 2001, 8, 1107–1121. [Google Scholar] [CrossRef][Green Version]
- Todd, B.; Moore, D.; Deivanayagam, C.C.; Lin, G.D.; Chattopadhyay, D.; Maki, M.; Wang, K.K.; Narayana, S.V. A structural model for the inhibition of calpain by calpastatin: Crystal structures of the native domain VI of calpain and its complexes with calpastatin peptide and a small molecule inhibitor. J. Mol. Biol. 2003, 328, 131–146. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Q.; Chi, C. Crystal structure of mung bean inhibitor lysine active fragment complex with bovine beta-trypsin at 1.8A resolution. J. Biomol. Struct. Dyn. 1999, 16, 1219–1224. [Google Scholar] [CrossRef]
- Rubio-Martínez, J.; Jiménez-Alesanco, A.; Ceballos-Laita, L.; Ortega-Alarcón, D.; Vega, S.; Calvo, C.; Benítez, C.; Abian, O.; Velázquez-Campoy, A.; Thomson, T.M.; et al. Discovery of Diverse Natural Products as inhibitors of SARS-CoV-2 Mpro Protease through Virtual Screening. J. Comp. Inf. Model. 2021, 61, 6094–6106. [Google Scholar] [CrossRef]
- Shramm, V.L. Enzymatic transition states and transition state analogs. Annu. Rev. Biochem. 1998, 67, 693–720. [Google Scholar] [CrossRef]
- Burger, A. Isosterism and bioisosterism in drug design. Prog. Drug Res. 1991, 37, 287–371. [Google Scholar]
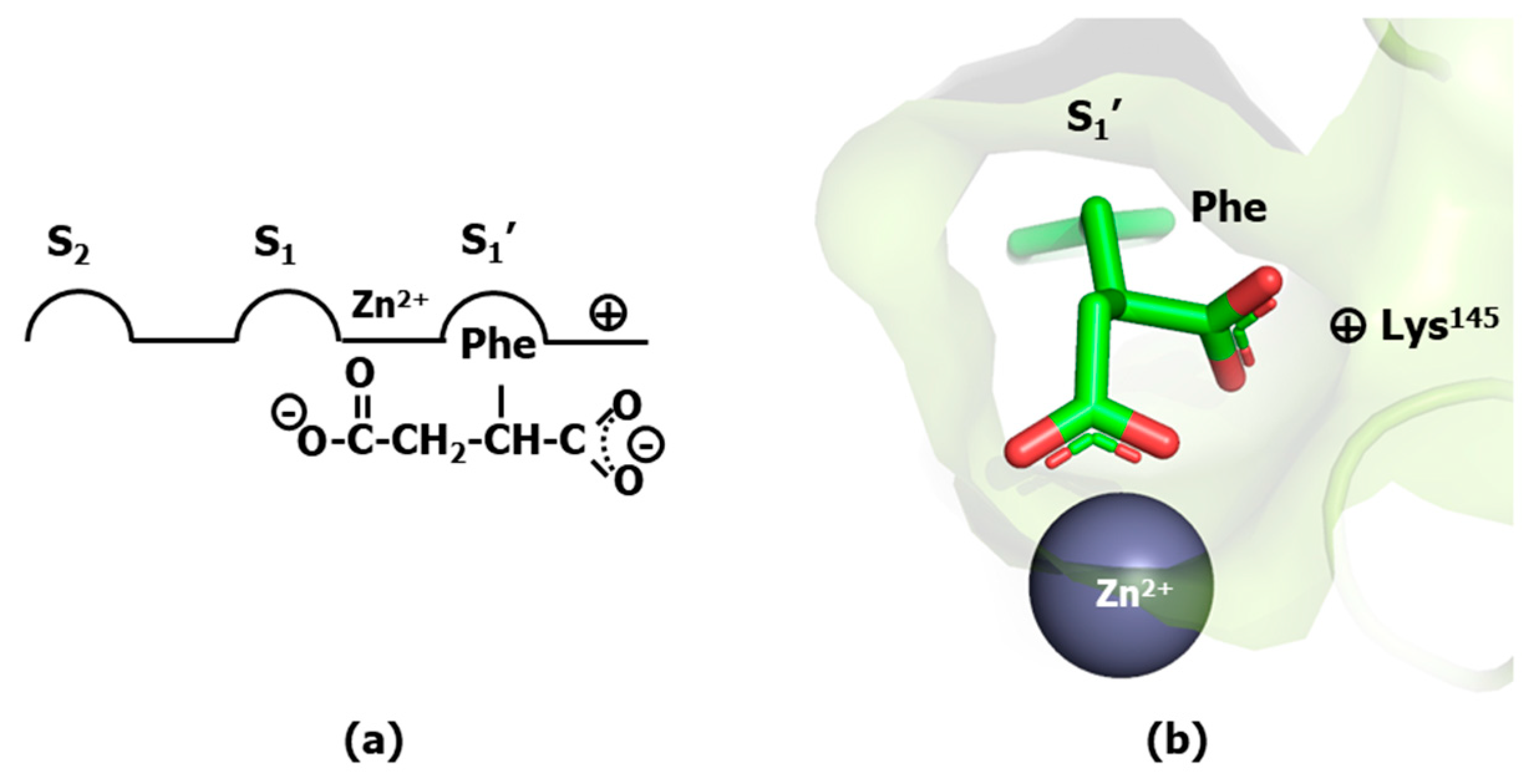
- Byers, L.D.; Wolfenden, R. Binding of by-product analog benzylsuccinic acid by carboxypeptidase A. Biochemistry 1973, 12, 2070–2078. [Google Scholar] [CrossRef]
- Mangani, S.; Carloni, P.; Orioli, P. Crystal structure of the complex between carboxypeptidase A and the biproduct analog inhibitor L-benzylsuccinate at 2.0 A resolution. J. Mol. Biol. 1992, 223, 573–578. [Google Scholar] [CrossRef]
- Matthews, B.W. Structural basis of the action of thermolysin and related zinc peptidases. Acc. Chem. Res. 1988, 21, 333–340. [Google Scholar] [CrossRef]
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. [Google Scholar] [CrossRef]
- Biron, E.; Chatterjee, J.; Ovadia, O.; Langenegger, D.; Brueggen, J.; Hoyer, D.; Schmid, H.A.; Jelinek, R.; Gilon, C.; Hoffman, A.; et al. Improving oral bioavailability of peptides by multiple N-methylation: Somatostatin analogues. Angew. Chem. Int. Ed. 2008, 47, 2595–2599. [Google Scholar] [CrossRef]
- Choudhary, A.; Raines, R.T. An Evaluation of Peptide-Bond Isosteres. ChemBioChem 2011, 12, 1801–1807. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Sasaki, K.; Nagai, K.; Shin-ya, K.; Furihata, K. Structure of Thioviridamide, a Novel Apoptosis Inducer from Streptomyces olivoviridis. J. Antibiot. 2006, 59, 6–10. [Google Scholar] [CrossRef]
- Beaulieu, P.L.; Wernic, D.; Abraham, A.; Anderson, P.C.; Bogri, T.; Bousquet, Y.; Croteau, G.; Guse, I.; Lamarre, D.; Liard, F.; et al. Potent HIV protease inhibitors containing a novel (hydroxyethyl)amide isostere. J. Med. Chem. 1997, 40, 2164–2176. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Kumaragurubaran, N.; Hong, L.; Kulkarni, S.; Xu, X.; Miller, H.B.; Reddy, D.S.; Weerasena, V.; Turner, R.; Chang, W.; et al. Potent memapsin 2 (β-secretase) inhibitors: Design, synthesis, protein-ligand X-ray structure, and in vivo evaluation. Bioorg. Med. Chem. Lett. 2008, 18, 1031–1036. [Google Scholar] [CrossRef]
- Kobayashi, K.; Otani, T.; Ijiri, S.; Kawasaki, Y.; Matsubara, H.; Miyagi, T.; Kitajima, T.; Iseki, R.; Ishizawa, K.; Shindo, N.; et al. Structure-activity relationship study of hydroxyethylamine isostere and P1′ site structure of peptide mimetic BACE1 inhibitors. Bioorg. Med. Chem. 2021, 50, 116459. [Google Scholar] [CrossRef]
- Kovada, V.; Withers-Martinez, C.; Bobrovs, R.; Ce Rule, H.N.; Liepins, E.; Grinberga, S.; Hackett, F.; Collins, C.R.; Kreicberga, A.; Jiménez-Díaz, M.B.; et al. Macrocyclic Peptidomimetic Plasmepsin X Inhibitors with Potent In Vitro and In Vivo Antimalarial Activity. J. Med. Chem. 2023, 66, 10658–10680. [Google Scholar] [CrossRef]
- Gradman, A.; Schmieder, R.; Lins, R.; Nussberger, J.; Chiang, Y.; Bedigian, M. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation 2005, 111, 1012–1018. [Google Scholar] [CrossRef]
- Ivkovic, J.; Jha, S.; Lembacher-Fadum, C.; Puschnig, J.; Kumar, P.; Reithofer, V.; Gruber, K.; Macheroux, P.; Breinbauer, R. Efficient Entropy-Driven Inhibition of Dipeptidyl Peptidase III by Hydroxyethylene Transition-State Peptidomimetics. Chemistry 2021, 27, 14108–14120. [Google Scholar] [CrossRef]
- Etoh, Y.; Miyazaki, M.; Saitoh, H.; Toda, N. KRI-1314: An orally effective inhibitor of human renin. Jpn. J. Pharmacol. 1993, 63, 109–119. [Google Scholar] [CrossRef][Green Version]
- Nguyen, J.T.; Hamada, Y.; Kimura, T.; Kiso, Y. Design of potent aspartic protease inhibitors to treat various diseases. Arch. Pharm. 2008, 341, 523–535. [Google Scholar] [CrossRef]
- Gupta, D.; Yedidi, R.S.; Varghese, S.; Kovari, L.C.; Woster, P.M. Mechanism-based inhibitors of the aspartyl protease plasmepsin II as potential antimalarial agents. J. Med. Chem. 2010, 53, 4234–4247. [Google Scholar] [CrossRef]
- Black, W.C.; Bayly, C.I.; Davis, D.E.; Desmarais, S.; Falgueyret, J.-P.; Leger, S.; Li, C.S.; Masse, F.; McKay, D.J.; Palmer, J.T.; et al. Trifluoroethylamines as amide isosteres in inhibitors of cathepsin K. Bioorg. Med. Chem. Lett. 2005, 15, 4741–4744. [Google Scholar] [CrossRef]
- Vacca, J.P. Design of tight-binding human-immunodeficiency-virus type-1 protease inhibitors. Methods Enzymol. 1994, 241, 311–334. [Google Scholar]
- Angelastro, M.R.; Marquart, A.L.; Mehdi, S.; Koehl, J.R.; Vaz, R.J.; Bey, P.; Peet, N.P. The synthesis of ketomethylene pseudopeptide analogues of dipeptide aldehyde inhibitors of calpain. Bioorg. Med. Chem. Lett. 1999, 9, 139–140. [Google Scholar] [CrossRef]
- Torbeev, V.Y.; Mandal, K.; Terechko, V.A.; Kent, S.B.H. Crystal structure of chemically synthesized HIV-1 protease and a ketomethylene isostere inhibitor based on the p2/NC cleavage site. Bioorg. Med. Chem. Lett. 2008, 18, 4554–4557. [Google Scholar] [CrossRef]
- Gelb, M.H.; Svaren, J.P.; Abeles, R.H. Fluoro ketone inhibitors of hydrolytic enzymes. Biochemistry 1985, 9, 1813–1817. [Google Scholar] [CrossRef]
- Schirlin, D.S.; Baltzer, V.; Van Dorsselaer, F.; Weber, D.; Weill, J.M.; Altenburger, B.; Neises, G.; Flynn, J.M.; Remy, C.; Tarnus, C. Short and unexpectedly potent difluorostatone type inhibitors of HIV-1 protease. Med. Chem. Lett. 1993, 3, 253–258. [Google Scholar] [CrossRef]
- Skiles, J.W.; Miao, C.; Sorek, R.; Jacober, S.; Mui, P.W.; Chow, G.; Weldon, S.W.; Possanza, G.; Skoog, M.; Keirns, J. Inhibition of human leukocyte elastase (HLE) by N-substituted peptidyl trifluoromethyl ketones. Eur. J. Med. Chem. 1992, 35, 641–662. [Google Scholar] [CrossRef]
- Eda, M.; Ashimori, A.; Akahoshi, F.; Yoshimura, T.; Inoue, Y.; Fukaya, C.; Nakajima, M.; Fukuyama, H.; Imada, T.; Nakamura, N. Peptidyl human heart chymase inhibitors. 1. Synthesis and inhibitory activity of difluoromethylene ketone derivatives bearing P′ binding subsites. Bioorg. Med. Chem. Lett. 1998, 8, 913–918. [Google Scholar] [CrossRef]
- Supuran, C.T.; Casini, A.; Scozzafava, A. Protease inhibitors of the sulfonamide type: Anticancer, antiinflammatory, and antiviral agents. Med. Res. Rev. 2003, 23, 535–558. [Google Scholar] [CrossRef]
- Ogden, R.C.; Flexner, C.W. (Eds.) Protease Inhibitors in AIDS Therapy; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Jung, S.; Fuchs, N.; Johe, P.; Wagner, A.; Diehl, E.; Yuliani, T.; Zimmer, C.; Barthels, F.; Zimmermann, R.A.; Klein, P.; et al. Fluorovinylsulfones and Sulfonates as Potent Covalent Reversible Inhibitors of the Trypanosomal Cysteine Protease Rhodesain: Structure-Activity Relationship, Inhibition Mechanism, Metabolism, and In Vivo Studies. J. Med. Chem. 2021, 64, 12322–12358. [Google Scholar] [CrossRef]
- Yang, K.-W.; Brand, J.J.; Chatwood, L.L.; Crowder, M.W. Phosphonamidate and Phosphothioate Dipeptides as Potential Inhibitors of VanX. Bioorg. Med. Chem. Lett. 2000, 10, 1085–1087. [Google Scholar] [CrossRef]
- Elliott, T.S.; Slowey, A.; Yeb, Y.; Conway, S.J. The use of phosphate bioisosteres in medicinal chemistry and chemical biology. Med. Chem. Commun. 2012, 3, 735–751. [Google Scholar] [CrossRef]
- Linares, M.; Devin, C.; Azay, J.; Bergé, G.; Fehrentz, J.A. Martinez, Syntheses and biological activities of bombesin analogs modified in the C-terminal dipeptide part. Eur. J. Med. Chem. 1997, 32, 767–780. [Google Scholar] [CrossRef]
- Wiktelius, D.; Luthman, K. Taking control of P1, P1′ and double bond stereochemistry in the synthesis of Phe-Phe (E)-alkene amide isostere dipeptidomimetics. Org. Biomol. Chem. 2007, 5, 603–605. [Google Scholar] [CrossRef]
- Dutheuil, G.; Couve-Bonnaire, S.; Pannecoucke, X. Diastereomeric Fluoroolefins as Peptide Bond Mimics Prepared by Asymmetric Reductive Amination of α-Fluoroenones. Angew. Chem. Int. Ed. 2007, 46, 1290–1292. [Google Scholar] [CrossRef]
- Van Marsenille, M.; Gysen, C.; Tourwe, D.; Van Binst, G. Synthesis of a Protected Acetylene Dipeptide Isostere. Bull. Soc. Chim. Belg. 1986, 95, 127–133. [Google Scholar] [CrossRef]
- Asgian, J.L.; James, K.E.; Li, Z.Z.; Carter, W.; Barrett, A.J.; Mikolajczyk, J.; Salvesen, G.S.; Powers, J.C. Aza-Peptide Epoxides: A New Class of Inhibitors Selective for Clan CD Cysteine Proteases. J. Med. Chem. 2002, 45, 4958–4960. [Google Scholar] [CrossRef]
- Lim, I.T.; Meroueh, S.O.; Lee, M.; Heeg, M.J.; Mobashery, S. Strategy in Inhibition of Cathepsin B, A Target in Tumor Invasion and Metastasis. J. Am. Chem. Soc. 2004, 126, 10271–10277. [Google Scholar] [CrossRef]
- Cushman, D.W.; Ondetti, M.A. Design of angiotensin converting enzyme inhibitors. Nat. Med. 1999, 5, 1110–1112. [Google Scholar] [CrossRef]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Laurent, S. Antihypertensive drugs. Pharmacol. Res. 2017, 124, 116–125. [Google Scholar] [CrossRef]
- Van Epps, H.L. Harry Goldblatt and the discovery of renin. J. Exp. Med. 2005, 201, 1351. [Google Scholar] [CrossRef]
- Antonaccio, M.J. Angiotensin convertin enzyme inhibitors. Annu. Rev. Pharmacol. Toxicol. 1982, 22, 57–87. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Ong, F.S.; Blackwell, W.L.; Shah, K.H.; Giani, J.F.; Gonzalez-Villalobos, R.A.; Shen, X.Z.; Fuchs, S.; Touyz, R.M. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol. Rev. 2012, 65, 1–46. [Google Scholar] [CrossRef]
- Ondetti, M.A.; Williams, N.J.; Sabo, E.F.; Pluscec, J.; Weaver, E.R.; Kocy, O. Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry 1971, 10, 4033–4039. [Google Scholar] [CrossRef]
- Collier, J.G.; Robinson, B.F.; Vane, J.R. Reduction of pressor effects of angiotensin I in man by synthetic nonapeptide (B.P.P.9a or SQ 20,881) which inhibits coverting enzyme. Lancet 1973, 1, 72–74. [Google Scholar] [CrossRef]
- Ondetti, M.A.; Rubin, B.; Cushman, D.W. Design of specific inhibitors of angiotensin-converting enzyme: New class of orally active antihypertensive agents. Science 1977, 196, 441–444. [Google Scholar] [CrossRef]
- Marzo, A.; Dal Bo, L.; Mazzucchelli, P.; Monti, N.C.; Crivelli, F.; Ismaili, S.; Giusti, A.; Uhr, M.R. Pharmacokinetic and pharmacodynamic comparative study of zofenopril and enalapril in healthy volunteers. Arzneimittelforschung 2002, 52, 233–242. [Google Scholar] [CrossRef]
- Yamauchi, H.; Nishimura, K.; Nakata, K.; Suda, H.; Iso, T.; Shimizu, M.; Hiramatsu, Y. General pharmacological properties of the potent angiotensin converting enzyme inhibitor rentiapril. Arzneimittelforschung 1987, 37, 157–164. [Google Scholar]
- Patchett, A.A.; Harris, E.W.; Tristram, W.M.J.; Wu, M.T.; Taub, D.; Peterson, E.R.; Ikeler, T.J.; Tenbroeke, J.; Payne, L.G.; Ondeyka, D.L.; et al. A new class of angiotensin converting enzyme inhibitors. Nature 1980, 288, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.T.; Douglas, A.W.; Ondeyka, D.L.; Payne, L.G.; Ikeler, T.J.; Joshua, H.; Patchett, A.A. Synthesis of N2-[(S)-1-carboxy-3-phenylpropyl]-L-lysyl-L-proline (lisinopril). J. Pharm. Sci. 1985, 74, 352–354. [Google Scholar] [CrossRef]
- Natesh, R.; Schwager, S.L.; Sturrock, E.D.; Acharya, K.R. Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature 2003, 421, 551–554. [Google Scholar] [CrossRef]
- DeForrest, J.M.; Waldron, T.L.; Harvey, C.; Scalese, B.; Rubin, B.; Powell, J.R.; Petrillo, E.W.; Cushman, D.W. Fosinopril, a phosphinic acid inhibitor of angiotensin I converting enzyme: In vitro and preclinical in vivo pharmacology. J. Cardiovasc. Pharmacol. 1989, 14, 730–736. [Google Scholar] [CrossRef]
- DeForrest, J.M.; Waldron, T.L.; Harvey, C.; Scalese, R.; Hammerstone, S.; Powell, J.R.; Karanewsky, D. Ceranapril (SQ 29,852), an orally active inhibitor of angiotensin converting enzyme (ACE). J. Cardiovasc. Pharmacol. 1990, 16, 121–127. [Google Scholar] [CrossRef]
- Wei, L.; Alhenc-Gelas, F.; Soubrier, F.; Michaud, A.; Corvol, P.; Clauser, E. Expression and characterization of recombinant human angiotensin I converting enzyme. Evidence for a C-terminal transmembrane anchor and for a proteolytic processing of the secreted recombinant and plasma enzymes. J. Biol. Chem. 1991, 266, 5540–5546. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Alhenc-Gelas, F.; Corvol, P.; Clauser, E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J. Biol. Chem. 1991, 266, 9002–9008. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, K.E.; Shen, X.Z.; Gonzalez-Villalobos, R.A.; Billet, S.; Okwan-Duodu, D.; Ong, F.S.; Fuchs, S. Different in vivo functions of the two catalytic domains of angiotensin-converting enzyme (ACE). Curr. Opin. Pharmacol. 2011, 11, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.R.; Gregory, K.S.; Sturrock, E.D. Advances in the structural basis for angiotensin-1 converting enzyme (ACE) inhibitors. Biosci. Rep. 2024, 44, BSR20240130. [Google Scholar] [CrossRef]
- Redelinghuys, P.; Nchinda, A.T.; Sturrock, E.D. Development of Domain-Selective Angiotensin I-Converting Enzyme Inhibitors. Ann. N. Y. Acad. Sci. 2005, 1056, 160–175. [Google Scholar] [CrossRef]
- Regulska, K.; Stanisz, B.; Regulski, M.; Murias, M. How to design a potent, specific, and stable angiotensin-converting enzyme inhibitor. Drug Discov. Today 2014, 19, 1731–1743. [Google Scholar] [CrossRef]
- UNAIDS. Global HIV & AIDS Statistics-Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 31 July 2025).
- Kaushik-Basu, N.; Harris, D. Peptide inhibition of HIV-1: Current status and future potential. BioDrugs 2008, 22, 161–175. [Google Scholar] [CrossRef]
- Scandlyn, J. When AIDS became a chronic disease. West. J. Med. 2000, 172, 130–133. [Google Scholar] [CrossRef]
- Roberts, N.A.; Martin, J.A.; Kinchington, D.; Broadhurst, A.V.; Craig, J.C.; Duncan, I.B.; Galpin, S.A.; Handa, B.K.; Kay, J.; Krohn, A.; et al. Rational design of peptide-based HIV proteinase inhibitors. Science 1990, 248, 358–361. [Google Scholar] [CrossRef]
- Wlodawer, A.; Vondrasek, J. Inhibitors of HIV-1 protease: A major success of structure-assisted drug design. Annu. Rev. Biophys. Biomol. Struct. 1998, 27, 249–284. [Google Scholar] [CrossRef]
- Navia, M.A.; Fitzgerald, P.M.D.; McKeever, B.M.; Leu, C.-T.; Heimbach, J.C.; Herber, W.K.; Sigal, I.S.; Darke, P.L.; Springer, J.P. Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature 1989, 337, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Schneider, J.; Sathyanarayana, B.K.; Toth, M.V.; Marshall, G.R.; Clawson, L.; Selk, L.; Kent, S.B.H.; Wlodawer, A. Structure of complex of synthetic HIV-1 protease with a substrate-based inhibitor at 2.3 Å resolution. Science 1989, 246, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Swain, A.L.; Miller, M.M.; Green, J.; Richo, D.H.; Schneider, J.; Kent, S.B.H.; Wlodawer, A. X-ray crystallographic structure of a complex between a synthetic protease of human immunodeficiency virus 1 and a substrate-based hydroxyethylamine inhibitor. Proc. Natl. Acad. Sci. USA 1990, 87, 8805–8809. [Google Scholar] [CrossRef]
- Pokorna, J.; Machala, L.; Rezacova, P.; Konvalinka, J. Current and Novel Inhibitors of HIV Protease. Viruses 2009, 1, 1209–1239. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.V.N.V.; Para, K.S.; Lunney, E.A.; Ortwine, D.F.; Dunbar, J.B., Jr.; Ferguson, D.; Tummino, P.J.; Hupe, D.; Tait, B.D.; Domagala, J.M.; et al. Novel Series of Achiral, Low Molecular Weight, and Potent HIV-1 Protease Inhibitors. J. Am. Chem. Soc. 1994, 116, 6989–6990. [Google Scholar] [CrossRef]
- Thaisrivongs, S.; Tomich, P.K.; Watenpaugh, K.D.; Chong, K.-T.; Howe, W.J.; Yang, C.-P.; Strohbach, J.W.; Turner, S.R.; McGrath, J.P.; Bohanon, M.J.; et al. Structure-Based Design of HIV Protease Inhibitors: 4-Hydroxycoumarins and 4-Hydroxy-2-pyrones as Non-peptidic Inhibitors. J. Med. Chem. 1994, 37, 3200–3204. [Google Scholar] [CrossRef]
- Tait, B.D.; Hagen, S.; Domagala, J.; Ellsworth, E.L.; Gajda, C.; Hamilton, H.W.; Prasad, J.V.N.V.; Ferguson, D.; Graham, N.; Hupe, D.; et al. 4-Hydroxy-5,6-dihydropyrones. 2. Potent Non-Peptide Inhibitors of HIV Protease. J. Med. Chem. 1997, 40, 3781–3792. [Google Scholar] [CrossRef]
- Prasad, J.V.N.V.; Boyer, F.E.; Domagala, J.M.; Ellsworth, E.L.; Gajda, C.; Hamilton, H.W.; Hagen, S.E.; Markoski, L.J.; Steinbaugh, B.A.; Tait, B.D.; et al. Nonpeptidic HIV Protease Inhibitors Possessing Excellent Antiviral Activities and Therapeutic Indices. PD 178390: A Lead HIV Protease Inhibitor. Bioorg. Med. Chem. 1999, 7, 2775–2800. [Google Scholar] [CrossRef]
- Flexner, C.; Bate, G.; Kirkpatrick, P. Tipranavir. Nat. Rev. Drug Discov. 2005, 4, 955–956. [Google Scholar] [CrossRef] [PubMed]
- Muzammil, S.; Armstrong, A.A.; Kang, L.W.; Jakalian, A.; Bonneau, P.R.; Schmelmer, V.; Amzel, L.M.; Freire, E. Unique Thermodynamic Response of Tipranavir to Human Immunodeficiency Virus Type 1 Protease Drug Resistance Mutations. J. Virol. 2007, 81, 5144–5154. [Google Scholar] [CrossRef]
- Grinspoon, S.; Carr, A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N. Engl. J. Med. 2005, 352, 48–62. [Google Scholar] [CrossRef]
- Lam, P.Y.; Jadhav, P.K.; Eyermann, C.J.; Hodge, C.N.; Ru, Y.; Bacheler, L.T.; Meek, J.L.; Otto, M.J.; Rayner, M.M.; Wong, Y.N.; et al. Rational design of potent, bioavailable, nonpeptide cyclic ureas as HIV protease inhibitors. Science 1994, 263, 380–384. [Google Scholar] [CrossRef]
- Hodge, C.N.; Aldrich, P.E.; Bacheler, L.T.; Chang, C.H.; Eyermann, C.J.; Garber, S.; Grubb, M.; Jackson, D.A.; Jadhav, P.K.; Korant, B.; et al. Improved cyclic urea inhibitors of the HIV-1 protease: Synthesis, potency, resistance profile, human pharmacokinetics and X-ray crystal structure of DMP 450. Chem. Biol. 1996, 3, 301–314. [Google Scholar] [CrossRef]
- Sham, H.L.; Zhao, C.; Stewart, K.D.; Betebenner, D.A.; Lin, S.; Park, C.H.; Kong, X.-P.; Rosenbrook, W., Jr.; Herrin, T.; Madigan, D.; et al. A Novel, Picomolar Inhibitor of Human Immunodeficiency Virus Type 1 Protease. J. Med. Chem. 1996, 39, 392–397. [Google Scholar] [CrossRef]
- Calugi, C.; Guarna, A.; Trabocchi, A. Heterocyclic HIV-Protease Inhibitors. Curr. Med. Chem. 2013, 20, 3693–3710. [Google Scholar] [CrossRef]
- Stellbrink, H.J.; Arastéh, K.; Schürmann, D.; Stephan, C.; Dierynck, I.; Smyej, I.; Hoetelmans, R.M.; Truyers, C.; Meyvisch, P.; Jacquemyn, B.; et al. Antiviral activity, pharmacokinetics, and safety of the HIV-1 protease inhibitor TMC310911, coadministered with ritonavir, in treatment-naive HIV-1-infected patients. J. Acquir. Immune Defic. Syndr. 2014, 65, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.T.B.; Zanardelli, S.; Chion, C.K.N.K.; Lane, D.A. The central role of thrombin in hemostasis. J. Thromb. Haemost. 2007, 5 (Suppl. S1), 95–101. [Google Scholar] [CrossRef]
- Blombäck, B.; Hessel, B.; Hogg, D.; Therkildsen, L. A two-step fibrinogen–fibrin transition in blood coagulation. Nature 1978, 275, 501–505. [Google Scholar] [CrossRef]
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to the global disease burden. J. Thromb. Haemost. 2014, 12, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Warkentin, T.E. The direct thrombin inhibitor hirudin. Thromb. Haemost. 2008, 99, 819–829. [Google Scholar] [CrossRef]
- Coppens, M.; Eikelboom, J.W.; Gustafsson, D.; Weitz, J.I.; Hirsch, J. Development of direct thrombin inhibitors. Circ. Res. 2012, 111, 920–929. [Google Scholar] [CrossRef]
- Bode, W. The structure of thrombin, a chameleon-like proteinase. J. Thromb. Haemost. 2005, 3, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Rydel, T.J.; Ravichandran, K.G.; Tulinsky, A.; Bode, W.; Huber, R.; Roitsch, C.; Fenton, J.W. The structure of a complex of recombinant hirudin and human alpha-thrombin. Science 1990, 249, 277–280. [Google Scholar] [CrossRef]
- Yue, S.Y.; DiMaio, J.; Szewczuk, Z.; Purisima, E.O.; Ni, F.; Konishi, Y. Characterization of the interactions of a bifunctional inhibitor with alpha-thrombin by molecular modeling and peptide-synthesis. Prot. Eng. 1992, 5, 77–85. [Google Scholar] [CrossRef]
- Blomback, B.; Blomback, M.; Olsson, P.; Svendsen, L.; Aberg, G. Synthetic peptides with anticoagulant and vasodilating activity. Scand. J. Clin. Lab. Investig. Suppl. 1969, 107, 59–61. [Google Scholar]
- Nakanishi, H.; Chrusciel, R.A.; Shen, R.; Bertenshaw, S.; Johnson, M.E.; Rydel, T.J.; Tulinsky, A.; Kahn, M. Peptide mimetics of the thrombin-bound structure of fibrinopeptide A. Proc. Natl. Acad. Sci. USA 1992, 89, 1705–1709. [Google Scholar] [CrossRef]
- Kettner, C.; Shaw, E. D-Phe-Pro-ArgCH2Cl-A selective affinity label for thrombin. Thromb. Res. 1979, 14, 969–973. [Google Scholar] [CrossRef]
- Powers, J.C.; Asgian, J.L.; Ekici, O.D.; James, K.E. Irreversible Inhibitors of Serine, Cysteine, and Threonine Proteases. Chem. Rev. 2002, 102, 4639–4750. [Google Scholar] [CrossRef]
- Mohler, M.A.; Refino, C.J.; Chen, S.A.; Chen, A.B.; Hotchkiss, A.J. D-Phe-Pro-Arg-chloromethylketone: Its potential use in inhibiting the formation of in vitro artifacts in blood collected during tissue-type plasminogen activator thrombolytic therapy. Thromb. Haemost. 1986, 56, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Troll, W.; Sherry, S.; Wachman, J. The action of plasmin on synthetic substrates. J. Biol. Chem. 1954, 208, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Hijikata, A.; Kikumoto, R.; Tonomura, S.; Hara, H.; Ninomiya, K.; Maruyama, A.; Sugano, M.; Tamao, Y. Potent inhibition of thrombin by the newly synthesized arginine derivative No. 805: The importance of stereo-structure of its hydrophobic carboxamide portion. Biochem. Biophys. Res. Commun. 1981, 101, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Stürzebecher, J.; Markwardt, E.; Voigt, B.; Wagner, G.; Walsmann, P. Cyclic amides of N-alpha-arylsulfonylaminoacylated 4-amidinophenylalanine-tight binding inhibitors of thrombin. Thromb. Res. 1983, 29, 635–642. [Google Scholar] [CrossRef]
- Nar, H. The role of structural information in the discovery of direct thrombin and factor Xa inhibitors. Trends Phamcol. Sci. 2012, 33, 279–288. [Google Scholar] [CrossRef]
- Gustafsson, D.; Antonsson, T.; Bylund, R. Effects of melagatran, a new low-molecular-weight thrombin inhibitor, on thrombin and fibrinolytic enzymes. Thromb. Haemost. 1998, 79, 110–118. [Google Scholar]
- Eriksson, U.G.; Bredberg, U.; Hoffmann, K.-J.; Thuresson, A.; Garielsson, M.; Erricsson, H.; Ahnhoff, M.; Gislén, K.; Fager, G.; Gustafsson, D. Absorption, distribution, metabolism, and excretion of ximelagatran, an oral direct thrombin inhibitor, in rats, dogs and humans. Drug Metab. Dispos. 2003, 31, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Brandstetter, H.; Turk, D.; Hoeffken, H.W.; Grosse, D.; Sturzebecher, J.; Martin, P.D.; Edwards, B.F.; Bode, W. Refined 2.3 Å X-ray crystal structure of bovine thrombin complexes formed with the benzamidine and arginine-based thrombin inhibitors NAPAP, 4-TAPAP and MQPA: A starting point for improving antithrombotics. J. Mol. Biol. 1992, 226, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Hauel, N.H.; Nar, H.; Priepke, H.; Ries, U.; Stassen, J.-M.; Wienen, W. Structure-Based Design of Novel Potent Nonpeptide Thrombin Inhibitors. J. Med. Chem. 2002, 45, 1757–1766. [Google Scholar] [CrossRef]
- Amm, I.; Sommer, T.; Wolf, D.H. Protein quality control and elimination of protein waste: The role of the ubiquitin-proteasome system. Biochim. Biophys. Acta 2014, 1843, 182–196. [Google Scholar] [CrossRef]
- Trivedi, P.C.; Bartlett, J.J.; Pulinilkunnil, T. Lysosomal Biology and Function: Modern View of Cellular Debris Bin. Cells 2020, 9, 1131. [Google Scholar] [CrossRef]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef]
- Tanaka, K. The proteasome: Overview of structure and functions. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 12–36. [Google Scholar] [CrossRef]
- Fenteany, G.; Schreiber, S.L. Lactacystin, proteasome function, and cell fate. J. Biol. Chem. 1998, 273, 8545–8548. [Google Scholar] [CrossRef] [PubMed]
- Arkwright, R.; Pham, T.M.; Zonder, J.A.; Dou, Q.P. The preclinical discovery and development of bortezomib for the treatment of mantle cell lymphoma. Exp. Opin. Drug Discov. 2017, 12, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.M. Carfilzomib: A new proteasome inhibitor for relapsed or refractory multiple myeloma. J. Oncol. Pharm. Pract. 2013, 19, 348–354. [Google Scholar] [CrossRef]
- Chauhan, D.; Tian, Z.; Zou, B.; Kuhn, D.; Orlowski, R.; Raje, N.; Richardson, P.; Anderson, K.C. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin. Cancer Res. 2011, 17, 5311–5321. [Google Scholar] [CrossRef]
- Tyrna, P.; Procyk, G.; Szeleszczuk, Ł.; Młynarczuk-Biały, I. Different Strategies to Overcome Resistance to Proteasome Inhibitors—A Summary 20 Years after Their Introduction. Int. J. Mol. Sci. 2024, 25, 8949. [Google Scholar] [CrossRef]
- Zhou, H.J.; Aujay, M.A.; Bennett, M.K.; Dajee, M.; Demo, S.D.; Fang, Y.; Ho, M.N.; Jiang, J.; Kirk, C.J.; Laidig, G.J.; et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047). J. Med. Chem. 2009, 52, 3028–3038. [Google Scholar] [CrossRef]
- Ge, Y.; Kazi, A.; Marsilio, F.; Luo, Y.; Jain, S.; Brooks, W.; Daniel, K.; Guida, W.; Sebti, S.; Lawrence, H. Discovery and Synthesis of Hydronaphthoquinones as Novel Proteasome Inhibitors. J. Med. Chem. 2012, 55, 1978–1998. [Google Scholar] [CrossRef] [PubMed]
- Feling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinospora. Angew. Chem. Int. Ed. Engl. 2003, 42, 355–357. [Google Scholar] [CrossRef]
- Roth, P.; Gorlia, T.; Reijneveld, J.C.; de Vos, F.; Idbaih, A.; Frenel, J.S.; Le Rhun, E.; Sepulveda, J.M.; Perry, J.; Masucci, G.L.; et al. Marizomib for patients with newly diagnosed glioblastoma: A randomized phase 3 trial. Neuro Oncol. 2024, 26, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.J. Designing Peptidomimetics. Curr. Top. Med. Chem. 2018, 18, 566–590. [Google Scholar] [CrossRef]
- Perez, J.J.; Corcho, F.; Llorens, O. Molecular modeling in the design of peptidomimetics and peptide surrogates. Curr. Med. Chem. 2002, 9, 2209–2229. [Google Scholar] [CrossRef]
- Markets and Data. Global ACE Inhibitors Market Assessment, Drugs, by Route of Administration, by Indication, by End-User, by Region, Opportunities and Forecast, 2017–2031F. Available online: https://www.marketsandata.com/industry-reports/ace-inhibitors-market (accessed on 31 July 2025).
- Beyrer, C. A pandemic anniversary: 40 years of HIV/AIDS. Lancet 2021, 397, 2142–2143. [Google Scholar] [CrossRef]
- Fortune Business Insight. HIV Drugs Market Size, Share & Industry Analysis, by Product Type, Combination HIV Medicines, by Distribution Channel, and Regional Forecast, 2024–2032. Report ID: Report ID: FBI100464. Available online: https://www.fortunebusinessinsights.com/industry-reports/protease-inhibitors-market-100464 (accessed on 31 July 2025).
- Fan, P.; Gao, Y.; Zheng, M.; Xu, T.; Schoenhagen, P.; Jin, Z. Recent progress and market analysis of anticoagulant drugs. J. Thorac. Dis. 2018, 10, 2011–2025. [Google Scholar] [CrossRef]
- Oktay, E. Will NOACs become the new standard of care in anticoagulation therapy? Int. J. Cardiovasc. Acad. 2015, 1, 1–4. [Google Scholar] [CrossRef]
- Grand View Research. Anticoagulants Market Size, Share & Trends Analysis Report by Drug Category (Novel Oral Anticoagulants, Direct Thrombin Inhibitors, Heparin), by Route of Administration, by Application, by Region, and Segment Forecasts, 2025–2030. Available online: https://www.grandviewresearch.com/industry-analysis/anticoagulants-market-report (accessed on 31 July 2025).
- UnivDatos. Proteasome Inhibitors Market: Current Analysis and Forecast (2023–2030). Available online: https://univdatos.com/reports/proteasome-inhibitors-market (accessed on 31 July 2025).
- Abbenante, G.; Fairlie, D.P. Protease inhibitors in the clinic. Med. Chem. 2005, 1, 71–104. [Google Scholar] [CrossRef] [PubMed]
- Kollet, O.; Das, A.; Karamanos, N.; Auf dem Keller, U.; Sagi, I. Redefining metalloproteases specificity through network proteolysis. Trends Mol. Med. 2024, 30, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Baidya, S.K.; Adhikari, N.; Jha, T. An updated patent review of matrix metalloproteinase (MMP) inhibitors (2021-present). Expert. Opin. Ther. Pat. 2023, 33, 631–649. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Gutierrez, P.; Perez, J.J. Small Molecule Protease Inhibitors as Model Peptidomimetics. Pharmaceuticals 2025, 18, 1377. https://doi.org/10.3390/ph18091377
Gomez-Gutierrez P, Perez JJ. Small Molecule Protease Inhibitors as Model Peptidomimetics. Pharmaceuticals. 2025; 18(9):1377. https://doi.org/10.3390/ph18091377
Chicago/Turabian StyleGomez-Gutierrez, Patricia, and Juan J. Perez. 2025. "Small Molecule Protease Inhibitors as Model Peptidomimetics" Pharmaceuticals 18, no. 9: 1377. https://doi.org/10.3390/ph18091377
APA StyleGomez-Gutierrez, P., & Perez, J. J. (2025). Small Molecule Protease Inhibitors as Model Peptidomimetics. Pharmaceuticals, 18(9), 1377. https://doi.org/10.3390/ph18091377







