Advances in Pharmacological Properties, Molecular Mechanisms, and Bioavailability Strategies of Chlorogenic Acid in Cardiovascular Diseases Therapy
Abstract
1. Introduction
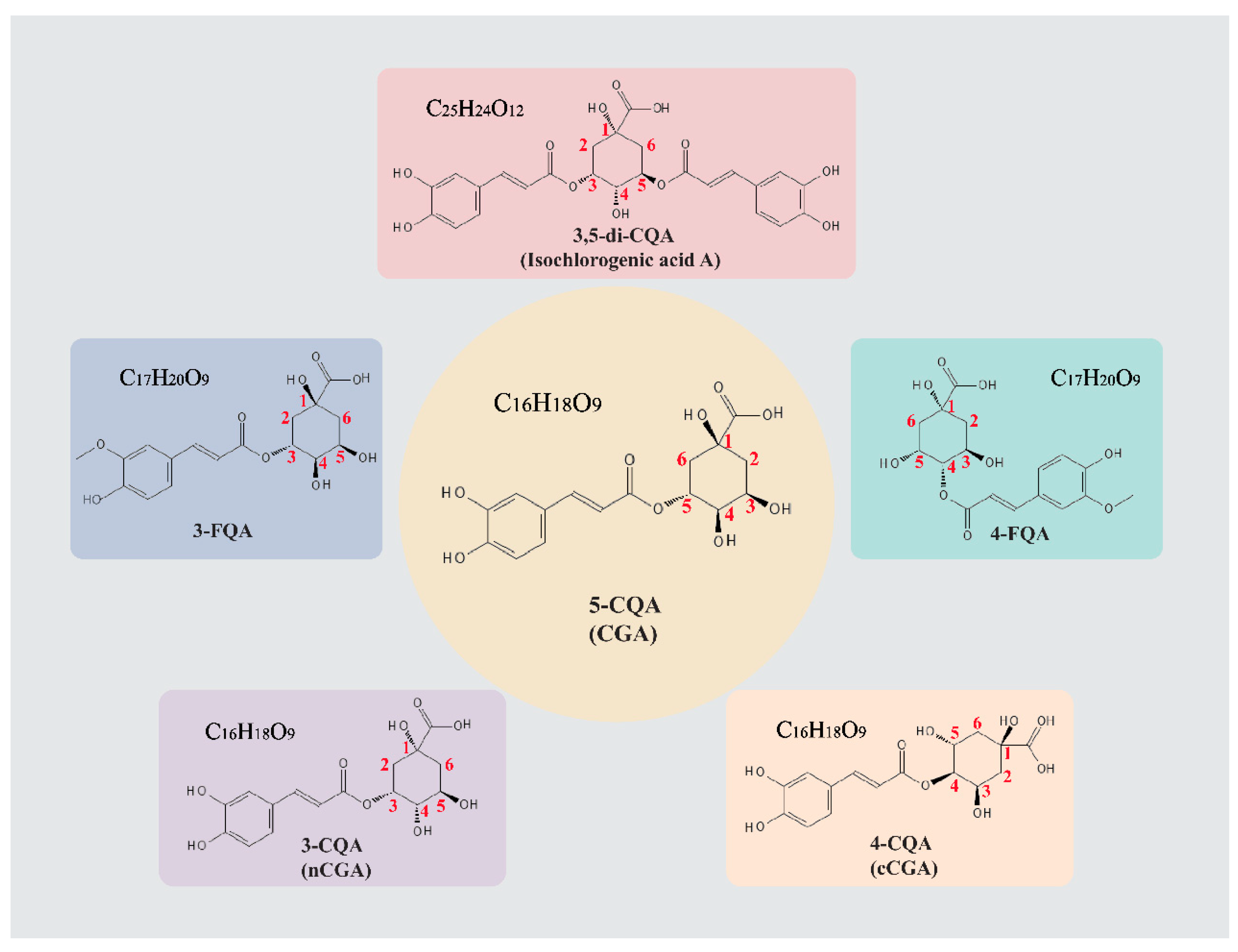
2. Preparation and Metabolic Pathways of CGA
2.1. Preparation
2.2. Metabolic Pathways
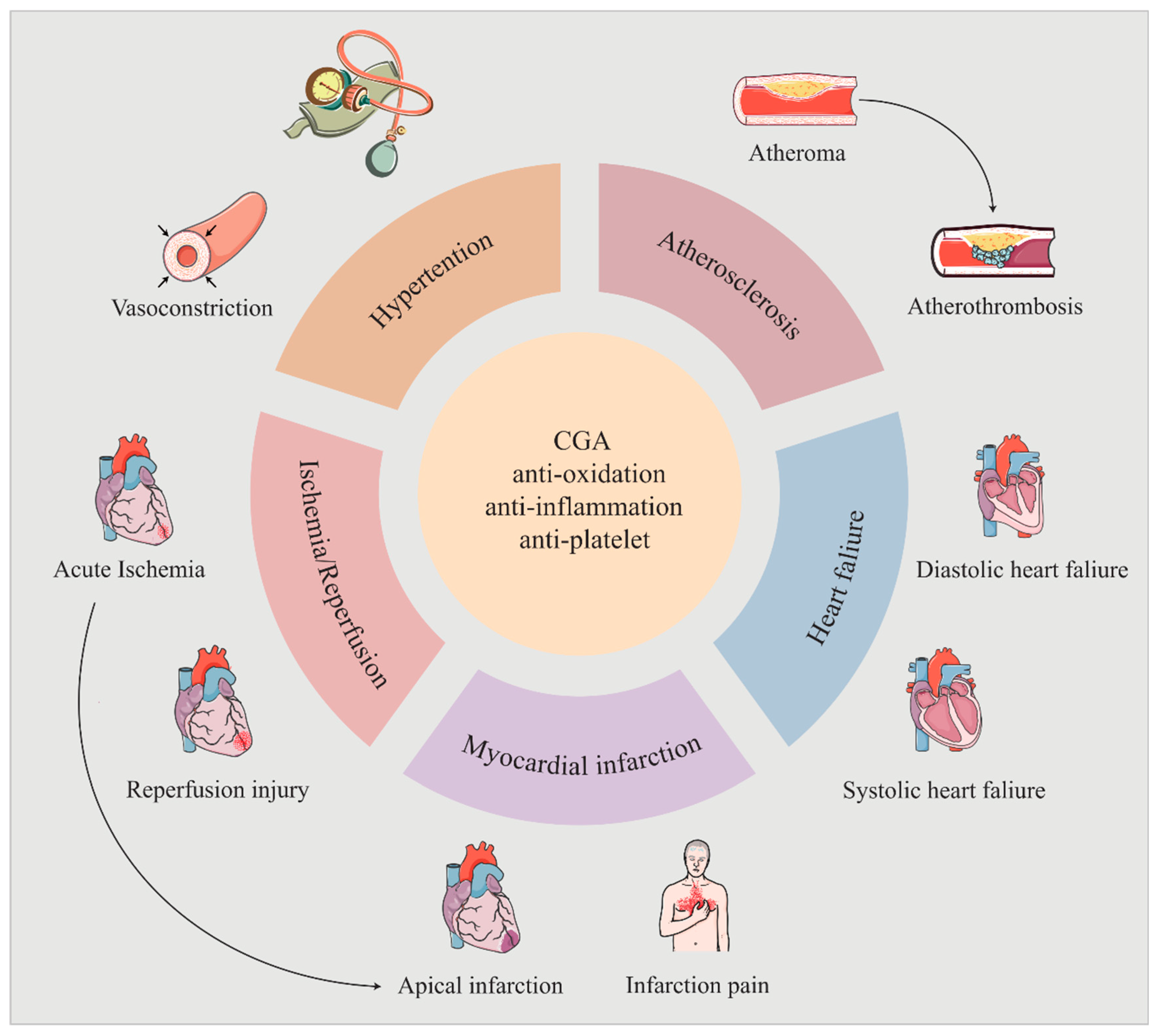
3. Cardiovascular Activity of CGA
3.1. Hypertensive
3.2. Atherosclerosis
3.3. Ischemia/Reperfusion Injury
3.4. Myocardial Infarction
3.5. Heart Failure
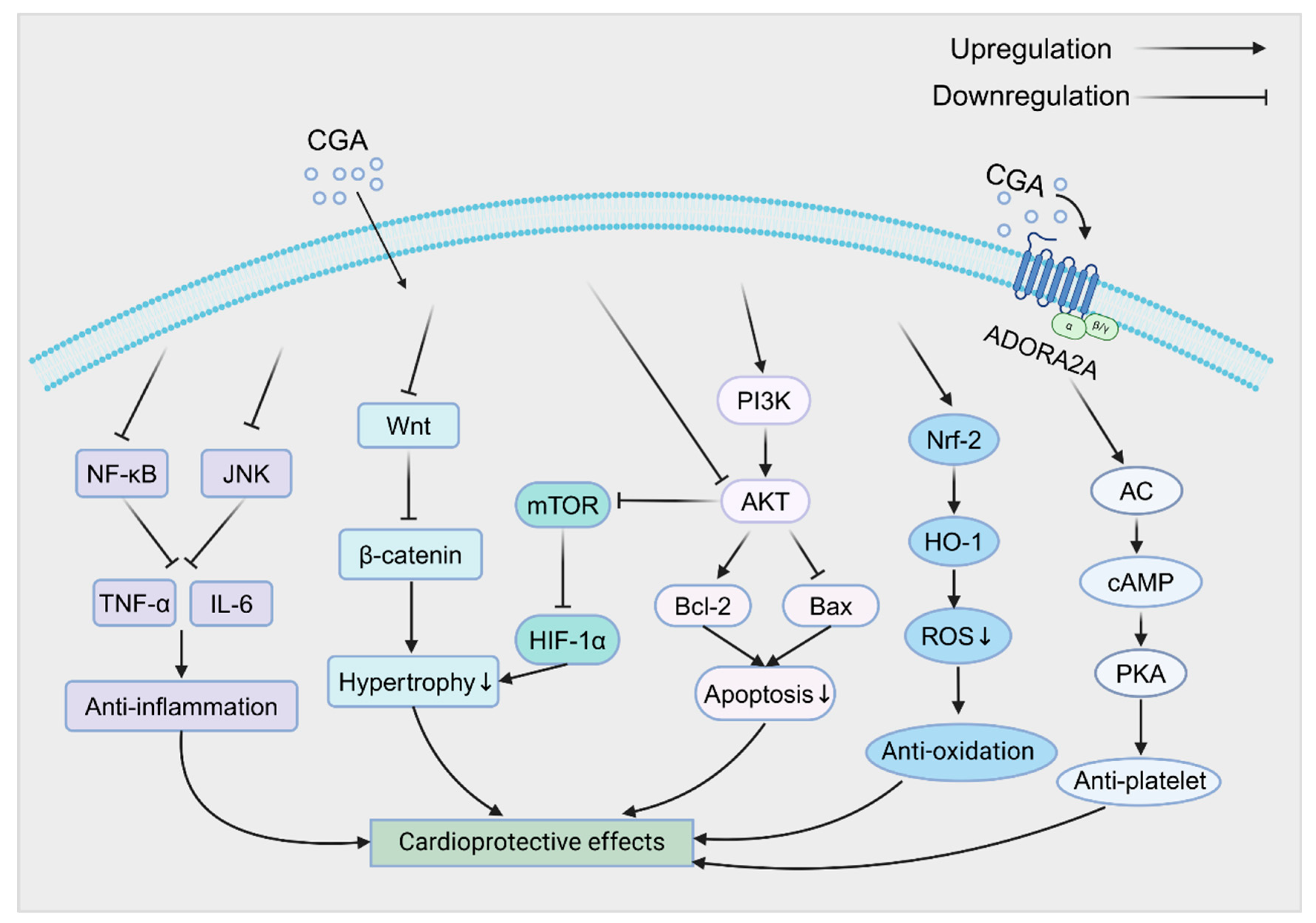
4. Regulation of Cardiovascular Signaling Pathways by CGA
5. Safety Research
6. Strategies to Improve the Bioavailability of CGA
6.1. Structural Modification
6.2. Optimization of Drug Delivery Systems
6.2.1. Liposomes
6.2.2. Lipid Microspheres
6.2.3. Self-Emulsifying Drug Delivery System
6.2.4. Hydrogels
6.2.5. Nanoparticles
7. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef]
- Xue, H.; Wei, M.; Ji, L. Chlorogenic acids: A pharmacological systematic review on their hepatoprotective effects. Phytomedicine 2023, 118, 154961. [Google Scholar] [CrossRef]
- Murai, T.; Matsuda, S. The Chemopreventive Effects of Chlorogenic Acids, Phenolic Compounds in Coffee, against Inflammation, Cancer, and Neurological Diseases. Molecules 2023, 28, 2381. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2020, 18, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1299–1352. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, S.; Zhang, J.; Wei, X.; Wang, Z.; Han, B. Osteogenic mechanism of chlorogenic acid and its application in clinical practice. Front. Pharmacol. 2024, 15, 1396354. [Google Scholar] [CrossRef]
- Zeng, L.; Xiang, R.; Fu, C.; Qu, Z.; Liu, C. The Regulatory effect of chlorogenic acid on gut-brain function and its mechanism: A systematic review. Biomed. Pharmacother. 2022, 149, 112831. [Google Scholar] [CrossRef]
- He, Y.; Mao, S.; Zhao, Y.; Yang, J. Research Advances in the Synthesis, Metabolism, and Function of Chlorogenic Acid. Foods 2025, 14, 1914. [Google Scholar] [CrossRef]
- Gómez-Juaristi, M.; Martínez-López, S.; Sarria, B.; Bravo, L.; Mateos, R. Bioavailability of hydroxycinnamates in an instant green/roasted coffee blend in humans. Identification of novel colonic metabolites. Food Funct. 2018, 9, 331–343. [Google Scholar] [CrossRef]
- Suzuki, A.; Kagawa, D.; Ochiai, R.; Tokimitsu, I.; Saito, I. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. Hypertens. Res. 2002, 25, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic Acids in Cardiovascular Disease: A Review of Dietary Consumption, Pharmacology, and Pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhu, Y.; Liu, Y.; Tao, Y.; Lin, Y.; Lai, S.; Liang, Z.; Chen, Y.; Chen, Y.; Wang, L. Moderation of gut microbiota and bile acid metabolism by chlorogenic acid improves high-fructose-induced salt-sensitive hypertension in mice. Food Funct. 2022, 13, 6987–6999. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Spencer, E.A.; Thompson, M.J.; Heneghan, C.J. The effect of chlorogenic acid on blood pressure: A systematic review and meta-analysis of randomized clinical trials. J. Hum. Hypertens. 2015, 29, 77–81. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamamoto, N.; Jokura, H.; Yamamoto, M.; Fujii, A.; Tokimitsu, I.; Saito, I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 1065–1073. [Google Scholar] [CrossRef]
- Carlström, M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 2021, 17, 575–590. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Agudelo-Ochoa, G.M.; Pulgarín-Zapata, I.C.; Velásquez-Rodriguez, C.M.; Duque-Ramírez, M.; Naranjo-Cano, M.; Quintero-Ortiz, M.M.; Lara-Guzmán, O.J.; Muñoz-Durango, K. Coffee Consumption Increases the Antioxidant Capacity of Plasma and Has No Effect on the Lipid Profile or Vascular Function in Healthy Adults in a Randomized Controlled Trial. J. Nutr. 2016, 146, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, M.; Maruhashi, T.; Hidaka, T.; Nakano, Y.; Kurisu, S.; Matsumoto, T.; Iwamoto, Y.; Kishimoto, S.; Matsui, S.; Aibara, Y.; et al. Coffee with a high content of chlorogenic acids and low content of hydroxyhydroquinone improves postprandial endothelial dysfunction in patients with borderline and stage 1 hypertension. Eur. J. Nutr. 2019, 58, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Zhao, Y.; Chen, Y.; Huang, R. Global and national burden of atherosclerosis from 1990 to 2019: Trend analysis based on the Global Burden of Disease Study 2019. Chin. Med. J. 2023, 136, 2442–2450. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Lara-Guzmán, O.J.; Álvarez, R.; Muñoz-Durango, K. Changes in the plasma lipidome of healthy subjects after coffee consumption reveal potential cardiovascular benefits: A randomized controlled trial. Free Radic. Biol. Med. 2021, 176, 345–355. [Google Scholar] [CrossRef]
- Wu, C.; Luan, H.; Zhang, X.; Wang, S.; Zhang, X.; Sun, X.; Guo, P. Chlorogenic acid protects against atherosclerosis in ApoE-/- mice and promotes cholesterol efflux from RAW264.7 macrophages. PLoS ONE 2014, 9, e95452. [Google Scholar] [CrossRef]
- Cho, H.-J.; Kang, H.-J.; Kim, Y.-J.; Lee, D.-H.; Kwon, H.-W.; Kim, Y.-Y.; Park, H.-J. Inhibition of platelet aggregation by chlorogenic acid via cAMP and cGMP-dependent manner. Blood Coagul. Fibrinolysis 2012, 23, 629–635. [Google Scholar] [CrossRef]
- Suzuki, A.; Nomura, T.; Jokura, H.; Kitamura, N.; Saiki, A.; Fujii, A. Chlorogenic acid-enriched green coffee bean extract affects arterial stiffness assessed by the cardio-ankle vascular index in healthy men: A pilot study. Int. J. Food Sci. Nutr. 2019, 70, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van den Eynde, J.; Oosterlinck, W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef]
- Fantozzi, E.T.; Breithaupt-Faloppa, A.C.; Ricardo-da-Silva, F.Y.; Rodrigues-Garbin, S.; Romero, D.C.; da Silva Rodrigues, A.; Riffo-Vasquez, Y.; Tavares-de-Lima, W. Estradiol mediates the long-lasting lung inflammation induced by intestinal ischemia and reperfusion. J. Surg. Res. 2018, 221, 1–7. [Google Scholar] [CrossRef]
- Li, K.; Feng, Z.; Wang, L.; Ma, X.; Wang, L.; Liu, K.; Geng, X.; Peng, C. Chlorogenic Acid Alleviates Hepatic Ischemia-Reperfusion Injury by Inhibiting Oxidative Stress, Inflammation, and Mitochondria-Mediated Apoptosis In Vivo and In Vitro. Inflammation 2023, 46, 1061–1076. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.; Yang, Y.; Lin, K.; Lin, Q.; Luo, S.; Zhou, X.; Lin, Q.; Zhang, F. Chlorogenic acid promotes angiogenesis and attenuates apoptosis following cerebral ischaemia-reperfusion injury by regulating the PI3K-Akt signalling. Pharm. Biol. 2022, 60, 1646–1655. [Google Scholar] [CrossRef]
- Arfian, N.; Wahyudi, D.A.P.; Zulfatina, I.B.; Citta, A.N.; Anggorowati, N.; Multazam, A.; Romi, M.M.; Sari, D.C.R. Chlorogenic Acid Attenuates Kidney Ischemic/Reperfusion Injury via Reducing Inflammation, Tubular Injury, and Myofibroblast Formation. Biomed. Res. Int. 2019, 2019, 5423703. [Google Scholar] [CrossRef]
- Chai, X.; Liang, Z.; Zhang, J.; Ding, J.; Zhang, Q.; Lv, S.; Deng, Y.; Zhang, R.; Lu, D. Chlorogenic acid protects against myocardial ischemia-reperfusion injury in mice by inhibiting Lnc Neat1/NLRP3 inflammasome-mediated pyroptosis. Sci. Rep. 2023, 13, 17803. [Google Scholar] [CrossRef]
- Tong, Y.; Li, G.; Shi, X.; Wang, L.; Zhou, J.; Chu, M.; Wang, Z.; Abd El-Aty, A.M.; Dang, J. Protection against myocardial ischemia/reperfusion injury in mice by 3-caffeoylquinic acid isomers isolated from Saxifraga tangutica. RSC Adv. 2024, 14, 6642–6655. [Google Scholar] [CrossRef] [PubMed]
- Kaier, T.E.; Alaour, B.; Marber, M. Cardiac troponin and defining myocardial infarction. Cardiovasc. Res. 2021, 117, 2203–2215. [Google Scholar] [CrossRef] [PubMed]
- Akila, P.; Asaikumar, L.; Vennila, L. Chlorogenic acid ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes. Biomed. Pharmacother. 2017, 85, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Akila, P.; Vennila, L. Chlorogenic acid a dietary polyphenol attenuates isoproterenol induced myocardial oxidative stress in rat myocardium: An in vivo study. Biomed. Pharmacother. 2016, 84, 208–214. [Google Scholar] [CrossRef]
- Wang, D.; Tian, L.; Lv, H.; Pang, Z.; Li, D.; Yao, Z.; Wang, S. Chlorogenic acid prevents acute myocardial infarction in rats by reducing inflammatory damage and oxidative stress. Biomed. Pharmacother. 2020, 132, 110773. [Google Scholar] [CrossRef]
- Kanno, Y.; Watanabe, R.; Zempo, H.; Ogawa, M.; Suzuki, J.-I.; Isobe, M. Chlorogenic acid attenuates ventricular remodeling after myocardial infarction in mice. Int. Heart J. 2013, 54, 176–180. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef]
- He, K.; Wang, X.; Li, T.; Li, Y.; Ma, L. Chlorogenic Acid Attenuates Isoproterenol Hydrochloride-Induced Cardiac Hypertrophy in AC16 Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway. Molecules 2024, 29, 760. [Google Scholar] [CrossRef]
- Ping, P.; Yang, T.; Ning, C.; Zhao, Q.; Zhao, Y.; Yang, T.; Gao, Z.; Fu, S. Chlorogenic acid attenuates cardiac hypertrophy via up-regulating Sphingosine-1-phosphate receptor1 to inhibit endoplasmic reticulum stress. ESC Heart Fail. 2024, 11, 1580–1593. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Li, Y.; Shen, D.; Tang, X.; Li, X.; Wo, D.; Yan, H.; Song, R.; Feng, J.; Li, P.; Zhang, J.; et al. Chlorogenic acid prevents isoproterenol-induced hypertrophy in neonatal rat myocytes. Toxicol. Lett. 2014, 226, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, F.; Tang, J.; Pu, H.; Sukhorukov, V.; Orekhov, A.N.; Guo, S. Chlorogenic acid ameliorates heart failure by attenuating cardiomyocyte ferroptosis. J. Tradit. Chin. Med. Sci. 2024, 11, 191–198. [Google Scholar] [CrossRef]
- Yang, X.; Kawasaki, N.K.; Min, J.; Matsui, T.; Wang, F. Ferroptosis in heart failure. J. Mol. Cell Cardiol. 2022, 173, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Su, C.P.; Wang, Q.; Wu, F.J.; Bai, R.; Zhang, H.M.; Liu, J.Y.; Lu, W.J.; Wang, W.; Lan, F.; et al. Chlorogenic acid: A potent molecule that protects cardiomyocytes from TNF-α–induced injury via inhibiting NF-κB and JNK signals. J. Cell. Mol. Med. 2019, 23, 4666–4678. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Ren, X.; Zhou, H.; Wang, K.; Zhang, H.; Luo, P. Caffeoylquinic Acid Derivatives Extract of Erigeron multiradiatus Alleviated Acute Myocardial Ischemia Reperfusion Injury in Rats through Inhibiting NF-KappaB and JNK Activations. Mediat. Inflamm. 2016, 2016, 7961940. [Google Scholar] [CrossRef]
- Fuentes, E.; Caballero, J.; Alarcón, M.; Rojas, A.; Palomo, I. Chlorogenic acid inhibits human platelet activation and thrombus formation. PLoS ONE 2014, 9, e90699. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Chen, X.-H.; Li, L.; Su, C.-P.; Zhang, Y.-L.; Jiang, Y.-Y.; Guo, S.-Z.; Liu, B. Deciphering the effective combinatorial components from Si-Miao-Yong-An decoction regarding the intervention on myocardial hypertrophy. J. Ethnopharmacol. 2021, 271, 113833. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Li, X.; Tang, J.; Li, Y.; Liu, B.; Guo, S. Cryptochlorogenic acid and its metabolites ameliorate myocardial hypertrophy through a HIF1α-related pathway. Food Funct. 2022, 13, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.-M.; Wu, Y.-T.; Chen, L.; Tan, Z.-B.; Fan, H.-J.; Xie, L.-P.; Zhang, W.-T.; Chen, H.-M.; Li, J.; Liu, B.; et al. 3,5-Dicaffeoylquinic acid protects H9C2 cells against oxidative stress-induced apoptosis via activation of the PI3K/Akt signaling pathway. Food Nutr. Res. 2018, 62, 1423. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, A.; Wu, X.; Shen, Z.; Chen, X.; Li, J.; Liu, L.; Lin, X.; Wu, M.; Chen, Y.; et al. Qingda granule attenuates angiotensin II-induced cardiac hypertrophy and apoptosis and modulates the PI3K/AKT pathway. Biomed. Pharmacother. 2021, 133, 111022. [Google Scholar] [CrossRef]
- Cicek, B.; Hacimuftuoglu, A.; Yeni, Y.; Danisman, B.; Ozkaraca, M.; Mokhtare, B.; Kantarci, M.; Spanakis, M.; Nikitovic, D.; Lazopoulos, G.; et al. Chlorogenic Acid Attenuates Doxorubicin-Induced Oxidative Stress and Markers of Apoptosis in Cardiomyocytes via Nrf2/HO-1 and Dityrosine Signaling. J. Pers. Med. 2023, 13, 649. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Doan, L.H.; Huang, Z.-Y.; Chu, L.-W.; Shi, T.-H.; Lee, Y.-R.; Wu, C.-T.; Lin, C.-H.; Chiang, S.-T.; Liu, H.-K.; et al. Honeysuckle (Lonicera japonica) and Huangqi (Astragalus membranaceus) Suppress SARS-CoV-2 Entry and COVID-19 Related Cytokine Storm in Vitro. Front. Pharmacol. 2021, 12, 765553. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Li, M.; Hao, D.; Yang, Y.; Zhang, C.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef]
- Lara-Guzmán, O.J.; Medina, S.; Álvarez, R.; Oger, C.; Durand, T.; Galano, J.-M.; Zuluaga, N.; Gil-Izquierdo, Á.; Muñoz-Durango, K. Oxylipin regulation by phenolic compounds from coffee beverage: Positive outcomes from a randomized controlled trial in healthy adults and macrophage derived foam cells. Free Radic. Biol. Med. 2020, 160, 604–617. [Google Scholar] [CrossRef]
- Venkatakrishna, K.; Hv, S.; Shyamprasad, K. Acute and sub-chronic toxicity evaluation of a standardized green coffee bean extract (CGA-7™) in Wistar albino rats. SAGE Open Med. 2021, 9, 2050312120984885. [Google Scholar] [CrossRef] [PubMed]
- Behne, S.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Chlorogenic and Isochlorogenic Acids in Coffee By-Products. Molecules 2023, 28, 5540. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.; Zock, P.L.; Katan, M.B. Consumption of high doses of chlorogenic acid, present in coffee, or of black tea increases plasma total homocysteine concentrations in humans. Am. J. Clin. Nutr. 2001, 73, 532–538. [Google Scholar] [CrossRef]
- Tom, E.N.L.; Girard-Thernier, C.; Demougeot, C. The Janus face of chlorogenic acid on vascular reactivity: A study on rat isolated vessels. Phytomedicine 2016, 23, 1037–1042. [Google Scholar] [CrossRef]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid. Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef]
- Joneidi, S.; Alizadeh, S.R.; Ebrahimzadeh, M.A. Chlorogenic Acid Derivatives: Structural Modifications, Drug Design, and Biological Activities: A Review. Mini Rev. Med. Chem. 2024, 24, 748–766. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wu, C.; Wu, T.; Yu, H.; Yang, S.; Hu, Y. Preparation and characterisation of Chlorogenic acid-gelatin: A type of biologically active film for coating preservation. Food Chem. 2017, 221, 657–663. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Y.-C.; Meng, F.-B.; Wang, Z.-W.; Liu, D.-Y.; Chen, W.-J.; Zou, L.-H. Simultaneously enhanced stability and biological activities of chlorogenic acid by covalent grafting with soluble oat β-glucan. Food Chem. X 2023, 17, 100546. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; Lio, C.; Sun, W.; Lai, K.; Liu, Y.; Zhang, Z.; Liang, J.; Zhou, H.; Liu, L.; et al. A chlorogenic acid-phospholipid complex ameliorates post-myocardial infarction inflammatory response mediated by mitochondrial reactive oxygen species in SAMP8 mice. Pharmacol. Res. 2018, 130, 110–122. [Google Scholar] [CrossRef]
- Li, X.; Zhu, S.; Yin, P.; Zhang, S.; Xu, J.; Zhang, Q.; Shi, S.; Zhang, T. Combination immunotherapy of chlorogenic acid liposomes modified with sialic acid and PD-1 blockers effectively enhances the anti-tumor immune response and therapeutic effects. Drug Deliv. 2021, 28, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, L.; Hou, Y.; He, W.; Wang, X.; Zhang, D.; Hu, J. Self-assembly of chlorogenic acid into hydrogel for accelerating wound healing. Colloids Surf. B Biointerfaces 2023, 228, 113440. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, J.; Hu, J.-F.; Hu, Q.-Y.; Fang, X.; Sun, Z.-J.; Xu, Z.; Zhang, L. Sustained release of chlorogenic acid-loaded nanomicelles alleviates bone loss in mouse periodontitis. Biomater. Sci. 2022, 10, 5583–5595. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Xiao, Q.; Fan, L.; Huang, D.; Chen, W.; He, W. Liposomes and liposome-like nanoparticles: From anti-fungal infection to the COVID-19 pandemic treatment. Asian J. Pharm. Sci. 2022, 17, 817–837. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Sun, C.; Yuan, Y.; Zhu, Y.; Wan, J.; Firempong, C.K.; Omari-Siaw, E.; Xu, Y.; Pu, Z.; Yu, J.; et al. Enhanced oral bioavailability and in vivo antioxidant activity of chlorogenic acid via liposomal formulation. Int. J. Pharm. 2016, 501, 342–349. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Yan, J.-H.; Zhou, Z.-E.; Geng, X.; Xiong, J.-H. Enhanced anti-inflammatory activity of chlorogenic acid via folic acid-TPGS-modified liposomes encapsulation: Characterization and In vivo evaluation on colitis mice. Front. Pharmacol. 2024, 15, 1437773. [Google Scholar] [CrossRef]
- Ye, J.; Yang, Y.; Jin, J.; Ji, M.; Gao, Y.; Feng, Y.; Wang, H.; Chen, X.; Liu, Y. Targeted delivery of chlorogenic acid by mannosylated liposomes to effectively promote the polarization of TAMs for the treatment of glioblastoma. Bioact. Mater. 2020, 5, 694–708. [Google Scholar] [CrossRef]
- Luo, L.; Wang, X.; Chen, Q.; Miao, L.; Zhuo, X.; Liu, L.; Xu, J.; Zhang, Y.; He, H.; Yin, T.; et al. A parenteral docetaxel-loaded lipid microsphere with decreased 7-epidocetaxel conversion in vitro and in vivo. Eur. J. Pharm. Sci. 2017, 109, 638–649. [Google Scholar] [CrossRef]
- Luo, Y.; Fan, Q.; Yu, Y.; Zhang, L.; Dong, L.; Luo, H. Efficacy of administrative intervention for neurosurgical patients with off-label use of alprostadil lipid microsphere. Sci. Rep. 2022, 12, 15363. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Luo, X.; Zhang, H.; An, H.; Feng, W.; Feng, Y. The Comparison of Plasma and Cerebrospinal Fluid R(−)- and S(+)-Flurbiprofen Concentration After Intravenous Injection of Flurbiprofen Axetil in Human Subjects. Front. Pharmacol. 2021, 12, 646196. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, G. Berberine-10-hydroxy camptothecine-loaded lipid microsphere for the synergistic treatment of liver cancer by inhibiting topoisomerase and HIF-1α. Drug Deliv. 2021, 28, 171–182. [Google Scholar] [CrossRef]
- Abdulkarim, M.; Sharma, P.K.; Gumbleton, M. Self-emulsifying drug delivery system: Mucus permeation and innovative quantification technologies. Adv. Drug Deliv. Rev. 2019, 142, 62–74. [Google Scholar] [CrossRef]
- Xin, J.; Qin, M.; Ye, G.; Gong, H.; Li, M.; Sui, X.; Liu, B.; Fu, Q.; He, Z. Hydrophobic ion pairing-based self-emulsifying drug delivery systems: A new strategy for improving the therapeutic efficacy of water-soluble drugs. Expert Opin. Drug Deliv. 2023, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maji, I.; Mahajan, S.; Sriram, A.; Medtiya, P.; Vasave, R.; Khatri, D.K.; Kumar, R.; Singh, S.B.; Madan, J.; Singh, P.K. Solid self emulsifying drug delivery system: Superior mode for oral delivery of hydrophobic cargos. J. Control. Release 2021, 337, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, C.-S.; Chen, Q.-Z.; Wang, S.; Xiong, Y.-A.; Jing, J.; Lv, J.-J. Characterization, pharmacokinetics and tissue distribution of chlorogenic acid-loaded self-microemulsifying drug delivery system. Eur. J. Pharm. Sci. 2017, 100, 102–108. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhao, R.; Chi, X.; Chen, T.; Li, Y.; Xu, Y.; Zhu, B.; Hu, J. Unleashing the power of chlorogenic acid: Exploring its potential in nutrition delivery and the food industry. Food Funct. 2024, 15, 4741–4762. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Wang, X.; Chen, L.; Huang, W.; Xiong, T.; Wang, N.; Guo, J.; Gao, Z.; Jin, M. Modulating macrophage phenotype for accelerated wound healing with chlorogenic acid-loaded nanocomposite hydrogel. J. Control. Release 2024, 369, 420–443. [Google Scholar] [CrossRef]
- Roy, T.; Dey, S.K.; Pradhan, A.; Chaudhuri, A.D.; Dolai, M.; Mandal, S.M.; Choudhury, S.M. Facile and Green Fabrication of Highly Competent Surface-Modified Chlorogenic Acid Silver Nanoparticles: Characterization and Antioxidant and Cancer Chemopreventive Potential. ACS Omega 2022, 7, 48018–48033. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, S.; Shi, L.; Shi, J.; Sun, C.; Wang, J.; Zhou, W.; Zhou, H.; Shan, F.; Wang, H.; et al. Osteogenic Induction and Anti-Inflammatory Effects of Calcium-Chlorogenic Acid Nanoparticles Remodel the Osteoimmunology Microenvironment for Accelerating Bone Repair. Adv. Healthc. Mater. 2024, 13, e2401114. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Zhang, D.; Wang, R.; Duan, J.; Hu, L.; Huang, F.; Liu, W.; Gu, J.; Li, S.; Yang, C.; et al. Advances in Pharmacological Properties, Molecular Mechanisms, and Bioavailability Strategies of Chlorogenic Acid in Cardiovascular Diseases Therapy. Pharmaceuticals 2025, 18, 1357. https://doi.org/10.3390/ph18091357
Huang K, Zhang D, Wang R, Duan J, Hu L, Huang F, Liu W, Gu J, Li S, Yang C, et al. Advances in Pharmacological Properties, Molecular Mechanisms, and Bioavailability Strategies of Chlorogenic Acid in Cardiovascular Diseases Therapy. Pharmaceuticals. 2025; 18(9):1357. https://doi.org/10.3390/ph18091357
Chicago/Turabian StyleHuang, Kai, Duosu Zhang, Ruting Wang, Jiahao Duan, Long Hu, Fan Huang, Wei Liu, Jia Gu, Songlin Li, Chun Yang, and et al. 2025. "Advances in Pharmacological Properties, Molecular Mechanisms, and Bioavailability Strategies of Chlorogenic Acid in Cardiovascular Diseases Therapy" Pharmaceuticals 18, no. 9: 1357. https://doi.org/10.3390/ph18091357
APA StyleHuang, K., Zhang, D., Wang, R., Duan, J., Hu, L., Huang, F., Liu, W., Gu, J., Li, S., Yang, C., & Yang, L. (2025). Advances in Pharmacological Properties, Molecular Mechanisms, and Bioavailability Strategies of Chlorogenic Acid in Cardiovascular Diseases Therapy. Pharmaceuticals, 18(9), 1357. https://doi.org/10.3390/ph18091357







