Pharmacogenetics and Molecular Ancestry of SLC22A1, SLC22A2, SLC22A3, ABCB1, CYP2C8, CYP2C9, and CYP2C19 in Ecuadorian Subjects with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Results
2.1. Genotype and Allelic Frequencies
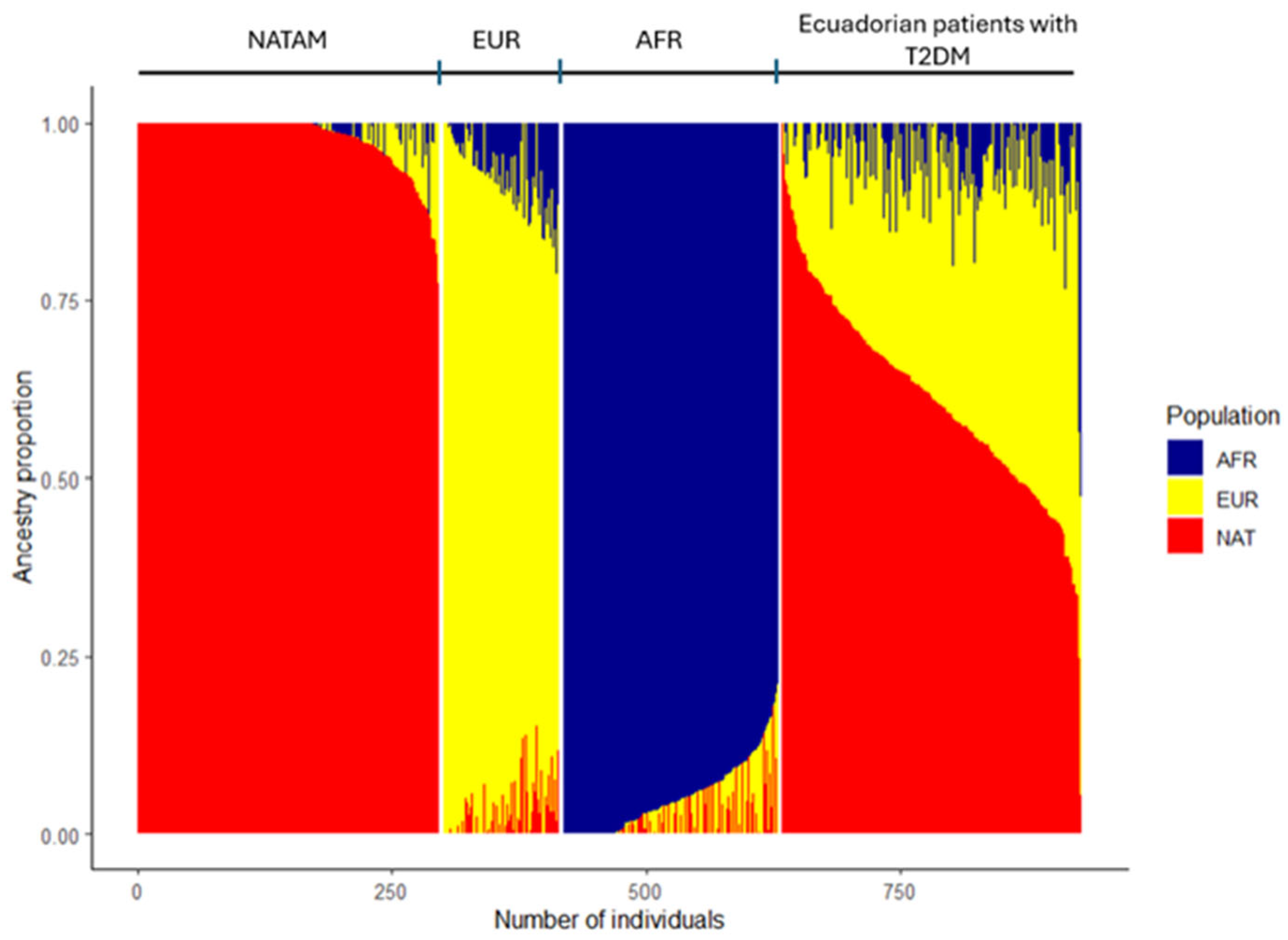
2.2. Description of Ancestry in Ecuadorian Patients with T2DM
2.3. Ancestry Inference Among CYP2C8, CYP2C9, and CYP2C19 Diplotypes and Activity Scores
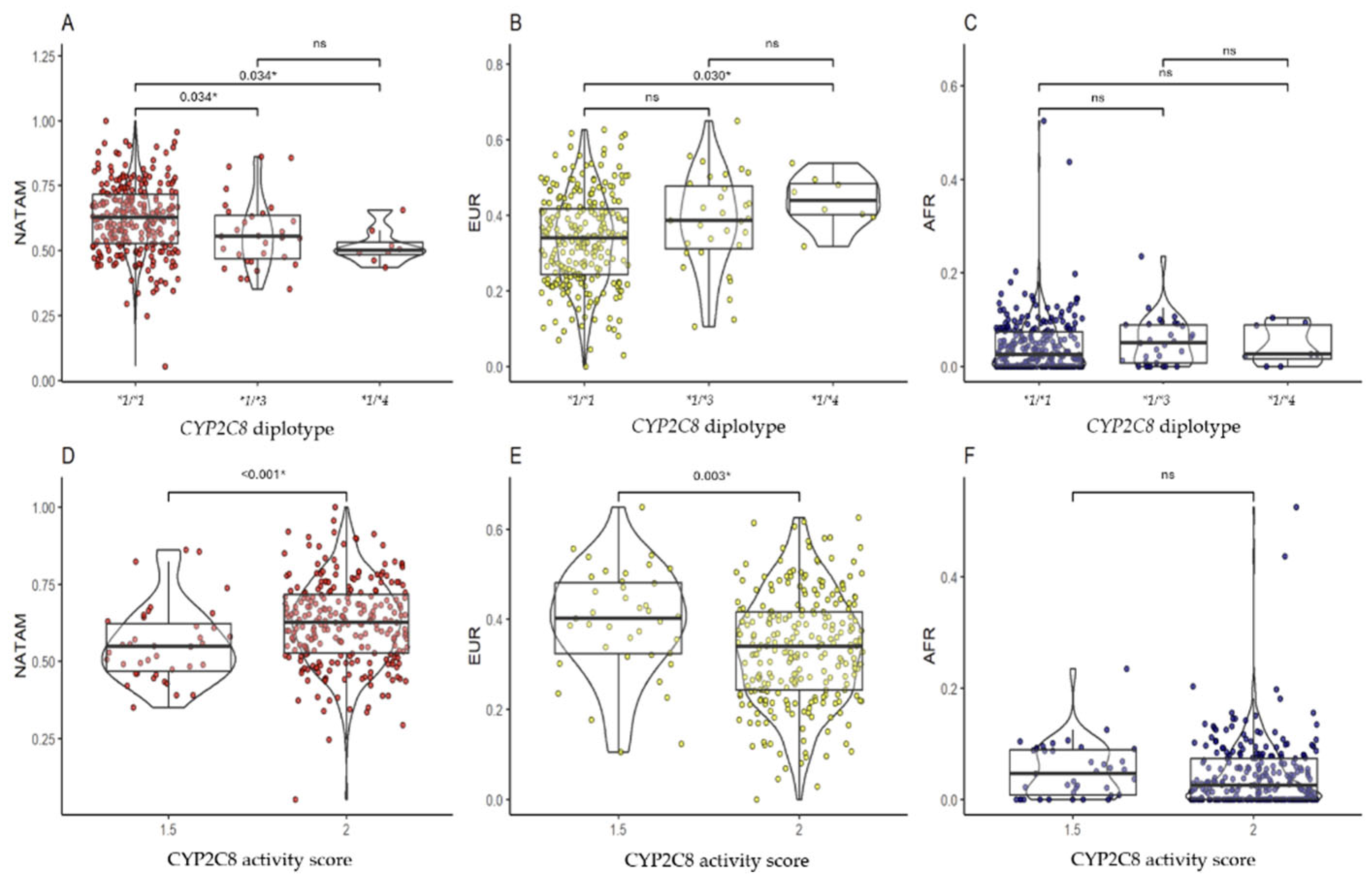
2.3.1. CYP2C8
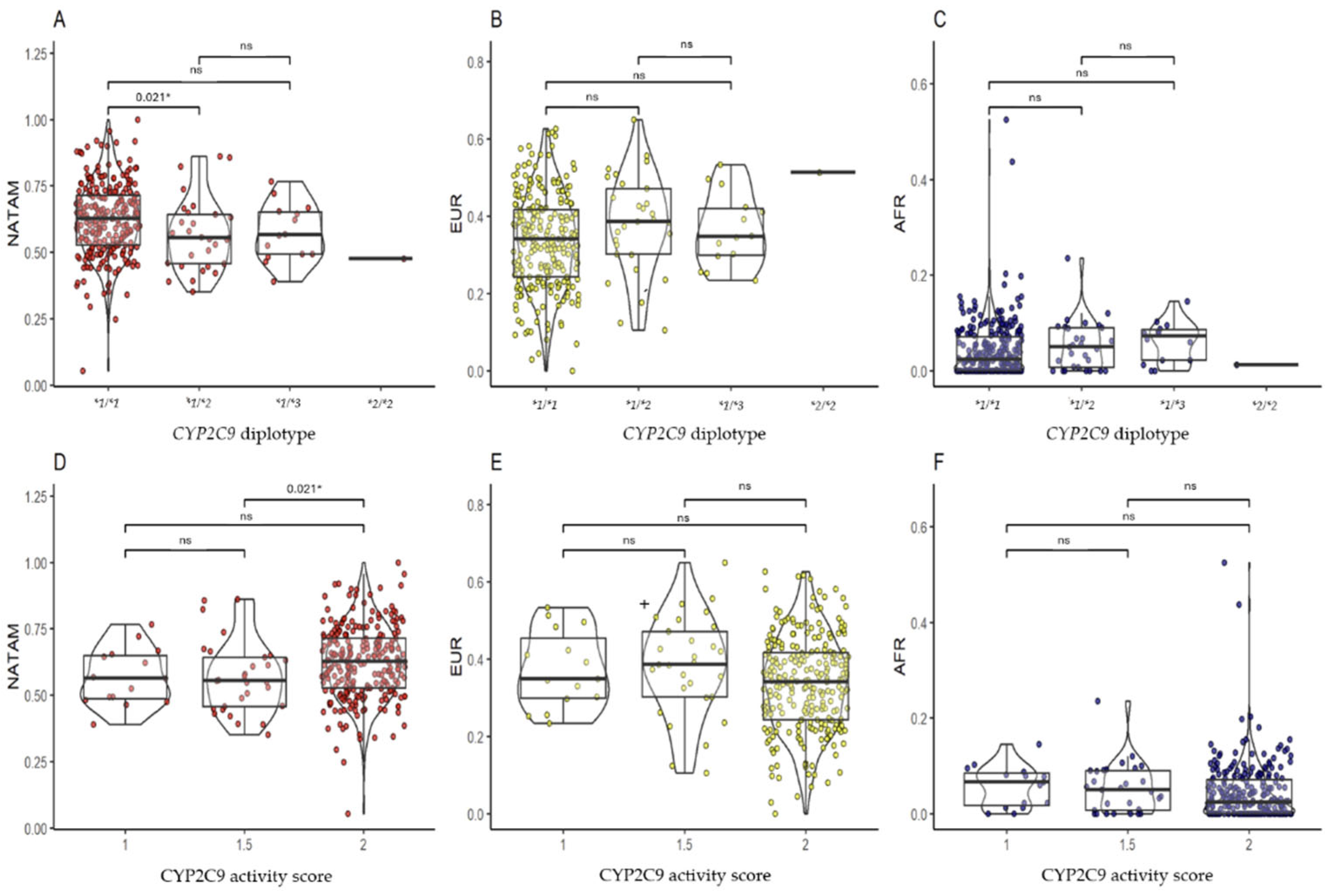
2.3.2. CYP2C9
2.3.3. CYP2C19
2.4. Ancestry Inference Among Transporter SNVs
2.4.1. SLC22A1
2.4.2. SLC22A3
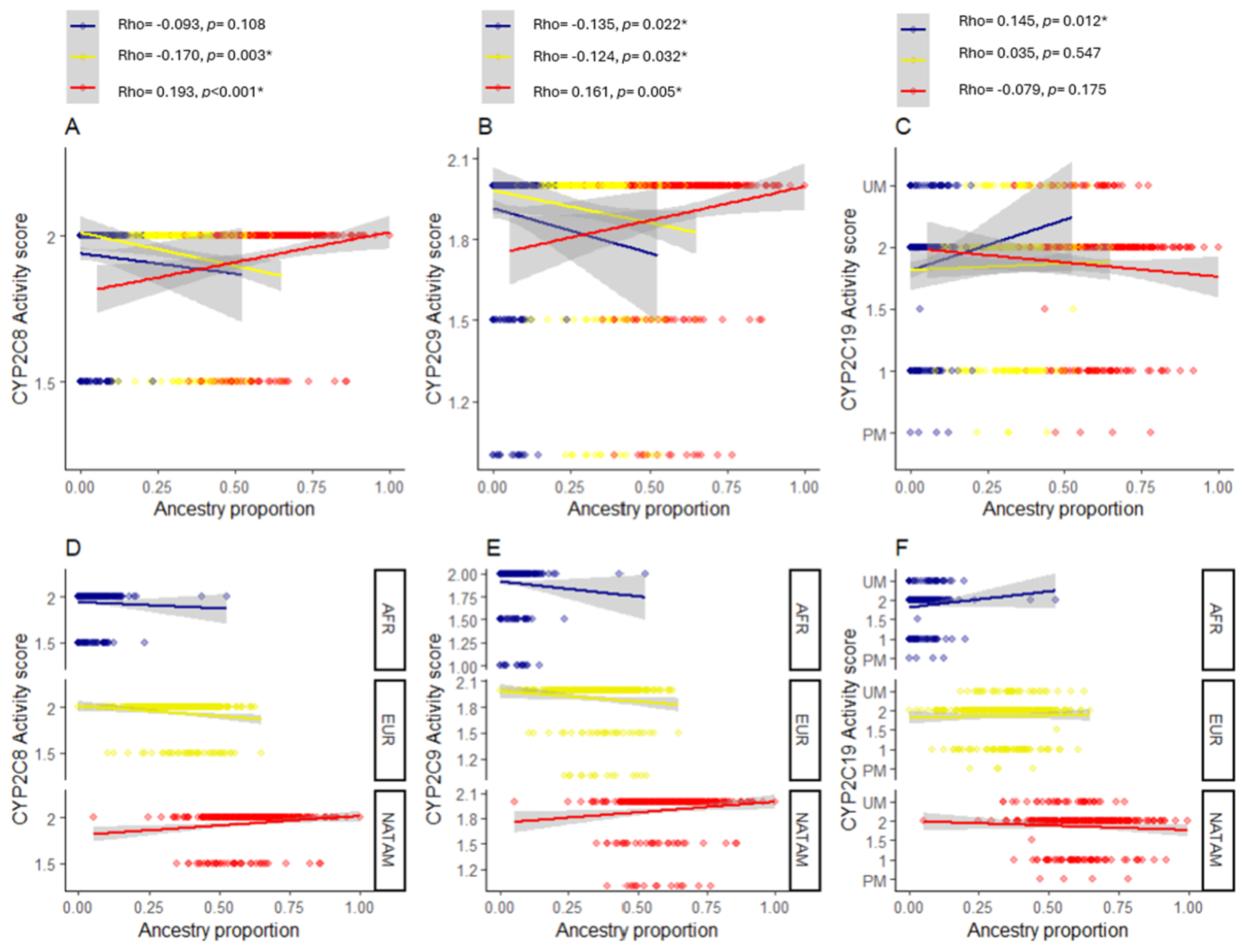
2.5. Correlation Analysis Between Genetic Variants and Ancestry Proportion
2.5.1. Correlation Analysis for CYP2C8 Variants
2.5.2. Correlation Analysis for CYP2C9 Variants
2.5.3. Correlation Analysis for CYP2C19 Variants
2.5.4. Correlation Analysis for Transporters SNVs
3. Discussion
3.1. Ancestry of the Ecuadorian Population
3.2. Ancestry and Pharmacogenetics of CYP450
3.2.1. CYP2C8
3.2.2. CYP2C9
3.2.3. CYP2C19
3.3. Ancestry and OCTs
3.3.1. SLC22A1
3.3.2. SLC22A2
3.3.3. SLC22A3
3.3.4. ABCB1
4. Materials and Methods
4.1. Study Design
4.2. Inclusion and Exclusion Criteria
4.3. Data Collection
4.4. Genotyping Procedure
4.5. Genomic Ancestry Analysis
4.6. Statistical Analysis
4.6.1. Descriptive Analysis
4.6.2. Inferential Analysis
4.6.3. Correlation Analysis
5. Conclusions
The Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCB1 | ATP Binding Cassette Subfamily B Member 1 |
| ADRs | Adverse drug reactions |
| AFR | African |
| AIMs | Ancestry informative markers |
| CPIC | Clinical Pharmacogenetics Implementation Consortium Guidelines |
| CYP450 | Cytochrome P450 |
| T2DM | Type 2 diabetes mellitus |
| EUR | European |
| IM | Intermediate metabolizer |
| NATAM | Native American |
| NM | Normal metabolizer |
| OCT | Organic cation transporters |
| PM | Poor metabolizer |
| P-gp | P-glycoprotein |
| SNV | Single nucleotide allelic variant |
| SU | Sulfonylureas |
| TZD | Thiazolidinediones |
References
- Puig-García, M.; Caicedo-Montaño, C.; Márquez-Figueroa, M.; Chilet-Rosell, E.; Montalvo-Villacis, G.; Benazizi-Dahbi, I.; Peralta, A.; Torres-Castillo, A.L.; Parker, L.A. Prevalence and gender disparities of type 2 diabetes mellitus and obesity in Esmeraldas, Ecuador: A population-based survey in a hard-to-reach setting. Int. J. Equity Health 2023, 22, 124. [Google Scholar] [CrossRef]
- Global Clinical Practice Recommendations-International Diabetes Federation. Available online: https://idf.org/what-we-do/education/idf-clinical-practice-recommendations-for-type-2-diabetes-2025/ (accessed on 18 July 2025).
- López-Jaramillo, P.; Barbosa, E.; Molina, D.I.; Sanchez, R.; Diaz, M.; Camacho, P.A.; Lanas, F.; Pasquel, M.; Accini, J.L.; Ponte-Negretti, C.I.; et al. Latin American Consensus on the Management of Hypertension in the Patient with Diabetes and the Metabolic Syndrome. J. Hypertens. 2019, 37, 1126–1147. [Google Scholar] [CrossRef]
- Ecuador Refuerza su Compromiso en la Lucha Contra la Diabetes–Ministerio de Salud Pública. Available online: https://www.salud.gob.ec/ecuador-refuerza-su-compromiso-en-la-lucha-contra-la-diabetes/ (accessed on 18 July 2025).
- Martin-Delgado, J.; Tovar, C.; Pazmiño, I.; Briones, A.; Carrillo, I.; Guilabert, M.; Mira, J.J. Indicators for Adequate Diabetes Care for the Indigenous Communities of Ecuador. Health Expect. 2022, 25, 3315–3325. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes. Diabetes Care 2025, 48 (Suppl. S1), S181–S206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lauschke, V.M. Population Pharmacogenomics: An Update on Ethnogeographic Differences and Opportunities for Precision Public Health. Hum. Genet. 2022, 141, 1113–1136. [Google Scholar] [CrossRef] [PubMed]
- Eichelbaum, M.; Ingelman-Sundberg, M.; Evans, W.E. Pharmacogenomics and individualized drug therapy. Annu. Rev. Med. 2006, 57, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Iinuma, S.; Yamamoto, M.; Niimura, T.; Osaki, Y.; Nishimura, S.; Harada, K.; Zamami, Y.; Hagiya, H. International Trends in Adverse Drug Event-Related Mortality from 2001 to 2019: An Analysis of the World Health Organization Mortality Database from 54 Countries. Drug Saf. 2024, 47, 237–249. [Google Scholar] [CrossRef]
- Ducoffe, A.R.; York, A.; Hu, D.J.; Perfetto, D.; Kerns, R.D. National action plan for adverse drug event prevention: Recommendations for safer outpatient opioid use. Pain Med. 2016, 17, 2291–2304. [Google Scholar] [CrossRef][Green Version]
- Damanhouri, Z.A.; Alkreathy, H.M.; Alharbi, F.A.; Abualhamail, H.; Ahmad, M.S. A Review of the Impact of Pharmacogenetics and Metabolomics on the Efficacy of Metformin in Type 2 Diabetes. Int. J. Med. Sci. 2023, 20, 142–150. [Google Scholar] [CrossRef]
- Peng, A.; Gong, C.; Xu, Y.; Liang, X.; Chen, X.; Hong, W.; Yan, J. Association between Organic Cation Transporter Genetic Polymorphisms and Metformin Response and Intolerance in T2DM Individuals: A Systematic Review and Meta-Analysis. Front. Public Health 2023, 11, 1183879. [Google Scholar] [CrossRef]
- Dujic, T.; Zhou, K.; Donnelly, L.A.; Tavendale, R.; Palmer, C.N.A.; Pearson, E.R. Association of organic cation transporter 1 with intolerance to metformin in type 2 diabetes: A GoDARTS study. Diabetes 2015, 64, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Dujic, T.; Causevic, A.; Bego, T.; Malenica, M.; Velija-Asimi, Z.; Pearson, E.R.; Semiz, S. Organic Cation Transporter 1 Variants and Gastrointestinal Side Effects of Metformin in Patients with Type 2 Diabetes. Diabet. Med. 2016, 33, 511–551. [Google Scholar] [CrossRef]
- Wang, K.; Yang, A.; Shi, M.; Tam, C.C.H.; Lau, E.S.H.; Fan, B.; Lim, C.K.P.; Lee, H.M.; Kong, A.P.S.; Luk, A.O.Y.; et al. CYP2C19 Loss-of-function Polymorphisms Are Associated with Reduced Risk of Sulfonylurea Treatment Failure in Chinese Patients with Type 2 Diabetes. Clin. Pharmacol. Ther. 2022, 111, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Dawed, A.Y.; Donnelly, L.; Tavendale, R.; Carr, F.; Leese, G.; Palmer, C.N.; Pearson, E.R.; Zhou, K. CYP2C8 and SLCO1B1 Variants and Therapeutic Response to Thiazolidinediones in Patients With Type 2 Diabetes. Diabetes Care 2016, 39, 1902–1908. [Google Scholar] [CrossRef]
- Gökalp, O.; Gunes, A.; Cam, H.; Cure, E.; Aydın, O.; Tamer, M.N.; Scordo, M.G.; Dahl, M.L. Mild hypoglycaemic attacks induced by sulphonylureas related to CYP2C9, CYP2C19 and CYP2C8 polymorphisms in routine clinical setting. Eur. J. Clin. Pharmacol. 2011, 67, 1223–1229. [Google Scholar] [CrossRef]
- Dujic, T.; Zhou, K.; Donnelly, L.A.; Leese, G.; Palmer, C.N.A.; Pearson, E.R. Interaction between variants in the CYP2C9 and POR genes and the risk of sulfonylurea-induced hypoglycaemia: A GoDARTS Study. Diabetes Obes. Metab. 2018, 20, 211–214. [Google Scholar] [CrossRef]
- Baye, A.M.; Fanta, T.G.; Siddiqui, M.K.; Dawed, A.Y. The Genetics of Adverse Drug Outcomes in Type 2 Diabetes: A Systematic Review. Front. Genet. 2021, 12, 675053. [Google Scholar] [CrossRef]
- Terán, E.; Cuautle, P.; Tana, L.; Rodríguez, N.; Bonilla, M.; Molina, J.; Llerena, A. Farmacogenética de la Diabetes Mellitus en Latinoamérica: Una Perspectiva desde la Red Iberoamericana de Farmacogenética. In Farmacogenómica y Medicina Personalizada en Latinoamérica, 1st ed.; Quiñonez, L., Redal, M.A., Eds.; Editorial Académica Española: London, UK, 2020; pp. 443–452. [Google Scholar]
- Peñas-Lledó, E.; Terán, E.; Sosa-Macías, M.; Galaviz-Hernández, C.; Gil, J.P.; Nair, S.; Diwakar, S.; Hernández, I.; Lara-Riegos, J.; Ramírez-Roa, R.; et al. Challenges and Opportunities for Clinical Pharmacogenetic Research Studies in Resource-limited Settings: Conclusions From the Council for International Organizations of Medical Sciences-Ibero-American Network of Pharmacogenetics and Pharmacogenomics Meeting. Clin. Ther. 2020, 42, 1595–1610.e5. [Google Scholar]
- Sosa-Macías, M.; Teran, E.; Waters, W.; Fors, M.M.; Altamirano, C.; Jung-Cook, H.; Galaviz-Hernández, C.; López-López, M.; Remírez, D.; Moya, G.E.; et al. Pharmacogenetics and ethnicity: Relevance for clinical implementation, clinical trials, pharmacovigilance and drug regulation in Latin America. Pharmacogenomics 2016, 17, 1741–1747. [Google Scholar] [CrossRef]
- Lteif, C.; Gawronski, B.E.; Cicali, E.J.; Martinez, K.A.; Newsom, K.J.; Starostik, P.; Cavallari, L.H.; Duarte, J.D. Development of an Ancestrally Inclusive Preemptive Pharmacogenetic Testing Panel. Clin. Transl. Sci. 2025, 18, e70230. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.; Hindorff, L.A.; Morales, J.; Ramos, E.M.; Manolio, T.A. Patterns of pharmacogenetic variation in nine biogeographic groups. Clin. Transl. Sci. 2024, 17, e70017. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, R.; González-Andrade, F.; Børsting, C.; Torroni, A.; Pereira, V.; Morling, N. Analysis of ancestry informative markers in three main ethnic groups from Ecuador supports a trihybrid origin of Ecuadorians. Forensic Sci. Int. Genet. 2017, 31, 29–33. [Google Scholar] [CrossRef]
- Naranjo, M.G.; Rodrigues-Soares, F.; Peñas-Lledó, E.M.; Tarazona-Santos, E.; Fariñas, H.; Rodeiro, I.; Terán, E.; Grazina, M.; Moya, G.E.; López-López, M.; et al. CEIBA-Consortium of the Ibero-American Network of Pharmacogenetics and Pharmacogenomics RIBEF, Interethnic Variability in CYP2D6, CYP2C9, and CYP2C19 Genes and Predicted Drug Metabolism Phenotypes Among 6060 Ibero- and Native Americans: RIBEF-CEIBA Consortium Report on Population Pharmacogenomics. Omics A J. Integr. Biol. 2018, 22, 575–588. [Google Scholar]
- Rodrigues-Soares, F.; Peñas-Lledó, E.M.; Tarazona-Santos, E.; Sosa-Macías, M.; Terán, E.; López-López, M.; Rodeiro, I.; Moya, G.E.; Calzadilla, L.R.; Ramírez-Roa, R.; et al. RIBEF Ibero-American Network of Pharmacogenetics and Pharmacogenomics, Genomic Ancestry, CYP2D6, CYP2C9, and CYP2C19 Among Latin Americans. Clin. Pharmacol. Ther. 2020, 107, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ayala, A.; de la Cruz, C.G.; Dorado, P.; Rodrigues-Soares, F.; Castillo-Nájera, F.; LLerena, A.; Molina-Guarneros, J. Molecular Ancestry Across Allelic Variants of SLC22A1, SLC22A2, SLC22A3, ABCB1, CYP2C8, CYP2C9, and CYP2C19 in Mexican-Mestizo DMT2 Patients. Biomedicines 2025, 13, 1156. [Google Scholar] [CrossRef]
- Ortega-Ayala, A.; De Andrés, F.; Llerena, A.; Bartolo-Montiel, C.M.; Molina-Guarneros, J.A. Impact of SLC22A1 variants rs622342 and rs72552763 on HbA1c and metformin plasmatic concentration levels in patients with type 2 diabetes mellitus. Biomed. Rep. 2024, 21, 117. [Google Scholar] [CrossRef]
- 1000 Genomes Project Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Peñas-Lledó, E.M.; Guillaume, S.; de Andrés, F.; Cortés-Martínez, A.; Dubois, J.; Kahn, J.P.; Leboyer, M.; Olié, E.; LLerena, A.; Courtet, P. A one-year follow-up study of treatment-compliant suicide attempt survivors: Relationship of CYP2D6-CYP2C19 and polypharmacy with suicide reattempts. Transl. Psychiatry 2022, 12, 451. [Google Scholar] [CrossRef]
- Homburger, J.R.; Moreno-Estrada, A.; Gignoux, C.R.; Nelson, D.; Sanchez, E.; Ortiz-Tello, P.; Pons-Estel, B.A.; Acevedo-Vasquez, E.; Miranda, P.; Langefeld, C.D.; et al. Genomic insights into the ancestry and demographic history of South America. PLoS Genet. 2015, 11, e1005602. [Google Scholar] [CrossRef]
- Farinango, C.; Gallardo-Cóndor, J.; Freire-Paspuel, B.; Flores-Espinoza, R.; Jaramillo-Koupermann, G.; López-Cortés, A.; Burgos, G.; Tejera, E.; Cabrera-Andrade, A. Genetic variations of the DPYD gene and its relationship with ancestry proportions in different Ecuadorian trihybrid populations. J. Pers. Med. 2022, 12, 950. [Google Scholar] [CrossRef]
- Nagar, S.D.; Conley, A.B.; Chande, A.T.; Rishishwar, L.; Sharma, S.; Mariño-Ramírez, L.; Aguinaga-Romero, G.; González-Andrade, F.; Jordan, I.K. Genetic ancestry and ethnic identity in Ecuador. HGG Adv. 2021, 2, 100050. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, J.R.; Salazar-Granara, A.; Acosta, O.; Castillo-Herrera, W.; Fujita, R.; Pena, S.D.; Santos, F.R. Tracing the genomic ancestry of Peruvians reveals a major legacy of pre-Columbian ancestors. J. Hum. Genet. 2013, 58, 627–634. [Google Scholar] [CrossRef]
- Mariño-Ramírez, L.; Sharma, S.; Hamilton, J.M.; Nguyen, T.L.; Gupta, S.; Natarajan, A.V.; Nagar, S.D.; Menuey, J.L.; Chen, W.A.; Sánchez-Gómez, A.; et al. The Consortium for Genomic Diversity, Ancestry, and Health in Colombia (CÓDIGO), building local capacity in genomics, bioinformatics, and precision medicine. bioRxiv 2025. [Google Scholar] [CrossRef]
- Pena, S.D.J.; Santos, F.R.; Tarazona-Santos, E. Genetic admixture in Brazil. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 928–938. [Google Scholar] [CrossRef]
- Guevara, M.; Rodrigues-Soares, F.; de la Cruz, C.G.; de Andrés, F.; Rodríguez, E.; Peñas-Lledó, E.; LLerena, A. CEIBA Consortium of the Ibero-American Network of Pharmacogenetics and Pharmacogenomics RIBEF. Afro-Latin American Pharmacogenetics of CYP2D6, CYP2C9, and CYP2C19 in Dominicans: A Study from the RIBEF-CEIBA Consortium. Pharmaceutics 2024, 16, 1399. [Google Scholar] [CrossRef]
- Nieves-Colón, M.A.; Ulrich, E.C.; Chen, L.; Torres Colón, G.A.; Rivera Clemente, M.; La Corporación Piñones Se Integra (COPI); Benn Torres, J. Genetic ancestry in Puerto Rican Afro-descendants illustrates diverse histories of African diasporic populations. Am. J. Biol. Anthropol. 2024, 185, e25029. [Google Scholar] [CrossRef]
- Sosa-Macías, M.; Fricke-Galindo, I.; Fariñas, H.; Monterde, L.; Ruiz-Cruz, E.D.; Molina-Guarneros, J.; Tarazona-Santos, E.; Rodrigues-Soares, F.; Galaviz-Hernández, C.; Peñas-Lledó, E.; et al. Pharmacogenetics, ethnicity, treatment and health in Latin American populations. Pharmacogenomics 2023, 24, 489–492. [Google Scholar] [CrossRef]
- Zhang, J.; Litvinova, M.; Liang, Y.; Wang, Y.; Wang, W.; Zhao, S.; Wu, Q.; Merler, S.; Viboud, C.; Vespignani, A.; et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science 2020, 368, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; González-Andrade, F.; Soriano, A.; Fanlo, A.; Martínez-Jarreta, B.; Sinués, B. Genetic polymorphisms of CYP2C8, CYP2C9 and CYP2C19 in Ecuadorian Mestizo and Spaniard populations: A comparative study. Mol. Biol. Rep. 2014, 41, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- ‘Pharmfreq’. Available online: https://www.pharmfreq.com/?_inputs_&gene=%22CYP2C8%22&allele=%22*3%22&go=1&mapping_method=%22Countries%22 (accessed on 8 July 2025).
- Chan, A.T.; Zauber, A.G.; Hsu, M.; Breazna, A.; Hunter, D.J.; Rosenstein, R.B.; Eagle, C.J.; Hawk, E.T.; Bertagnolli, M.M. Cytochrome P450 2C9 variants influence response to celecoxib for prevention of colorectal adenoma. Gastroenterology 2009, 136, 2127–2136.e1. [Google Scholar] [CrossRef]
- Theken, K.N.; Lee, C.R.; Gong, L.; Caudle, K.E.; Formea, C.M.; Gaedigk, A.; Klein, T.E.; Agúndez, J.A.G.; Grosser, T. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clin. Pharmacol. Ther. 2020, 108, 191–200. [Google Scholar] [CrossRef]
- Dorado, P.; Cavaco, I.; Cáceres, M.C.; Piedade, R.; Ribeiro, V.; LLerena, A. Relationship between CYP2C8 genotypes and diclofenac 5-hydroxylation in healthy Spanish volunteers. Eur. J. Clin. Pharmacol. 2008, 64, 967–970. [Google Scholar] [CrossRef]
- Ochoa, D.; Prieto-Pérez, R.; Román, M.; Talegón, M.; Rivas, A.; Galicia, I.; Abad-Santos, F. Effect of Gender and CYP2C9 and CYP2C8 Polymorphisms on the Pharmacokinetics of Ibuprofen Enantiomers. Pharmacogenomics 2015, 16, 939–948. [Google Scholar] [CrossRef]
- Campodónico, D.M.; Zubiaur, P.; Soria-Chacartegui, P.; Casajús, A.; Villapalos-García, G.; Navares-Gómez, M.; Gómez-Fernández, A.; Parra-Garcés, R.; Mejía-Abril, G.; Román, M.; et al. CYP2C8*3 and *4 Define CYP2C8 Phenotype: An Approach with the Substrate Cinitapride. Clin. Transl. Sci. 2022, 15, 2613–2624. [Google Scholar] [CrossRef]
- Céspedes-Garro, C.; Fricke-Galindo, I.; Naranjo, M.E.; Rodrigues-Soares, F.; Fariñas, H.; de Andrés, F.; López-López, M.; Peñas-Lledó, E.M.; LLerena, A. Worldwide interethnic variability and geographical distribution of CYP2C9 genotypes and phenotypes. Expert. Opin. Drug Metab. Toxicol. 2015, 11, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Nevosadová, L.; Eliasson, E.; Lauschke, V.M. Global distribution of functionally important CYP2C9 alleles and their inferred metabolic consequences. Hum. Genom. 2023, 17, 4–13. [Google Scholar] [CrossRef]
- Dorado, P.; Beltrán, L.J.; MacHín, E.; Peñas-Lledó, E.M.; Terán, E.; Llerena, A. Losartan hydroxylation phenotype in an Ecuadorian population: Influence of CYP2C9 genetic polymorphism, habits and gender. Pharmacogenomics 2012, 13, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Karnes, J.H.; Rettie, A.E.; Somogyi, A.A.; Huddart, R.; Fohner, A.E.; Formea, C.M.; Ta Michael Lee, M.; Llerena, A.; Whirl-Carrillo, M.; Klein, T.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C9 and HLA-B Genotypes and Phenytoin Dosing: 2020 Update. Clin. Pharmacol. Ther. 2021, 109, 302–309. [Google Scholar] [CrossRef]
- Johnson, J.A.; Caudle, K.E.; Gong, L.; Whirl-Carrillo, M.; Stein, C.M.; Scott, S.A.; Lee, M.T.; Gage, B.F.; Kimmel, S.E.; Perera, M.A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Pharmacogenetics-Guided Warfarin Dosing: 2017 Update. Clin. Pharmacol. Ther. 2017, 102, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.; Heo, Y.; Kim, H.; Yoon, H.Y.; Song, G.; Gwak, H.S. Association Between the CYP2C9 Genotype and Hypoglycemia Among Patients With Type 2 Diabetes Receiving Sulfonylurea Treatment: A Meta-analysis. Clin. Ther. 2021, 43, 836–843.e4. [Google Scholar] [CrossRef]
- Koren, S.; Koren, R. Any Polymorphisms of CYP2C9 Affects the Biochemical Profile of Diabetic Patients Receiving Glibenclamide. Clin. Med. Biochem. Open Access 2015, 1, 1–4. [Google Scholar] [CrossRef]
- Salam, R.F.A.; Zeyada, R.; Osman, N.A. Effect of CYP2C9 gene polymorphisms on response to treatment with sulfonylureas in a cohort of Egyptian type 2 diabetes mellitus patients. Comp. Clin. Path 2014, 23, 341–346. [Google Scholar] [CrossRef]
- Castelán-Martínez, O.D.; Hoyo-Vadillo, C.; Bazán-Soto, T.B.; Cruz, M.; Tesoro-Cruz, E.; Valladares-Salgado, A. CYP2C9*3 gene variant contributes independently to glycaemic control in patients with type 2 diabetes treated with glibenclamide. J. Clin. Pharm. Ther. 2018, 43, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Saeed, M.; Mothana, R.A.; Muhammad, T.; Rahman, N.; Alanzi, A.R.; Akbar, R. Association of CYP2C9*2 Allele with Sulphonylurea-Induced Hypoglycaemia in Type 2 Diabetes Mellitus Patients: A Pharmacogenetic Study in Pakistani Pashtun Population. Biomedicines 2023, 11, 2282. [Google Scholar] [CrossRef]
- Scheen, A.J. Sulphonylureas in the management of type 2 diabetes: To be or not to be? Diabetes Epidemiol. Manag. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Alonso Llorente, A.; Salgado Garrido, J.; Teijido Hermida, Ó.; González Andrade, F.; Valiente Martín, A.; Fanlo Villacampa, A.J.; Vicente Romero, J. Genetic Polymorphisms of CYP2C19 in Ecuadorian Population: An Interethnic Approach. Heliyon 2024, 10, e28566. [Google Scholar] [CrossRef]
- De Andrés, F.; Terán, S.; Hernández, F.; Terán, E.; Llerena, A. To Genotype or Phenotype for Personalized Medicine? CYP450 Drug Metabolizing Enzyme Genotype-Phenotype Concordance and Discordance in the Ecuadorian Population. Omi. A J. Integr. Biol. 2016, 20, 699–710. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Jung-Cook, H.; Llerena, A.; López-López, M. Interethnic Variability of Pharmacogenetic Biomarkers in Mexican Healthy Volunteers: A Report from the RIBEF (Ibero-American Network of Pharmacogenetics and Pharmacogenomics). Drug Metab. Pers. Ther. 2016, 31, 61–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, D.; Chen, X.; Lin, N.; Guo, Y.; Zhou, H.; Zhong, D. Influence of CYP2C9 and CYP2C19 Genetic Polymorphisms on Pharmacokinetics of Gliclazide MR in Chinese Subjects. Br. J. Clin. Pharmacol. 2007, 64, 67–74. [Google Scholar] [CrossRef]
- Tan, B.; Zhang, Y.F.; Chen, X.Y.; Zhao, X.H.; Li, G.X.; Zhong, D.F. The effects of CYP2C9 and CYP2C19 genetic polymorphisms on the pharmacokinetics and pharmacodynamics of glipizide in Chinese subjects. Eur. J. Clin. Pharmacol. 2010, 66, 145–151. [Google Scholar] [CrossRef]
- Lee, C.R.; Luzum, J.A.; Sangkuhl, K.; Gammal, R.S.; Sabatine, M.S.; Stein, C.M.; Kisor, D.F.; Limdi, N.A.; Lee, Y.M.; Scott, S.A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clin. Pharmacol. Ther. 2022, 112, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Sangkuhl, K.; Swen, J.J.; Ellingrod, V.L.; Müller, D.J.; Shimoda, K.; Bishop, J.R.; Kharasch, E.D.; Skaar, T.C.; Gaedigk, A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2D6 and CYP2C19 Genotypes and Dosing of Tricyclic Antidepressants: 2016 Update. Clin. Pharmacol. Ther. 2017, 102, 37–44. [Google Scholar] [CrossRef]
- Bousman, C.A.; Stevenson, J.M.; Ramsey, L.B.; Sangkuhl, K.; Hicks, J.K.; Strawn, J.R.; Singh, A.B.; Ruaño, G.; Mueller, D.J.; Tsermpini, E.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin. Pharmacol. Ther. 2023, 114, 51–68. [Google Scholar] [CrossRef]
- Dyer, S.C.; Austine-Orimoloye, O.; Azov, A.G.; Barba, M.; Barnes, I.; Barrera-Enriquez, V.P.; Becker, A.; Bennett, R.; Beracochea, M.; Berry, A.; et al. Ensembl 2025. Nucleic Acids Res. 2025, 53, D948–D957. [Google Scholar] [CrossRef]
- Sundelin, E.; Gormsen, L.C.; Jensen, J.B.; Vendelbo, M.H.; Jakobsen, S.; Munk, O.L.; Christensen, M.; Brøsen, K.; Frøkiaer, J.; Jessen, N. Genetic Polymorphisms in Organic Cation Transporter 1 Attenuates Hepatic Metformin Exposure in Humans. Clin. Pharmacol. Ther. 2017, 102, 841–848. [Google Scholar] [CrossRef]
- Christensen, M.M.H.; Brasch-Andersen, C.; Green, H.; Nielsen, F.; Damkier, P.; Beck-Nielsen, H.; Brosen, K. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet. Genom. 2011, 21, 837–850. [Google Scholar] [CrossRef]
- Meyer, M.J.; Seitz, T.; Brockmöller, J.; Tzvetkov, M.V. Effects of genetic polymorphisms on the OCT1 and OCT2-mediated uptake of ranitidine. PLoS ONE 2017, 12, e0189521. [Google Scholar] [CrossRef]
- Fukuda, T.; Chidambaran, V.; Mizuno, T.; Venkatasubramanian, R.; Ngamprasertwong, P.; Olbrecht, V.; Esslinger, H.R.; Vinks, A.A.; Sadhasivam, S. OCT1 Genetic Variants Influence the Pharmacokinetics of Morphine in Children. Pharmacogenomics 2013, 14, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Ayala, A.; De Andrés, F.; Llerena, A.; Bartolo-Montiel, C.M.; Acosta-Altamirano, G.; Molina-Guarneros, J.A. Longitudinal Assessment of SNPs rs72552763 and rs622342 in SLC22A1 over HbA1c Control among Mexican-Mestizo Diabetic Type 2 Patients. Front. Pharmacol. 2024, 15, 1433519. [Google Scholar] [CrossRef]
- Ahmed, A.; Elsadek, H.M.; Shalaby, S.M.; Elnahas, H.M. Association of SLC22A1, SLC47A1, and KCNJ11 polymorphisms with efficacy and safety of metformin and sulfonylurea combination therapy in Egyptian patients with type 2 diabetes. Res. Pharm. Sci. 2023, 18, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Guo, Y.; Li, X.; Yin, J.Y.; Zheng, W.; Qiu, X.W.; Xiao, L.; Liu, R.R.; Wang, S.Y.; Gong, W.J.; et al. The Impacts of SLC22A1 rs594709 and SLC47A1 rs2289669 Polymorphisms on Metformin Therapeutic Efficacy in Chinese Type 2 Diabetes Patients. Int. J. Endocrinol. 2016, 2016, 4350712. [Google Scholar] [CrossRef] [PubMed]
- Reséndiz-Abarca, C.A.; Flores-Alfaro, E.; Suárez-Sánchez, F.; Cruz, M.; Valladares-Salgado, A.; Del Carmen Alarcón-Romero, L.; Vázquez-Moreno, M.A.; Wacher-Rodarte, N.A.; Gómez-Zamudio, J.H. Altered Glycemic Control Associated with Polymorphisms in the SLC22A1 (OCT1) Gene in a Mexican Population with Type 2 Diabetes Mellitus Treated with Metformin: A Cohort Study. J. Clin. Pharmacol. 2019, 59, 1384–1390. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, A.; Kong, F.; Chen, J.; Lan, B.; He, G.; Gao, K.; Cheng, L.; Sun, X.; Yan, C.; et al. Structural Insights into Human Organic Cation Transporter 1 Transport and Inhibition. Cell Discov. 2024, 10, 30. [Google Scholar] [CrossRef]
- Ningrum, V.D.A.; Sadewa, A.H.; Ikawati, Z.; Yuliwulandari, R.; Ikhsan, M.R.; Fajriyah, R. The influence of metformin transporter gene SLC22A1 and SLC47A1 variants on steady-state pharmacokinetics and glycemic response. PLoS ONE 2022, 17, e0271410. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, W.; Wang, Y.; Jiang, Z.; Meng, X.; Xiao, Q.; Zhao, Q.; Yan, J. Genetic Variants of OCT1 Influence Glycemic Response to Metformin in Han Chinese Patients with Type-2 Diabetes Mellitus in Shanghai. Int. J. Clin. Exp. Pathol. 2015, 8, 9533–9542. [Google Scholar]
- Moreno-González, J.G.; Reza-López, S.A.; González-Rodríguez, E.; Siqueiros-Cendón, T.S.; Escareño Contreras, A.; Rascón-Cruz, Q.; Leal-Berumen, I. Genetic Variants of SLC22A1 rs628031 and rs622342 and Glycemic Control in T2DM Patients from Northern Mexico. Genes 2025, 16, 139. [Google Scholar] [CrossRef]
- Emami-Riedmaier, A.; Schaeffeler, E.; Nies, A.T.; Mörike, K.; Schwab, M. Stratified medicine for the use of antidiabetic medication in treatment of type II diabetes and cancer: Where do we go from here? J. Intern. Med. 2015, 277, 235–247. [Google Scholar] [CrossRef]
- Santoro, A.B.; Botton, M.R.; Struchiner, C.J.; Suarez-Kurtz, G. Influence of pharmacogenetic polymorphisms and demographic variables on metformin pharmacokinetics in an admixed Brazilian cohort. Br. J. Clin. Pharmacol. 2018, 84, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Phani, N.M.; Vohra, M.; Kakar, A.; Adhikari, P.; Nagri, S.K.; D’Souza, S.C.; Umakanth, S.; Satyamoorthy, K.; Rai, P.S. Implication of critical pharmacokinetic gene variants on therapeutic response to metformin in Type 2 diabetes. Pharmacogenomics 2018, 19, 905–911. [Google Scholar] [CrossRef]
- Chen, E.C.; Liang, X.; Yee, S.W.; Geier, E.G.; Stocker, S.L.; Chen, L.; Giacomini, K.M. Targeted Disruption of Organic Cation Transporter 3 Attenuates the Pharmacologic Response to Metformin. Mol. Pharmacol. 2015, 88, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Musi, N.; Hirshman, M.F.; Nygren, J.; Svanfeldt, M.; Bavenholm, P.; Rooyackers, O.; Zhou, G.; Williamson, J.M.; Ljunqvist, O.; Efendic, S.; et al. Metformin Increases AMP-Activated Protein Kinase Activity in Skeletal Muscle of Subjects with Type 2 Diabetes. Diabetes 2002, 51, 2074–2081. [Google Scholar] [CrossRef]
- Zazuli, Z.; Duin, N.J.C.B.; Jansen, K.; Vijverberg, S.J.H.; Maitland-Van der Zee, A.H.; Masereeuw, R. The impact of genetic polymorphisms in organic cation transporters on renal drug disposition. Int. J. Mol. Sci. 2020, 21, 6627. [Google Scholar] [CrossRef]
- Hemauer, S.J.; Nanovskaya, T.N.; Abdel-Rahman, S.Z.; Patrikeeva, S.L.; Hankins, G.D.V.; Ahmed, M.S. Modulation of human placental P-glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem. Pharmacol. 2010, 79, 921–925. [Google Scholar] [CrossRef]
- Gallardo-Cóndor, J.; Naranjo, P.; Atarihuana, S.; Coello, D.; Guevara-Ramírez, P.; Flores-Espinoza, R.; Burgos, G.; López-Cortés, A.; Cabrera-Andrade, A. Population-Specific Distribution of TPMT Deficiency Variants and Ancestry Proportions in Ecuadorian Ethnic Groups: Towards Personalized Medicine. Ther. Clin. Risk Manag. 2023, 19, 1005–1018. [Google Scholar] [CrossRef]
- Florez, J.C.; Price, A.L.; Campbell, D.; Riba, L.; Parra, M.V.; Yu, F.; Duque, C.; Saxena, R.; Gallego, N.; Tello-Ruiz, M.; et al. Strong Association of Socioeconomic Status with Genetic Ancestry in Latinos: Implications for Admixture Studies of Type 2 Diabetes. Diabetologia 2009, 52, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Chande, A.T.; Rishishwar, L.; Conley, A.B.; Valderrama-Aguirre, A.; Medina-Rivas, M.A.; Jordan, I.K. Ancestry Effects on Type 2 Diabetes Genetic Risk Inference in Hispanic/Latino Populations. BMC Med. Genet. 2020, 21 (Suppl. S2), 132. [Google Scholar] [CrossRef] [PubMed]
- Cooper-DeHoff, R.M.; Niemi, M.; Ramsey, L.B.; Luzum, J.A.; Tarkiainen, E.K.; Straka, R.J.; Gong, L.; Tuteja, S.; Wilke, R.A.; Wadelius, M.; et al. The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 Genotypes and Statin-Associated Musculoskeletal Symptoms. Clin. Pharmacol. Ther. 2022, 111, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]


| NATAM | EUR | AFR | NATAM | EUR | AFR | ||
|---|---|---|---|---|---|---|---|
| CYP2C8 | Activity score | ||||||
| *1/*1 | 62.61 (52.71–71.70) | 33.88 (24.35–41.58) | 2.48 (0.00–7.31) | 1 | - | - | - |
| *1/*3 | 55.41 (46.78–63.68) | 38.58 (31.13–47.70) | 5.01 (0.77–8.92) | 1.5 | 54.83 (46.85–62.21) | 40.23 (32.32–48.16) | 4.58 (0.77–8.92) |
| *1/*4 | 49.98 (48.43–53.26) | 43.80 (40.05–48.37) | 2.54 (1.55–8.87) | 2 | 62.61 (52.71–71.70) | 33.88 (24.35–41.58) | 2.48 (0.00–7.31) |
| *3/*4 | - | - | - | ||||
| pKW | 0.002 * | 0.005 * | 0.262 | pU | <0.001 * | 0.003 * | 0.109 |
| CYP2C9 | |||||||
| *1/*1 | 62.55 (52.67–71.49) | 34.09 (24.34–41.68) | 2.40 (0.00–7.06) | 1 | 56.36 (48.72–64.91) | 34.89 (29.90–45.35) | 6.61 (1.72–8.45) |
| *1/*2 | 55.51 (45.76–64.34) | 38.58 (30.19–47.06) | 5.01 (0.68–9.01) | 1.5 | 55.51 (45.76–64.34) | 38.58 (30.19–47.07) | 5.01 (0.68–9.01) |
| *1/*3 | 56.63 (49.29–65.18) | 34.72 (29.79–41.99) | 7.18 (2.18–8.59) | 2 | 62.55 (52.66–71.49) | 34.09 (24.34–41.68) | 2.40 (0.00–7.06) |
| *2/*2 | - | - | - | ||||
| *2/*3 | - | - | - | ||||
| pKW | 0.031 * | 0.132 | 0.053 | pKW | 0.021 * | 0.097 | 0.070 |
| CYP2C19 | |||||||
| *1/*1 | 62.42 (51.50–72.79) | 33.63 (24.07–42.50) | 2.44 (0.00–6.60) | PM | 60.54 (53.34–68.82) | 31.93 (29.27–35.16) | 5.50 (1.90–9.47) |
| *1/*2 | 60.94 (54.63–67.25) | 34.44 (28.14–41.31) | 1.89 (0.00–5.97) | 1 | 60.94 (54.63–67.25) | 34.44 (28.14–41.31) | 1.89 (0.00–5.97) |
| *1/*4 | - | - | - | 1.5 | - | - | - |
| *1/*17 | 58.93 (50.04–64.08) | 36.27 (30.13–44.09) | 6.92 (0.58–10.27) | 2 | 62.42 (51.50–72.79) | 33.63 (24.07–42.50) | 2.44 (0.00–6.60) |
| *2/*2 | 60.54 (53.34–68.82) | 31.93 (29.27–35.16) | 5.50 (1.90–9.47) | UM | 57.31 (50.10–63.82) | 36.33 (30.15–43.71) | 7.06 (0.92–10.19) |
| *17/*17 | - | - | - | ||||
| *2/*17 | - | - | - | ||||
| pKW | 0.183 | 0.601 | 0.025 * | pKW | 0.183 | 0.601 | 0.025* |
| Gene | ID | Genotype | NATAM | EUR | AFR |
|---|---|---|---|---|---|
| SLC22A1 | rs72552763 | GAT/GAT | 59.61 (49.30–67.77) | 35.66 (27.29–44.39) | 3.13 (0.00–7.51) |
| GAT/del | 61.96 (52.55–72.83) | 34.42 (22.95–41.17) | 2.56 (0.00–7.55) | ||
| del/del | 64.98 (60.31–76.51) | 30.11 (21.94–36.37) | 1.89 (0.00–6.70) | ||
| pKW | 0.022 * | 0.017 * | 0.842 | ||
| rs622342 | A/A | 60.46 (49.38–67.91) | 35.53 (26.25–43.23) | 2.70 (0.00–7.86) | |
| A/C | 60.28 (51.47–71.26) | 35.30 (24.37–42.77) | 2.77 (0.00–7.35) | ||
| C/C | 64.98 (60.31–76.51) | 30.11 (21.94–36.37) | 1.89 (0.00–6.70) | ||
| PKW | 0.247 | 0.243 | 0.397 | ||
| rs12208357 | C/C | 61.41 (51.78–70.61) | 34.44 (25.55–42.08) | 2.62 (0.00–7.39) | |
| C/T | 51.21 (44.62–64.92) | 44.73 (34.13–48.11) | 0.66 (0.93–8.83) | ||
| T/T | - | - | - | ||
| pU | 0.339 | 0.265 | 0.884 | ||
| rs2282143 | C/C | 61.41 (51.78–70.61) | 34.44 (25.55–42.08) | 2.62 (0.00–7.39) | |
| C/T | 51.21 (44.62–64.92) | 44.73 (34.13–48.11) | 0.93 (0.66–8.83) | ||
| T/T | - | - | - | ||
| pU | 0.339 | 0.265 | 0.884 | ||
| rs594709 | A/A | 62.58 (52.90–71.94) | 33.17 (22.91–41.83) | 2.57 (0.00–7.76) | |
| A/G | 58.46 (49.93–66.18) | 37.13 (27.70–45.61) | 3.38 (0.00–6.57) | ||
| G/G | 56.09 (42.93–68.57) | 41.27 (31.42–53.12) | 0.00 (0.00–1.31) | ||
| pKW | 0.040 * | 0.013 * | 0.298 | ||
| rs683369 | C/C | 61.10 (51.52–70.60) | 34.53 (24.27–42.47) | 2.60 (0.00–7.68) | |
| C/G | 61.96 (52.16–70.55) | 34.13 (27.19–41.71) | 3.14 (0.00–6.61) | ||
| G/G | - | - | |||
| pU | 0.843 | 0.625 | 0.849 | ||
| rs628031 | G/G | 62.21 (52.85–72.00) | 33.63 (23.17–42.00) | 2.47 (0.00–7.64) | |
| G/A | 57.96 (49.51–65.48) | 38.47 (29.54–45.10) | 4.02 (0.00–6.96) | ||
| A/A | 67.71 (44.48–71.17) | 32.28 (28.82–50.25) | 0.00 (0.00–0.19) | ||
| pKW | 0.042 * | 0.040 * | 0.143 | ||
| SLC22A2 | rs316019 | C/C | 61.97 (51.93–70.79) | 34.33 (25.55–41.90) | 2.54 (0.00–7.36) |
| C/A | 54.99 (46.89–66.91) | 39.39 (26.97–44.43) | 5.26 (1.03–10.60) | ||
| A/A | - | - | - | ||
| pU | 0.163 | 0.369 | 0.120 | ||
| SLC22A3 | rs2076828 | C/C | 63.16 (53.56–72.81) | 32.89 (22.99–40.97) | 2.31 (0.00–6.61) |
| C/G | 55.40 (47.32–65.61) | 39.52 (31.02–47.14) | 4.32 (0.00–8.31) | ||
| G/G | 53.25 (46.62–57.56) | 40.07 (38.26–43.06) | 7.32 (2.70–9.14) | ||
| pKW | <0.001 * | <0.001 * | 0.560 | ||
| ABCB1 | rs2032582 | G/G | 57.27 (49.30–65.98) | 36.21 (28.09–44.97) | 3.57 (0.00–7.35) |
| G/A | 60.11 (47.98–67.88) | 36.29 (28.13–47.83) | 3.84 (1.77–9.51) | ||
| A/A | - | - | - | ||
| G/T | 61.33 (50.31–70.44) | 34.39 (26.05–42.52) | 2.10 (0.00–7.51) | ||
| T/T | 62.73 (54.13–72.24) | 33.70 (24.05–41.62) | 2.22 (0.00–6.44) | ||
| T/A | 66.02 (60.04–71.07) | 30.90 (23.53–35.07) | 3.09 (0.00–7.99) | ||
| pKW | 0.139 | 0.181 | 0.623 | ||
| rs1128503 | C/C | 57.93 (49.23–65.52) | 36.37 (30.03–44.99) | 3.59 (0.00–7.19) | |
| C/T | 60.95 (50.37–70.38) | 33.88 (25.74–41.68) | 2.31 (0.00–7.75) | ||
| T/T | 63.16 (53.14–72.79) | 33.18 (23.40–41.76) | 2.21 (0.00–6.56) | ||
| pKW | 0.072 | 0.127 | 0.481 | ||
| rs1045642 | C/C | 58.04 (49.91–67.67) | 34.72 (28.91–44.13) | 3.48 (0.00–6.96) | |
| C/T | 61.26 (50.63–69.90) | 34.42 (25.96–41.58) | 2.40 (0.00–7.69) | ||
| T/T | 63.16 (53.27–72.44) | 32.72 (22.87–42.43) | 2.14 (0.00–5.50) | ||
| pKW | 0.206 | 0.318 | 0.730 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Ayala, A.; de la Cruz, C.G.; Mora, L.; Bonilla, M.; Tana, L.; Rodrigues-Soares, F.; Dorado, P.; LLerena, A.; Terán, E. Pharmacogenetics and Molecular Ancestry of SLC22A1, SLC22A2, SLC22A3, ABCB1, CYP2C8, CYP2C9, and CYP2C19 in Ecuadorian Subjects with Type 2 Diabetes Mellitus. Pharmaceuticals 2025, 18, 1335. https://doi.org/10.3390/ph18091335
Ortega-Ayala A, de la Cruz CG, Mora L, Bonilla M, Tana L, Rodrigues-Soares F, Dorado P, LLerena A, Terán E. Pharmacogenetics and Molecular Ancestry of SLC22A1, SLC22A2, SLC22A3, ABCB1, CYP2C8, CYP2C9, and CYP2C19 in Ecuadorian Subjects with Type 2 Diabetes Mellitus. Pharmaceuticals. 2025; 18(9):1335. https://doi.org/10.3390/ph18091335
Chicago/Turabian StyleOrtega-Ayala, Adiel, Carla González de la Cruz, Lorena Mora, Mauro Bonilla, Leandro Tana, Fernanda Rodrigues-Soares, Pedro Dorado, Adrián LLerena, and Enrique Terán. 2025. "Pharmacogenetics and Molecular Ancestry of SLC22A1, SLC22A2, SLC22A3, ABCB1, CYP2C8, CYP2C9, and CYP2C19 in Ecuadorian Subjects with Type 2 Diabetes Mellitus" Pharmaceuticals 18, no. 9: 1335. https://doi.org/10.3390/ph18091335
APA StyleOrtega-Ayala, A., de la Cruz, C. G., Mora, L., Bonilla, M., Tana, L., Rodrigues-Soares, F., Dorado, P., LLerena, A., & Terán, E. (2025). Pharmacogenetics and Molecular Ancestry of SLC22A1, SLC22A2, SLC22A3, ABCB1, CYP2C8, CYP2C9, and CYP2C19 in Ecuadorian Subjects with Type 2 Diabetes Mellitus. Pharmaceuticals, 18(9), 1335. https://doi.org/10.3390/ph18091335










